Abstract
Receptor interacting protein kinase 3 (RIPK3) is an essential serine/threonine kinase for necroptosis, a type of regulated necrosis. A variety of stimuli can cause RIPK3 activation through phosphorylation. Activated RIPK3 in turn phosphorylates and activates the downstream necroptosis executioner mixed lineage kinase domain-like (MLKL). Necroptosis is a highly inflammatory type of cell death because of the release of intracellular immunogenic contents from disrupted plasma membrane. Indeed, RIPK3-deficient mice exhibited reduced inflammation in many inflammatory disease models. These results have been interpreted as evidence that necroptosis is a key driver for RIPK3-induced inflammation. Interestingly, recent studies show that RIPK3 also regulates NF-κB, inflammasome activation, and kinase-independent apoptosis. These studies also reveal that these non-necroptotic functions contribute significantly to disease pathogenesis. In this review, we summarize our current understanding of necroptotic and non-necroptotic functions of RIPK3 and discuss how these effects contribute to RIPK3-mediated inflammation.
Keywords: RIPK3, RIPK1, MLKL, necroptosis, NF-κB, inflammasome, caspase 8, inflammation, TNF, apoptosis
Introduction
In pathology, the term necrosis is used to describe gross histological damage caused by cell death. It is defined at the cellular and morphological level by cell and organelle swelling and membrane rupture. On the other hand, the term apoptosis describes cell death marked by cellular shrinkage, chromatin condensation, and cellular fragmentation (Kerr et al., 1972). Although the different cell death modes were originally defined by morphology, we now know that they also exhibit distinct biological functions and are regulated by unique mechanisms. Genetic studies in C. elegans laid the foundation for discovery of numerous apopotosis genes and the signaling network that regulates it. These discoveries unveiled the important physiological roles of apoptosis in embryonic development (Ellis and Horvitz, 1986), immune homeostasis (Burger et al., 2014), and cancer (Hockenbery et al., 1990; Tsujimoto et al., 1985). In contrast, since necrotic cell death is often observed when cells are exposed to excessive physical or chemical stresses, it was considered to be an un-programmed and accidental cell death. However, accumulating evidence shows that necrosis can in fact be induced by dedicated regulatory signaling pathways and thus the long-standing dogma that necrosis represents un-regulated cell death is being challenged.
Necroptosis is a type of regulated necrosis which is controlled by receptor interacting protein kinase 3 (RIPK3) and its downstream effector mixed lineage kinase domain-like (MLKL) (Chan et al., 2014). Upon ligand binding, a variety of cell surface receptors, such as tumor necrosis factor (TNF) superfamily death receptors (Vercammen et al., 1998a; Vercammen et al., 1998b), toll like receptors (TLRs) (He et al., 2011), interferon receptors (IFNRs) (Thapa et al., 2011; Thapa et al., 2013), and T cell receptor (Ch'en et al., 2011; Lu et al., 2011; Osborn et al., 2010; Zhang et al., 2011), induce necroptosis through phosphorylation-driven activation of the RIPK3-MLKL signaling pathway. Germline Ripk3-deficient (Ripk3−/−) mice are widely used to examine the physiological functions of necroptosis. Many infectious and noninfectious inflammatory disease models were attenuated in Ripk3−/− mice (Chan et al., 2014). These observations bolster the premise that RIPK3 promotes the release of intracellular immunogenic contents through necroptosis to elicit inflammatory responses (Kaczmarek et al., 2013). However, recent evidence indicates that RIPK3 also exhibits necroptosis-independent functions and that the amelioration of inflammatory phenotypes in Ripk3−/− mice could at least in part be attributed to these non-necroptotic signaling functions (Moriwaki and Chan, 2014). In this review, we summarize our current knowledge of how RIPK3 executes necrotic and non-necrotic functions. We will also discuss how these distinct functions of RIPK3 cooperate to promote inflammation in physiology.
RIPK3 functions as an essential adaptor for necroptosis
Phosphorylation-driven activation of RIPK3
RIPK3 is a cytosolic serine/threonine kinase that consists of an active kinase domain at the amino terminus (Moriwaki and Chan, 2013). Essential amino acids for enzymatic activity of typical protein kinases are conserved in RIPK3, including the catalytic triad (Lys50, Glu60, and Asp160 in human RIPK3) and the DFG motif (Asp160, Phe161, and Gly162 in human RIPK3). RIPK3 also carries a unique homotypic protein-protein interaction domain, called RIP homotypic interaction motif (RHIM), at the carboxy terminus. The RHIM is characterized by a hydrophobic β-sheet dominant polypeptide sequence flanking a highly conserved tetra-peptide core (458-VQVG-461 in human RIPK3) (Sun et al., 2002). Both the kinase activity and the RHIM are indispensable for necroptosis in response to different physiological stimuli (Cho et al., 2009; He et al., 2009; Zhang et al., 2009).
RIPK3 is phosphorylated at multiple sites upon necroptosis induction (Chen et al., 2013; He et al., 2009). Although RIPK3 phosphorylation is key for necroptosis, the upstream events that trigger RIPK3 activation are not fully understood. In TNF-induced necroptosis, the upstream adaptor RIPK1, another RHIM-containing RIP kinase family protein, interacts with RIPK3 through the RHIM. This interaction is essential for TNF-induced necroptosis (Cho et al., 2009). Because RIPK1 kinase inhibitors block RIPK3 phosphorylation and necrotic cell death, RIPK1 is widely considered to be the upstream kinase that phosphorylates RIPK3 (Degterev et al., 2008; Degterev et al., 2005). However, RIPK1 failed to phosphorylate RIPK3 in in vitro kinase assay (Cho et al., 2009), suggesting that RIPK1 may function as an adaptor to provide a scaffold for RIPK3 to be activated by auto-phosphorylation. In this scenario, RIPK1 kinase activity may mediate its own auto-phosphorylation, which leads to changes in conformation and interaction with RIPK3. This alternative viewpoint is supported by the fact that RIPK3 is also activated by other necroptosis inducers such as the TLR3 ligand polyI:C and murine cytomegalovirus that signal independently of RIPK1 (Dillon et al., 2014; Upton et al., 2012; Weng et al., 2014). In these cases, RIPK3 interacts with the RHIM-containing proteins Toll/interleukin-1 (IL-1) receptor domain-containing adaptor protein inducing interferon β (TRIF) or DNA-dependent activator of interferon regulatory factor (DAI), both of which do not possess kinase activity. Thus, it is likely that RHIM-RHIM interaction provides the scaffold for RIPK3 activation rather than direct activation of RIPK3 through trans-phosphorylation.
Phospho-proteomic analysis has identified multiple phosphorylation sites on RIPK3 during TNF-induced necroptosis. Among them, Ser227 in human RIPK3 (Thr231/Ser232 in mouse RIPK3) has been reported to be crucial for necroptosis induction (Chen et al., 2013). Alanine substitution of Ser227 did not impair RIPK3 kinase activity, but blocked TNF-induced RIPK3-MLKL interaction. This is consistent with crystal structure analysis that phosphorylated Ser227 forms hydrogen bond with Ser404 in the pseudokinase domain of MLKL at the interface of the RIPK3-MLKL complex (Xie et al., 2013). Since phospho-mimetic glutamate mutation also blocked this interaction (McQuade et al., 2013), the negative charge from phosphorylation may not be key for this interaction. Rather, Ser288 phosphorylation may alter the conformation to facilitate binding to MLKL. In contrast to Ser232, site-directed mutagenesis analysis of conserved serine/threonine residues of RIPK3 identified Ser204 in mouse RIPK3 (Ser199 in human RIPK3) as an important residue for its kinase activity (McQuade et al., 2013). The phospho-mimetic mutant S204D, but not S204A, could mediate necroptosis in response to TNF. Interestingly, necroptosis mediated by RIPK3-S204D is no longer dependent on RIPK1 (McQuade et al., 2013), again indicating that RIPK3 can be activated downstream of TNF receptor without RIPK1.
RHIM-mediated formation of RIPK3 oligomer during necroptosis
Recent biochemical and structural studies showed that signaling adaptors often organize into higher-order and repetitive structures and that this organization is essential for full activity of the signaling complexes (Kagan et al., 2014). Similarly, the RHIM of RIPK3 mediates amyloid-like filamentous signaling complex formation with RIPK1 during TNF-induced necroptosis (Li et al., 2012). Single amino acid substitutions in the tetra-peptide core sequence of the RHIM prevented formation of this filamentous scaffold and TNF-induced necroptosis. Similar higher order structures also mediate RIPK3-induced necroptosis in models of chemically enforced dimerization of RIPK3 (Orozco et al., 2014).
How is the assembly of the RHIM-driven amyloid oligomer regulated? Curiously, the kinase activity of RIPK3 was also attenuated when the RIPK3 RHIM is mutated. On the other hand, RIPK3 kinase-dead mutant failed to form the amyloid oligomer (Li et al., 2012). These results suggest a feed-forward mechanism in which phosphorylation of residues in the kinase domain relieves the steric hindrance on the RHIM to promote polymerization. RHIM-mediated polymerization serves to further stimulate maximal kinase activity. Thus, in the quiescent state, the kinase domain of RIPK3 masks and prevents the RHIM from polymerization. In addition to phosphorylation, we recently found that RIPK3 undergoes K48-linked polyubiquitination in the kinase domain during normal turnover of the protein. Normally, this event does not lead to necroptosis as the unmasked RIPK3 is rapidly degraded by the proteasome. However, inhibition of the proteasome can trigger receptor-independent necroptosis (Moriwaki and Chan, 2016). Hence, the requirement for oligomerization-induced activation may serve to keep RIPK3 in check and to prevent inadvertent necroptosis during normal protein turnover.
Negative regulatory mechanisms of necroptosis
Endogenous necroptosis inhibitory proteins
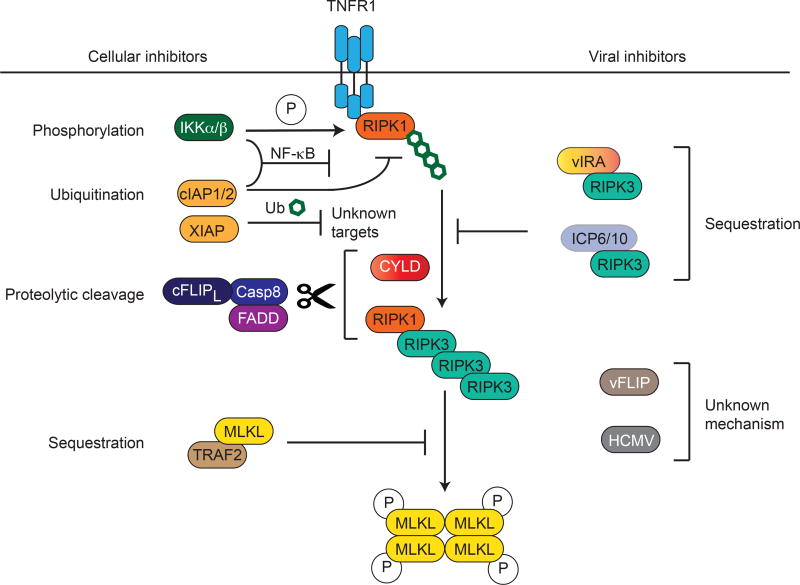
Necrotic cell death is a highly inflammatory type of cell death due to the release intracellular immunogenic contents that stimulates innate immune cells and subsequently inflammation. As such, there are cellular mechanisms put in place to keep in check the potential deleterious effects of necroptosis. For instance, the initiator caspase, caspase 8, not only induces apoptosis, but also has a critical role in necroptosis inhibition by cleavage of the crucial necroptosis regulators RIPK1, RIPK3, and cylindromatosis (CYLD) (Chan et al., 2003; Feng et al., 2007; Lin et al., 1999; O'Donnell et al., 2011). In fact, pharmacologic or genetic inhibition of caspase 8 is a trick that is widely used to induce necroptosis in the laboratory. In addition, the caspase 8 binding partner Fas-associated via death domain (FADD) is also required for caspase 8-mediated inhibition of necroptosis. Genetic inactivation of caspase 8 or FADD led to excessive RIPK1 and RIPK3-dependent necroptosis and embryonic lethality (Kaiser et al., 2011; Oberst et al., 2011; Zhang et al., 2011). Tissue-specific deletion of these two proteins in the intestine or the skin also caused severe RIPK3-dependent necroptosis and inflammation (Bonnet et al., 2011; Gunther et al., 2011; Welz et al., 2011). Hence, caspase 8 is a key checkpoint regulator that dictates the cell death mode (Fig. 1).
Figure 1. Cellular and viral inhibitors of necroptosis.
Multiple inhibitors of adaptors of necroptosis have been identified. These include cellular inhibitors such as IKKα/β, which directly phosphorylate RIPK1 to prevent it from engaging downstream cell death effectors, cellular IAPs and XIAP, which poly-ubiquitinate RIPK1 and other unknown substrates to prevent its interaction with RIPK3, cFLIPL/Caspase 8 heterodimer and their adaptor FADD, which cleave and inactivate RIPK1, RIPK3 and CYLD, and TRAF2, which sequesters MLKL and prevents it from interacting with RIPK3. Note that the IKKs and the IAPs can also inhibit necroptosis through NF-kB dependent induction of survival factors. On the right, the known viral and putative viral necroptosis inhibitors are shown. Both vIRA (M45) and ICP6/10 function to sequester RIPK3 from binding to other RHIM-containing adaptors.
Nuclear factor-κB (NF-κB) is a family of transcription factors that plays a central role in expression of inflammatory and pro-survival molecules such as cellular FLICE (caspase 8)-like IL-1β-converting enzyme-inhibitory protein (cFLIP). cFLIP is an enzymatically inactive caspase 8 homolog that binds to caspase 8 and inhibits its apoptotic activity (Irmler et al., 1997). Germline or tissue-specific deletion of cFLIP caused excessive caspase 8-mediated apoptosis (Dillon et al., 2012; Panayotova-Dimitrova et al., 2013; Piao et al., 2012; Weinlich et al., 2013; Yeh et al., 2000). Interestingly, the long isoform of cFLIP (cFLIPL) forms a heterodimer with caspase 8 that inhibits caspase-dependent apoptosis, but retains cleavage activity against RIPK1 and RIPK3 (Oberst et al., 2011) (Fig. 1). In contrast, the short isoform cFLIPS also inhibits this anti-necroptotic activity and thus functions as a pro-necroptotic factor (Feoktistova et al., 2011). Hence, necroptosis is tightly controlled by the balance between the two cFLIP isoforms.
In addition to cFLIP, the cellular inhibitor of apoptosis proteins (cIAPs) are also transcriptional targets of NF-κB. The cIAPs are E3 ubiquitin ligases that mediate RIPK1 ubiquitination upon TNF stimulation. RIPK1, cIAP1 and cIAP2 are recruited to TNFR1 complex along with TRADD, TNFR-associated factor 2 (TRAF2), and the linear ubiquitin chain assembly complex (LUBAC). RIPK1 and other polyubiquitinated adaptors within the TNF receptor 1 complex function as a scaffold to recruit IKKα/β/NEMO and TAK1/TAB1/TAB2 complexes to induce NF-κB-dependent pro-survival gene expression (Bertrand et al., 2008; Gerlach et al., 2011; Mahoney et al., 2008; Samuel et al., 2006; Vince et al., 2009). Although the primary function of IKKα/β is to stimulate NF-κB activation, a recent report showed that IKKα/β may also directly phosphorylate RIPK1 to block formation of the death-inducing RIPK1-FADD-caspase 8 complex (complex IIa) and RIPK1-RIPK3 complex (complex IIb) (Dondelinger et al., 2015) (Fig. 1). TRAF2 was reported to inhibit necroptosis by recruiting cIAPs to the TNFR1 complex and by direct interaction with MLKL to preclude it from binding to RIPK3 (Petersen et al., 2015). Another IAP protein, X-linked IAP (XIAP), also protected RIPK3-dependent cell death through unknown mechanism perhaps mediated by its E3 ligase function (Yabal et al., 2014) (Fig. 1). A20, a unique ubiquitin editing protein that carries both deubiquitination and E3 ligase activities, was also reported to block necroptosis through stabilization of linear ubiquitin chains in TNFR1 complex and direct deubiquitination of RIPK3 (Draber et al., 2015; Onizawa et al., 2015; Wertz et al., 2015). However, the precise lysine residue mediating this effect is controversial (Moriwaki and Chan, 2016). As such, many regulators of the NF-κB pathway negatively control RIPK3-dependent necroptosis in NF-κB-dependent and independent manners.
Viral necroptosis inhibitory proteins
Many viruses have acquired the ability to inhibit apoptosis during evolution. Specifically, large DNA viruses are adept at inhibiting caspase 8 to protect the viral factory within infected host cells from destruction. However, blocking caspase 8 activity sensitizes cells to necroptosis. Hence, cells infected by the poxvirus vaccinia virus, which encodes the caspase inhibitor B13R/Spi2, are highly sensitive to TNF- and RIPK3-dependent necroptosis (Cho et al., 2009). Since viruses have developed strategies to evade apoptosis, it raises the possibility that viruses may have also acquired anti-necroptotic functions throughout evolution. In addition to the M36-encoded viral inhibitor of caspase 8 activation (vICA), murine cytomegalovirus (MCMV) also carries the M45-encoded viral inhibitor of RIP activation (vIRA). M45/vIRA is homologous to the viral ribonucleotide reductase found in other herpesviruses, but exhibit no enzymatic activity. Interestingly, vIRA contains a RHIM at the N-terminus that interacts with the RHIM of RIPK3 (Upton et al., 2008, 2010). As a result, vIRA sequesters RIPK3 and prevents it from interacting with its cellular RHIM-containing partners RIPK1, TRIF and DAI (Fig. 1). Recently, RHIM-containing viral necroptosis inhibitors have been identified in herpes simplex virus type I (HSV-1) and HSV-2. In contrast to M45, the HSV inhibitors (ICP6 for HSV-1 and ICP10 for HSV2) are dual apoptosis and necroptosis inhibitors that bind to caspase 8 at the N-terminus and RIPK3 through the C-terminal RHIM (Dufour et al., 2011; Guo et al., 2015). ICP6 and ICP10 block necroptosis in human cells, their natural host, but induce necroptosis in mouse cells. This suggests that viral necroptosis inhibitors may also function as species restriction factors (Huang et al., 2015; Wang et al., 2014). Although human CMV also inhibits necroptosis, the responsible viral inhibitor has not been identified (Fig. 1) (Omoto et al., 2015). Interestingly, certain viral FLIPs have been shown to also inhibit TNF-induced necroptosis (Chan et al., 2003; Upton and Chan, 2014). However, the underlying mechanism has not been fully elucidated (Fig. 1).
How does RIPK3 promote inflammation?
The germline Ripk3−/− mice generated by Newton and Dixit have been instrumental in the study of physiological functions of RIPK3 and necroptosis over the years (Newton et al., 2004). Ripk3−/− mice exhibited reduced inflammatory phenotypes in viral infection models as well as sterile inflammatory diseases in the kidney (Linkermann et al., 2013), heart (Luedde et al., 2014; Zhang et al., 2016), blood vessel (Lin et al., 2013; Meng et al., 2015), pancreas (He et al., 2009), brain (Vitner et al., 2014), liver (Afonso et al., 2015; Dara et al., 2015; Deutsch et al., 2015; Gautheron et al., 2014; Kang et al., 2015b; Ramachandran et al., 2013; Roychowdhury et al., 2013; Vucur et al., 2013; Wang et al., 2016), intestine (Gunther et al., 2011; Moriwaki et al., 2014; Welz et al., 2011), skin (Bonnet et al., 2011), and retina (Murakami et al., 2014; Murakami et al., 2012; Trichonas et al., 2010). This has led to the current dogma that RIPK3 drives inflammation through necroptosis-associated release of intracellular immunogenic contents. Electron microscopy or standard histology is often used to detect necrotic cells in tissues. However, these methods are neither quantitative nor specific for necroptosis. Hence, it is difficult to evaluate whether necroptosis is truly a driver of these diseases in vivo. Moreover, it is noteworthy that optimal induction of necroptosis requires caspase 8 and cIAPs inhibition. Because deletion of FADD, caspase 8 or the IAPs often compromises survival of mice, it is questionable that they represent physiological disease conditions. As such, while certain virus infections can lead to caspase 8 and cIAPs inhibition, it remains unclear if these cellular necroptosis inhibitors are indeed disabled in other sterile inflammation models. Recently, RIPK3 was reported to possess multiple necroptosis-independent functions. Importantly, these novel functions of RIPK3 often do not require inhibition of FADD, caspase 8 or the IAPs. These observations thus raise the interesting possibility that RIPK3 may promote inflammation without induction of necroptosis.
Non-necrotic functions of RIPK3
RIPK3 in NF-κB activation
RIPK3 was originally identified as a RIPK1 binding protein with homology to RIPK1 and RIPK2, both of which were known NF-κB inducers (Sun et al., 1999; Yu et al., 1999). Similar to these other RIP kinase family members, early studies showed that over-expression of RIPK3 also alter NF-κB activation (Kasof et al., 2000; Meylan et al., 2004; Sun et al., 1999; Yu et al., 1999). However, the results were confusing since different studies have shown activating as well as inhibitory effects on NF-κB activation. Ripk3−/− mouse embryonic fibroblasts and bone marrow derived macrophages (BMDMs) exhibited normal TNF- and TLR ligand-induced phosphorylation and degradation of inhibitor κB α (IκBα) (Newton et al., 2004). These results led to the view that RIPK3 is dispensable for NF-κB activation. However, we recently found that RIPK3 is a cell type-specific NF-κB activator (Moriwaki et al., 2014). In GM-CSF and IL-4 derived bone marrow derived dendritic cells (BMDCs), TLR4-induced NF-κB activation and cytokine production was critically dependent on RIPK3. Instead of pairing with its more widely known partner p52 as a “non-canonical” NF-κB, RelB partners with p50 in DCs to regulate TLR-mediated cytokine expression (Shih et al., 2012). Consistent with previous report (Newton et al., 2004), phosphorylation and degradation of IκBα was normal in TLR4-stimulated Ripk3−/− BMDCs. Rather, nuclear translocation of RelB-p50 heterodimer was severely impaired in Ripk3−/− BMDCs. It is noteworthy that cell type-specific control of NF-κB activation and cytokine expression by RIPK3 has also been reported in aortic smooth muscle cells (Wang et al., 2015). In both BMDCs and aortic smooth muscle cells, RIPK3 controls NF-κB activation downstream of IκBα phosphorylation and degradation. The precise mechanism by which RIPK3 facilitates RelB-p50 heterodimer nuclear translocation in specific cell types is unknown at present. However, the RHIM, but not the kinase activity of RIPK3, appears to be crucial for this function ((Moriwaki et al., 2014) and unpublished observation). Nonetheless, these studies suggest that RIPK3 can drive powerful inflammation directly through cytokine production in a necroptosis-independent manner.
RIPK3 in inflammasome activation
Inflammasome is a macromolecular cytosolic protein complex in which the effector protease caspase 1 is activated in response to various stimuli (Latz et al., 2013). Activated caspase 1 cleaves pro-IL-1β and pro-IL-18 to produce the mature cytokines, both of which are key mediators of inflammatory pathologies. BMDCs are distinct from macrophages in that TLR4 stimulation alone is sufficient to induce processing and secretion of mature IL-1β, although an inflammasome-activating signal does result in higher level of IL-1β production (He et al., 2013). TLR4-induced IL-1β production was strongly suppressed in the absence of RIPK3 (Moriwaki et al., 2014). Consistent with its role in pro-IL-1β processing, caspase 1 activation was also blunted in Ripk3−/− BMDCs. In addition to caspase 1, the initiator caspase for death receptors, caspase 8, can also mediate pro-IL-1β cleavage in some cases (Maelfait et al., 2008). Similar to caspase 1, TLR4-induced caspase 8 activation was also abolished in Ripk3−/− BMDCs (Moriwaki et al., 2015). RIPK3-dependent cleavage of pro-IL-1β is negatively controlled by the IAPs and A20, since RIPK3-dependent IL-1β production was strongly enhanced in the absence of IAPs or A20 (Duong et al., 2015; Vince et al., 2012). These results suggest a crucial role for ubiquitination in regulating the non-necroptotic function of RIPK3.
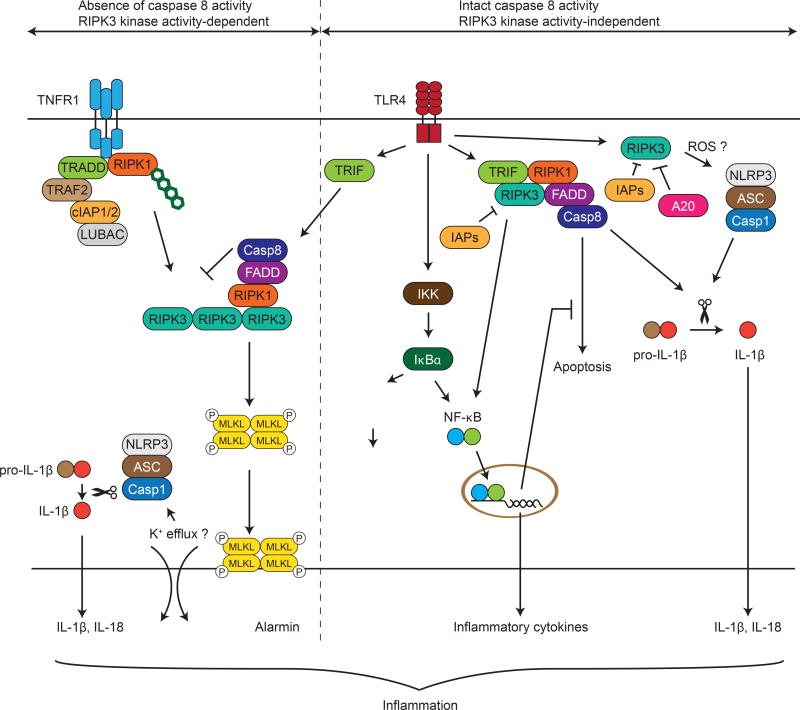
In contrast to necroptosis, RIPK3 kinase activity is dispensable for pro-IL-1β processing. This observation further bolsters the argument that RIPK3 promotes pro-IL-1β cleavage independent of necroptosis. Interestingly, reactive oxygen species (ROS) scavengers strongly inhibited LPS-induced caspase 1 activation and IL-1β secretion by BMDCs (Moriwaki et al., 2014). This is similar to RIPK3-induced necroptosis, which is also inhibited by ROS scavengers (Cho et al., 2009; Zhang et al., 2009). Since mitochondrial ROS has been implicated in inflammasome activation (Zhou et al., 2010; Zhou et al., 2011), these results suggest a model in which RIPK3 stimulates mitochondrial ROS production, which in turn activates the NLRP3 inflammasome and caspase 1 (Fig. 2).
Figure 2. Necroptotic and non-necroptotic functions of RIPK3.
Stimulation of TNF receptor 1 (TNFR1) and other innate immune receptors such as TLR4 in the presence of caspase 8 inhibition results in complex formation between RIPK1 and RIPK3. This complex also contains FADD and caspase 8 and is dependent on intact RIPK1 and RIPK3 kinase activities. Activated RIPK3 in turn phosphorylates MLKL, which leads to formation of membrane lesion through which intracellular immunogenic contents (alarmins) are released. Although MLKL may not directly induce membrane lesions, it eventually triggers pore formation to facilitate potassium efflux, a process that can lead to NLRP3 inflammasome activation. In BMDCs, RIPK3 facilitates NLRP3 inflammasome activation through ROS production, and caspase 8 activation through assembly of a TRIF-RIPK1-RIPK3-FADD-caspase 8 complex. These two pathways collectively promote processing of pro-IL-1β and pro-IL-18. Apoptosis is prevented through concomitant induction of pro-survival genes such as cFLIPL. In addition to pro-IL-1β and pro-IL-18 cleavage, TLR4 also stimulates RelB-p50 (NF-κB) activation downstream of IκBα phosphorylation and degradation, which is possibly mediated by ROS. RIPK3 promotes pro-IL-1β processing and NF-κB activation independent of its kinase activity.
Although ROS scavengers potently inhibited RIPK3-dependent caspase 1 activity, they had no effects on RIPK3-dependent caspase 8 activation (Moriwaki et al., 2014), indicating that RIPK3 regulates caspase 8-mediated pro-IL-1β processing through a distinct mechanism. Interestingly, Fadd−/−, Trif−/− and Ripk1−/− BMDCs also failed to activate caspase 8 in response to TLR4 stimulation, suggesting that these adaptors are required for RIPK3-dependent caspase 8 activation. Indeed, a complex containing RIPK1, RIPK3, FADD and caspase 8 is formed in BMDCs in response to LPS stimulation. Because TRIF is also a RHIM-containing adaptor, it is tantalizing to speculate that TRIF recruits and activates RIPK1 and RIPK3 through RHIM-RHIM interaction. Subsequently, RIPK1 further recruits FADD and caspase 8 through their respective death domains and death effector domains, resulting in the assembly of the RIPK3-RIPK1-FADD-caspase 8 complex. Thus, while caspase 8 cleaves and inactivates RIPK3 to inhibit necroptosis, RIPK3 can in turn act as an activator of caspase 8 in specific immune effectors such as DCs.
Although caspase 8 promotes IL-1β maturation and secretion in wild type DCs, caspase 8−/− DCs actually produced elevated level of IL-1β in response to TLR4 stimulation. The increase in IL-1β release is due to increased RIPK3-dependent NLRP3 inflammasome and caspase 1 activation. Moreover, the necroptosis effector MLKL is also required for elevated IL-1β release in caspase 8−/− DCs (Kang et al., 2013). As a result of the exaggerated IL-1β response, DC-specific caspase 8-deficient mice were highly vulnerable to LPS-induced septic shock (Kang et al., 2013). Similar requirement for RIPK3 kinase activity and MLKL for NLRP3 inflammasome and caspase 1 activation was observed in BMDMs stimulated with LPS and IAP antagonist or with poly(I:C) in the presence of pan-caspase inhibitor (Kang et al., 2015a; Lawlor et al., 2015). How RIPK3 and MLKL activate pro-IL-1β processing and secretion is unknown at present. One possible mechanism is that activated MLKL may induce pore formation at the plasma membrane to facilitate potassium efflux, which in turn activates the NLRP3 inflammasome (Fig. 2). Interestingly, while poly(I:C) alone induced NLRP3 inflammasome activation in the presence of pan-caspase inhibitor, it did not do so in caspase 8-deficient BMDMs (Kang et al., 2015a). In addition, caspase 8 has been implicated to promote priming of the inflammasome (Gurung et al., 2014; Weng et al., 2014). Based on these results, caspase 8 may function as a scaffold or a NF-κB activator to promote NLRP3 inflammasome activation. Whether caspase 8 activates or inhibits pro-IL-1β processing may depend on the cell type and activities of caspase 8 and cIAPs. Taken together, these results highlight the complex and paradoxical relationship between RIPK3 and caspase 8 in cell death and inflammasome activation.
RIPK3 functions in apoptosis
Besides processing of pro-IL-1β and pro-IL-18, the atypical RIPK1-RIPK3-FADD-caspase 8 complex may also play a role in apoptosis. In fact, this complex and similar versions of it, which were also known as the “ripoptosome”, was originally identified as macro-molecular complexes that mediate cell death (Feoktistova et al., 2011; Tenev et al., 2011). Recently, we found that high doses of RIPK3 kinase inhibitors can induce conformational change of RIPK3 that leads to assembly of the same RIPK3-caspase 8 complex that mediates pro-IL-1β processing (Mandal et al., 2014; Moriwaki et al., 2015). Formation of this complex and apoptosis can also be induced by expression of the kinase inactive RIPK3 mutant D161N mutation or in knock-in mice that harbor this mutation (Mandal et al., 2014; Newton et al., 2014). Apoptosis induced by RIPK3 kinase inhibitors or expression of RIPK3-D161N was rescued by inactivation of caspase 8. However, not all kinase inactive RIPK3 mutants promote formation of the caspase 8 complex and apoptosis (Mandal et al., 2014). Moreover, the death-inducing effect of the RIPK3 inhibitors was prominent in mouse cells, but much more subdued in human cells (unpublished observation). Hence, RIPK3 from different species exhibits differential sensitivity to conformation-induced caspase 8 and apoptosis activation. Although the same complex was also formed in LPS-treated BMDCs, they did not undergo apoptosis due to the more limited extent of caspase 8 activation. However, when de novo protein synthesis was blocked by cycloheximide, LPS did cause apoptosis of BMDCs in a RIPK3-dependent manner (Moriwaki et al., 2015). This suggests that LPS likely induces expression of pro-survival proteins that block caspase 8-mediated apoptosis. One such pro-survival factor could be the enzyme-inactive caspase 8 homolog cFLIPL, which is a transcriptional target of NF-κB. Caspase 8-cFLIPL heterodimer is known to exhibit altered substrate specificity compared with caspase 8 homodimer (Pop et al., 2011). TLR4-induced expression of cFLIPL can therefore alter the cleavage specificity of caspase 8 so that it retains cleavage activity on pro-IL-1β while blocking cleavage of apoptosis substrates.
Necroptosis-independent functions of RIPK3 in inflammatory diseases
As we have discussed already, necroptosis is thought to be a major mechanism by which RIPK3 mediates inflammation. To date, the majority of studies have used mice with compromised functions in FADD, caspase 8 or the cIAPs. As the biochemical studies indicate, these genetic manipulations strongly skew the response toward necroptosis. However, studies in Ripk3−/− mice with intact FADD, caspase 8 and cIAP functions indicate that RIPK3 can also regulate inflammation without inactivating the function of these necroptosis inhibitors. Dextran sulfate sodium (DSS) causes intestinal epithelial cell (IEC) injury and is a popular model for studying the tissue repair mechanism in experimental colitis. Because RIPK3 deficiency reversed the spontaneous colitis found in IEC-specific FADD- or caspase 8-deficient mice (Gunther et al., 2011; Welz et al., 2011), it was widely assumed that RIPK3 promotes colitis through necroptosis. In contrast to this model, Ripk3−/− mice developed more severe colitis in response to medium dose of DSS when compared with their wild type littermates (Moriwaki et al., 2014). Radiation chimera experiment showed that hematopoietic cell-derived IL-23 and IL-1β production was responsible for the protective function of RIPK3. As we have already described in previous sections, RIPK3 is essential for IL-23 and IL-1β production in BMDCs. This suggests that DCs in the intestinal lamina propria may similarly control IL-23 and IL-1β production during DSS-induced IEC injury. Both IL-23 and IL-1β are potent inducers of IL-22, a cytokine produced by type 3 innate lymphoid cells in the lamina propria that stimulates tissue repair in colonic tissues (Mizoguchi, 2012). Indeed, IL-22 expression and tissue repair were both retarded in DSS-treated Ripk3−/− mice. Importantly, the extent of IEC injury early during the reaction was similar between Ripk3−/− mice and their wild type littermates. These results strongly suggest that necroptosis is not a major driver for the sustained intestinal injury found in Ripk3−/− mice.
Another example of RIPK3 controlling inflammation independent of necroptosis comes from the K/B × N serum transfer mouse arthritis model. Disease pathology in this model is highly dependent on IL-1β. Interestingly, Ripk3−/− mice, but not Mlkl−/− or Casp1−/− mice, showed reduced IL-1β production and attenuated disease severity (Lawlor et al., 2015). Disease severity was further attenuated in Ripk3−/−Casp8−/− mice. These results suggest that IL-1β production might be mediated by the RIPK3-caspase 8 ripoptosome. In addition, genetic deletion of cIAP1, cIAP2 and XIAP in the myeloid lineage or administration of IAP antagonist in mice led to systemic inflammatory cytokine storm and granulocytosis that was attenuated by RIPK3 deletion (Wong et al., 2014). Again, IAP antagonist-induced TNF production was reveresed in Ripk3−/−, but not Mlkl−/− BMDMs. These results indicate RIPK3 can promote inflammation through necroptosis as well as independent of necroptosis. Cellular context and environmental cues likely determines whether one response dominates over the other one in any particular inflammatory disease. Moreover, one cannot rule out the possibility that both responses cooperate with each other to maximize the impact of RIPK3 signaling on inflammation.
Concluding remarks
Genetic evidence has provided strong rationale that RIPK3 is a therapeutic target in treating various inflammatory diseases. Indeed, several types of RIPK3 inhibitors targeting its kinase activity have been reported in recent years (Fauster et al., 2015; Mandal et al., 2014; Najjar et al., 2015; Rodriguez et al., 2016). However, as we have discussed here, RIPK3 can promote inflammation independent of its kinase activity and necroptosis. Therefore, it will be important to determine which RIPK3 function dominantly drives inflammation in each disease condition. Another hurdle to overcome is the potential difference between human and mouse RIPK3. For example, human RIPK3 does not interact with mouse MLKL and vice versa (Chen et al., 2013). There is also evidence to suggest that the molecular chaperon HSP90 may preferentially bind to human, but not mouse RIPK3 (Jacobsen et al., 2016; Li et al., 2015). Furthermore, we have found that RIPK3 kinase inhibitors were much more potent in inducing apoptosis through mouse RIPK3 than human RIPK3 (unpublished observation). These observations highlight the potential deficiency of mouse models in interrogating the role of RIPK3 in different human diseases. We therefore argue that a holistic understanding of RIPK3 biology and therapeutic potential will require detailed studies on the structural determinants that control human RIPK3 activation. For example, we currently have little knowledge on how phosphorylation and ubiquitination alter the folding between the kinase domain and RHIM domain of RIPK3. We also do not know how this conformational change facilitates recruitment and activation of MLKL. We also do not know whether the same changes occur when RIPK3 functions in a non-necroptotic manner. These are but some of the future challenges that await the scientific community to resolve.
References
- Afonso MB, Rodrigues PM, Carvalho T, Caridade M, Borralho P, Cortez-Pinto H, Castro RE, Rodrigues CM. Necroptosis is a key pathogenic event in human and experimental murine models of non-alcoholic steatohepatitis. Clin Sci (Lond) 2015;129:721–739. doi: 10.1042/CS20140732. [DOI] [PubMed] [Google Scholar]
- Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Bonnet MC, Preukschat D, Welz PS, van Loo G, Ermolaeva MA, Bloch W, Haase I, Pasparakis M. The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity. 2011;35:572–582. doi: 10.1016/j.immuni.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Burger ML, Leung KK, Bennett MJ, Winoto A. T cell-specific inhibition of multiple apoptotic pathways blocks negative selection and causes autoimmunity. Elife. 2014;3 doi: 10.7554/eLife.03468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch'en IL, Tsau JS, Molkentin JD, Komatsu M, Hedrick SM. Mechanisms of necroptosis in T cells. J Exp Med. 2011;208:633–641. doi: 10.1084/jem.20110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan FK, Luz NF, Moriwaki K. Programmed Necrosis in the Cross Talk of Cell Death and Inflammation. Annu Rev Immunol. 2014 doi: 10.1146/annurev-immunol-032414-112248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278:51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhou Z, Li L, Zhong CQ, Zheng X, Wu X, Zhang Y, Ma H, Huang D, Li W, et al. Diverse sequence determinants control human and mouse receptor interacting protein 3 (RIP3) and mixed lineage kinase domain-like (MLKL) interaction in necroptotic signaling. J Biol Chem. 2013;288:16247–16261. doi: 10.1074/jbc.M112.435545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dara L, Johnson H, Suda J, Win S, Gaarde W, Han D, Kaplowitz N. Receptor interacting protein kinase 1 mediates murine acetaminophen toxicity independent of the necrosome and not through necroptosis. Hepatology. 2015;62:1847–1857. doi: 10.1002/hep.27939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- Deutsch M, Graffeo CS, Rokosh R, Pansari M, Ochi A, Levie EM, Van Heerden E, Tippens DM, Greco S, Barilla R, et al. Divergent effects of RIP1 or RIP3 blockade in murine models of acute liver injury. Cell Death Dis. 2015;6:e1759. doi: 10.1038/cddis.2015.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon CP, Oberst A, Weinlich R, Janke LJ, Kang TB, Ben-Moshe T, Mak TW, Wallach D, Green DR. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep. 2012;1:401–407. doi: 10.1016/j.celrep.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F, Gong YN, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–1202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondelinger Y, Jouan-Lanhouet S, Divert T, Theatre E, Bertin J, Gough PJ, Giansanti P, Heck AJ, Dejardin E, Vandenabeele P, Bertrand MJ. NF-kappaB-Independent Role of IKKalpha/IKKbeta in Preventing RIPK1 Kinase-Dependent Apoptotic and Necroptotic Cell Death during TNF Signaling. Mol Cell. 2015;60:63–76. doi: 10.1016/j.molcel.2015.07.032. [DOI] [PubMed] [Google Scholar]
- Draber P, Kupka S, Reichert M, Draberova H, Lafont E, de Miguel D, Spilgies L, Surinova S, Taraborrelli L, Hartwig T, et al. LUBAC-Recruited CYLD and A20 Regulate Gene Activation and Cell Death by Exerting Opposing Effects on Linear Ubiquitin in Signaling Complexes. Cell Rep. 2015;13:2258–2272. doi: 10.1016/j.celrep.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour F, Sasseville AM, Chabaud S, Massie B, Siegel RM, Langelier Y. The ribonucleotide reductase R1 subunits of herpes simplex virus types 1 and 2 protect cells against TNFalpha- and FasL-induced apoptosis by interacting with caspase-8. Apoptosis. 2011;16:256–271. doi: 10.1007/s10495-010-0560-2. [DOI] [PubMed] [Google Scholar]
- Duong BH, Onizawa M, Oses-Prieto JA, Advincula R, Burlingame A, Malynn BA, Ma A. A20 restricts ubiquitination of pro-interleukin-1beta protein complexes and suppresses NLRP3 inflammasome activity. Immunity. 2015;42:55–67. doi: 10.1016/j.immuni.2014.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis HM, Horvitz HR. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986;44:817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- Fauster A, Rebsamen M, Huber KV, Bigenzahn JW, Stukalov A, Lardeau CH, Scorzoni S, Bruckner M, Gridling M, Parapatics K, et al. A cellular screen identifies ponatinib and pazopanib as inhibitors of necroptosis. Cell Death Dis. 2015;6:e1767. doi: 10.1038/cddis.2015.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Yang Y, Mei Y, Ma L, Zhu DE, Hoti N, Castanares M, Wu M. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19:2056–2067. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, MacFarlane M, Hacker G, Leverkus M. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautheron J, Vucur M, Reisinger F, Cardenas DV, Roderburg C, Koppe C, Kreggenwinkel K, Schneider AT, Bartneck M, Neumann UP, et al. A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol Med. 2014;6:1062–1074. doi: 10.15252/emmm.201403856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, Webb AI, Rickard JA, Anderton H, Wong WW, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- Gunther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF, Becker C. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Omoto S, Harris PA, Finger JN, Bertin J, Gough PJ, Kaiser WJ, Mocarski ES. Herpes simplex virus suppresses necroptosis in human cells. Cell Host Microbe. 2015;17:243–251. doi: 10.1016/j.chom.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P, Anand PK, Malireddi RK, Vande Walle L, Van Opdenbosch N, Dillon CP, Weinlich R, Green DR, Lamkanfi M, Kanneganti TD. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. 2014;192:1835–1846. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci U S A. 2011;108:20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- He Y, Franchi L, Nunez G. TLR agonists stimulate Nlrp3-dependent IL-1beta production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J Immunol. 2013;190:334–339. doi: 10.4049/jimmunol.1202737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Huang Z, Wu SQ, Liang Y, Zhou X, Chen W, Li L, Wu J, Zhuang Q, Chen C, Li J, et al. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe. 2015;17:229–242. doi: 10.1016/j.chom.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Jacobsen AV, Lowes KN, Tanzer MC, Lucet IS, Hildebrand JM, Petrie EJ, van Delft MF, Liu Z, Conos SA, Zhang JG, et al. HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis. 2016;7:e2051. doi: 10.1038/cddis.2015.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Kagan JC, Magupalli VG, Wu H. SMOCs: supramolecular organizing centres that control innate immunity. Nat Rev Immunol. 2014;14:821–826. doi: 10.1038/nri3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Fernandes-Alnemri T, Rogers C, Mayes L, Wang Y, Dillon C, Roback L, Kaiser W, Oberst A, Sagara J, et al. Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat Commun. 2015a;6:7515. doi: 10.1038/ncomms8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity. 2013;38:27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Bang BR, Han KH, Hong L, Shim EJ, Ma J, Lerner RA, Otsuka M. Regulation of NKT cell-mediated immune responses to tumours and liver inflammation by mitochondrial PGAM5-Drp1 signalling. Nat Commun. 2015b;6:8371. doi: 10.1038/ncomms9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasof GM, Prosser JC, Liu D, Lorenzi MV, Gomes BC. The RIP-like kinase, RIP3, induces apoptosis and NF-kappaB nuclear translocation and localizes to mitochondria. FEBS Lett. 2000;473:285–291. doi: 10.1016/s0014-5793(00)01473-3. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D'Cruz AA, Hall C, Kaur Spall S, Anderton H, Masters SL, et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun. 2015;6:6282. doi: 10.1038/ncomms7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Xu T, Cao Y, Wang H, Li L, Chen S, Wang X, Shen Z. A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. Proc Natl Acad Sci U S A. 2015;112:5017–5022. doi: 10.1073/pnas.1505244112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, Damko E, Moquin D, Walz T, McDermott A, et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150:339–350. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Li H, Yang M, Ren J, Huang Z, Han F, Huang J, Ma J, Zhang D, Zhang Z, et al. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep. 2013;3:200–210. doi: 10.1016/j.celrep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkermann A, Brasen JH, Darding M, Jin MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H, et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2013;110:12024–12029. doi: 10.1073/pnas.1305538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JV, Weist BM, van Raam BJ, Marro BS, Nguyen LV, Srinivas P, Bell BD, Luhrs KA, Lane TE, Salvesen GS, Walsh CM. Complementary roles of Fas-associated death domain (FADD) and receptor interacting protein kinase-3 (RIPK3) in T-cell homeostasis and antiviral immunity. Proc Natl Acad Sci U S A. 2011;108:15312–15317. doi: 10.1073/pnas.1102779108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedde M, Lutz M, Carter N, Sosna J, Jacoby C, Vucur M, Gautheron J, Roderburg C, Borg N, Reisinger F, et al. RIP3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovasc Res. 2014;103:206–216. doi: 10.1093/cvr/cvu146. [DOI] [PubMed] [Google Scholar]
- Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S, Beyaert R. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med. 2008;205:1967–1973. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E, Arora V, Mak TW, Lacasse EC, Waring J, Korneluk RG. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci U S A. 2008;105:11778–11783. doi: 10.1073/pnas.0711122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, Lich JD, Finger J, Kasparcova V, Votta B, et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell. 2014;56:481–495. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade T, Cho Y, Chan FK. Positive and Negative Phosphorylation Regulates RIP1 and RIP3-Induced Programmed Necrosis. Biochem J. 2013 doi: 10.1042/BJ20130860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Jin W, Wang X. RIP3-mediated necrotic cell death accelerates systematic inflammation and mortality. Proc Natl Acad Sci U S A. 2015;112:11007–11012. doi: 10.1073/pnas.1514730112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, Tschopp J. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A. Healing of intestinal inflammation by IL-22. Inflamm Bowel Dis. 2012;18:1777–1784. doi: 10.1002/ibd.22929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki K, Balaji S, McQuade T, Malhotra N, Kang J, Chan FK. The Necroptosis Adaptor RIPK3 Promotes Injury-Induced Cytokine Expression and Tissue Repair. Immunity. 2014;41:567–578. doi: 10.1016/j.immuni.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki K, Bertin J, Gough PJ, Chan FKM. A RIPK3-Caspase 8 complex mediates atypical pro-IL-1b processing. J Immunol. 2015 doi: 10.4049/jimmunol.1402167. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki K, Chan FK. RIP3: a molecular switch for necrosis and inflammation. Genes Dev. 2013;27:1640–1649. doi: 10.1101/gad.223321.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki K, Chan FK. Necrosis-dependent and independent signaling of the RIP kinases in inflammation. Cytokine Growth Factor Rev. 2014;25:167–174. doi: 10.1016/j.cytogfr.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki K, Chan FK. Regulation of RIPK3 and RHIM-dependent necroptosis by the proteasome. J Biol Chem. 2016 doi: 10.1074/jbc.M115.700997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Matsumoto H, Roh M, Giani A, Kataoka K, Morizane Y, Kayama M, Thanos A, Nakatake S, Notomi S, et al. Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsRNA-induced retinal degeneration. Cell Death Differ. 2014;21:270–277. doi: 10.1038/cdd.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Matsumoto H, Roh M, Suzuki J, Hisatomi T, Ikeda Y, Miller JW, Vavvas DG. Receptor interacting protein kinase mediates necrotic cone but not rod cell death in a mouse model of inherited degeneration. Proc Natl Acad Sci U S A. 2012;109:14598–14603. doi: 10.1073/pnas.1206937109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar M, Suebsuwong C, Ray SS, Thapa RJ, Maki JL, Nogusa S, Shah S, Saleh D, Gough PJ, Bertin J, et al. Structure guided design of potent and selective ponatinib-based hybrid inhibitors for RIPK1. Cell Rep. 2015;10:1850–1860. doi: 10.1016/j.celrep.2015.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Dugger DL, Wickliffe KE, Kapoor N, de Almagro MC, Vucic D, Komuves L, Ferrando RE, French DM, Webster J, et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343:1357–1360. doi: 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]
- Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell MA, Perez-Jimenez E, Oberst A, Ng A, Massoumi R, Xavier R, Green DR, Ting AT. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol. 2011;13:1437–1442. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoto S, Guo H, Talekar GR, Roback L, Kaiser WJ, Mocarski ES. Suppression of RIP3-dependent necroptosis by human cytomegalovirus. J Biol Chem. 2015;290:11635–11648. doi: 10.1074/jbc.M115.646042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onizawa M, Oshima S, Schulze-Topphoff U, Oses-Prieto JA, Lu T, Tavares R, Prodhomme T, Duong B, Whang MI, Advincula R, et al. The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis. Nat Immunol. 2015;16:618–627. doi: 10.1038/ni.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco S, Yatim N, Werner MR, Tran H, Gunja SY, Tait SW, Albert ML, Green DR, Oberst A. RIPK1 both positively and negatively regulates RIPK3 oligomerization and necroptosis. Cell Death Differ. 2014;21:1511–1521. doi: 10.1038/cdd.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn SL, Diehl G, Han SJ, Xue L, Kurd N, Hsieh K, Cado D, Robey EA, Winoto A. Fas-associated death domain (FADD) is a negative regulator of T-cell receptor-mediated necroptosis. Proc Natl Acad Sci U S A. 2010;107:13034–13039. doi: 10.1073/pnas.1005997107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayotova-Dimitrova D, Feoktistova M, Ploesser M, Kellert B, Hupe M, Horn S, Makarov R, Jensen F, Porubsky S, Schmieder A, et al. cFLIP regulates skin homeostasis and protects against TNF-induced keratinocyte apoptosis. Cell Rep. 2013;5:397–408. doi: 10.1016/j.celrep.2013.09.035. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Chen TT, Lawrence DA, Marsters SA, Gonzalvez F, Ashkenazi A. TRAF2 is a biologically important necroptosis suppressor. Cell Death Differ. 2015;22:1846–1857. doi: 10.1038/cdd.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao X, Komazawa-Sakon S, Nishina T, Koike M, Piao JH, Ehlken H, Kurihara H, Hara M, Van Rooijen N, Schutz G, et al. c-FLIP Maintains Tissue Homeostasis by Preventing Apoptosis and Programmed Necrosis. Sci Signal. 2012;5:ra93. doi: 10.1126/scisignal.2003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop C, Oberst A, Drag M, Van Raam BJ, Riedl SJ, Green DR, Salvesen GS. FLIP(L) induces caspase 8 activity in the absence of interdomain caspase 8 cleavage and alters substrate specificity. Biochem J. 2011;433:447–457. doi: 10.1042/BJ20101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, McGill MR, Xie Y, Ni HM, Ding WX, Jaeschke H. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013;58:2099–2108. doi: 10.1002/hep.26547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez DA, Weinlich R, Brown S, Guy C, Fitzgerald P, Dillon CP, Oberst A, Quarato G, Low J, Cripps JG, et al. Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis. Cell Death Differ. 2016;23:76–88. doi: 10.1038/cdd.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychowdhury S, McMullen MR, Pisano SG, Liu X, Nagy LE. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology. 2013;57:1773–1783. doi: 10.1002/hep.26200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel T, Welsh K, Lober T, Togo SH, Zapata JM, Reed JC. Distinct BIR domains of cIAP1 mediate binding to and ubiquitination of tumor necrosis factor receptor-associated factor 2 and second mitochondrial activator of caspases. J Biol Chem. 2006;281:1080–1090. doi: 10.1074/jbc.M509381200. [DOI] [PubMed] [Google Scholar]
- Shih VF, Davis-Turak J, Macal M, Huang JQ, Ponomarenko J, Kearns JD, Yu T, Fagerlund R, Asagiri M, Zuniga EI, Hoffmann A. Control of RelB during dendritic cell activation integrates canonical and noncanonical NF-kappaB pathways. Nat Immunol. 2012;13:1162–1170. doi: 10.1038/ni.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Lee J, Navas T, Baldwin DT, Stewart TA, Dixit VM. RIP3, a novel apoptosis-inducing kinase. J Biol Chem. 1999;274:16871–16875. doi: 10.1074/jbc.274.24.16871. [DOI] [PubMed] [Google Scholar]
- Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem. 2002;277:9505–9511. doi: 10.1074/jbc.M109488200. [DOI] [PubMed] [Google Scholar]
- Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, MacFarlane M, Cain K, Meier P. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Thapa RJ, Basagoudanavar SH, Nogusa S, Irrinki K, Mallilankaraman K, Slifker MJ, Beg AA, Madesh M, Balachandran S. NF-kappaB protects cells from gamma interferon-induced RIP1-dependent necroptosis. Mol Cell Biol. 2011;31:2934–2946. doi: 10.1128/MCB.05445-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, Rall GF, Degterev A, Balachandran S. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci U S A. 2013;110:E3109–3118. doi: 10.1073/pnas.1301218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trichonas G, Murakami Y, Thanos A, Morizane Y, Kayama M, Debouck CM, Hisatomi T, Miller JW, Vavvas DG. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc Natl Acad Sci U S A. 2010;107:21695–21700. doi: 10.1073/pnas.1009179107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228:1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- Upton JW, Chan FK. Staying alive: cell death in antiviral immunity. Mol Cell. 2014;54:273–280. doi: 10.1016/j.molcel.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton JW, Kaiser WJ, Mocarski ES. Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. J Biol Chem. 2008;283:16966–16970. doi: 10.1074/jbc.C800051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, Grooten J, Fiers W, Vandenabeele P. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998a;187:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercammen D, Brouckaert G, Denecker G, Van de Craen M, Declercq W, Fiers W, Vandenabeele P. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J Exp Med. 1998b;188:919–930. doi: 10.1084/jem.188.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince JE, Pantaki D, Feltham R, Mace PD, Cordier SM, Schmukle AC, Davidson AJ, Callus BA, Wong WW, Gentle IE, et al. TRAF2 must bind to cellular inhibitors of apoptosis for tumor necrosis factor (tnf) to efficiently activate nf-{kappa}b and to prevent tnf-induced apoptosis. J Biol Chem. 2009;284:35906–35915. doi: 10.1074/jbc.M109.072256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O'Reilly L, Mason K, Gross O, Ma S, Guarda G, et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Vitner EB, Salomon R, Farfel-Becker T, Meshcheriakova A, Ali M, Klein AD, Platt FM, Cox TM, Futerman AH. RIPK3 as a potential therapeutic target for Gaucher's disease. Nat Med. 2014;20:204–208. doi: 10.1038/nm.3449. [DOI] [PubMed] [Google Scholar]
- Vucur M, Reisinger F, Gautheron J, Janssen J, Roderburg C, Cardenas DV, Kreggenwinkel K, Koppe C, Hammerich L, Hakem R, et al. RIP3 inhibits inflammatory hepatocarcinogenesis but promotes cholestasis by controlling caspase-8- and JNK-dependent compensatory cell proliferation. Cell Rep. 2013;4:776–790. doi: 10.1016/j.celrep.2013.07.035. [DOI] [PubMed] [Google Scholar]
- Wang Q, Liu Z, Ren J, Morgan S, Assa C, Liu B. Receptor-interacting protein kinase 3 contributes to abdominal aortic aneurysms via smooth muscle cell necrosis and inflammation. Circ Res. 2015;116:600–611. doi: 10.1161/CIRCRESAHA.116.304899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Ni HM, Dorko K, Kumer SC, Schmitt TM, Nawabi A, Komatsu M, Huang H, Ding WX. Increased hepatic receptor interacting protein kinase 3 expression due to impaired proteasomal functions contributes to alcohol-induced steatosis and liver injury. Oncotarget. 2016 doi: 10.18632/oncotarget.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li Y, Liu S, Yu X, Li L, Shi C, He W, Li J, Xu L, Hu Z, et al. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc Natl Acad Sci U S A. 2014;111:15438–15443. doi: 10.1073/pnas.1412767111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinlich R, Oberst A, Dillon CP, Janke LJ, Milasta S, Lukens JR, Rodriguez DA, Gurung P, Savage C, Kanneganti TD, Green DR. Protective roles for caspase-8 and cFLIP in adult homeostasis. Cell Rep. 2013;5:340–348. doi: 10.1016/j.celrep.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernandez-Majada V, Ermolaeva M, Kirsch P, Sterner-Kock A, van Loo G, Pasparakis M. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477:330–334. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- Weng D, Marty-Roix R, Ganesan S, Proulx MK, Vladimer GI, Kaiser WJ, Mocarski ES, Pouliot K, Chan FK, Kelliher MA, et al. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc Natl Acad Sci U S A. 2014;111:7391–7396. doi: 10.1073/pnas.1403477111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz IE, Newton K, Seshasayee D, Kusam S, Lam C, Zhang J, Popovych N, Helgason E, Schoeffler A, Jeet S, et al. Phosphorylation and linear ubiquitin direct A20 inhibition of inflammation. Nature. 2015;528:370–375. doi: 10.1038/nature16165. [DOI] [PubMed] [Google Scholar]
- Wong WW, Vince JE, Lalaoui N, Lawlor KE, Chau D, Bankovacki A, Anderton H, Metcalf D, O'Reilly L, Jost PJ, et al. cIAPs and XIAP regulate myelopoiesis through cytokine production in an RIPK1- and RIPK3-dependent manner. Blood. 2014;123:2562–2572. doi: 10.1182/blood-2013-06-510743. [DOI] [PubMed] [Google Scholar]
- Xie T, Peng W, Yan C, Wu J, Gong X, Shi Y. Structural insights into RIP3-mediated necroptotic signaling. Cell Rep. 2013;5:70–78. doi: 10.1016/j.celrep.2013.08.044. [DOI] [PubMed] [Google Scholar]
- Yabal M, Muller N, Adler H, Knies N, Gross CJ, Damgaard RB, Kanegane H, Ringelhan M, Kaufmann T, Heikenwalder M, et al. XIAP restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell Rep. 2014;7:1796–1808. doi: 10.1016/j.celrep.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Yeh WC, Itie A, Elia AJ, Ng M, Shu HB, Wakeham A, Mirtsos C, Suzuki N, Bonnard M, Goeddel DV, Mak TW. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633–642. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- Yu PW, Huang BC, Shen M, Quast J, Chan E, Xu X, Nolan GP, Payan DG, Luo Y. Identification of RIP3, a RIP-like kinase that activates apoptosis and NFkappaB. Curr Biol. 1999;9:539–542. doi: 10.1016/s0960-9822(99)80239-5. [DOI] [PubMed] [Google Scholar]
- Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–376. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv F, Liu Y, Zheng W, Shang H, Zhang J, et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med. 2016;22:175–182. doi: 10.1038/nm.4017. [DOI] [PubMed] [Google Scholar]
- Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]