Abstract
People diagnosed with neuropsychiatric disorders such as depression, anxiety, addiction or schizophrenia often have dysregulated memory, mood, pattern separation and/or reward processing. These symptoms are indicative of a disrupted function of the dentate gyrus (DG) subregion of the brain, and they improve with treatment and remission. The dysfunction of the DG is accompanied by structural maladaptations, including dysregulation of adult-generated neurons. An increasing number of studies using modern inducible approaches to manipulate new neurons show that the behavioral symptoms in animal models of neuropsychiatric disorders can be produced or exacerbated by the inhibition of DG neurogenesis. Thus, here we posit that the connection between neuropsychiatric disorders and dysregulated DG neurogenesis is beyond correlation or epiphenomenon, and that the regulation of adult-generated DG neurogenesis merits continued and focused attention in the ongoing effort to develop novel treatments for neuropsychiatric disorders.
By 2020, neuropsychiatric disorders are predicted to be the second highest cause of global disease burden1. Current treatment for disorders such as major depressive disorder (MDD), bipolar disorder, schizophrenia, post-traumatic stress disorder (PTSD) and substance-related or addictive disorders includes pharmacological intervention, which provides relief for many people. However, these therapies are ineffective for as many as 30% of individuals, and they are often accompanied by substantial side effects2–4. Equally concerning is that a high percentage of treated individuals relapse5–8. These facts call for aggressive expansion of the current neuropsychiatric-disorder treatment toolkit.
Clues to treatment and understanding of neuropsychiatric disorders come from the fact that many symptoms of these disorders are reminiscent of abnormal DG function. Similarly to the hippocampus that surrounds it, the DG has a role in memory and mood regulation, and it is also instrumental in the processing of contextual similarities and differences known as ‘pattern separation,’ as well as the processing of intrinsically positive or rewarding stimuli (Box 1)9–16. The DG is also highly sensitive to stressful experiences, and baseline DG function can be enhanced or inhibited by stress (Box 1)17–19. Illustrative of aberrant DG function, many people diagnosed with MDD, bipolar disorder, schizophrenia, PTSD or substance-related or addictive disorders have memory dysfunction and mood fluctuations or abnormalities20–22. Humans diagnosed with MDD, PTSD or schizophrenia show aberrant pattern separation22–24, whereas those diagnosed with MDD, bipolar disorder, schizophrenia or substance-related and addictive disorders have abnormal processing of rewarding stimuli25.
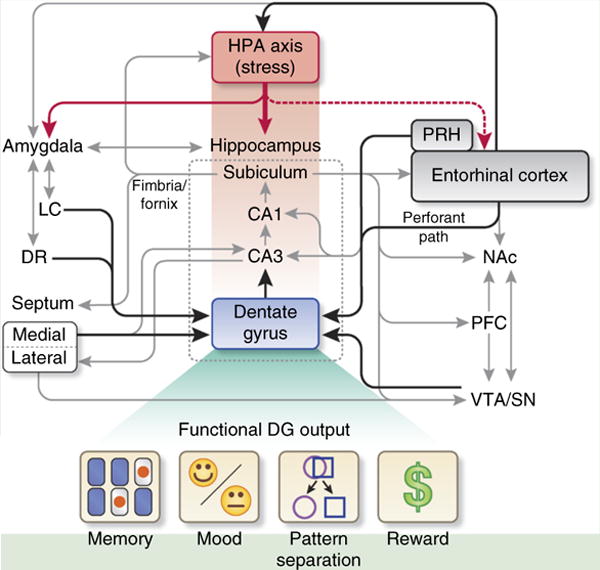
Box 1. The circuits and functional output of the hippocampal DG.
The healthy hippocampal DG receives input (black lines) from the limbic system (Entorhinal cortex (Ent) via perforant path (PP), perirhinal cortex (PRH), medial septum and (not shown) contralateral DG and recurrent collaterals from hippocampal CA3) and midbrain and hindbrain modulatory regions (VTA/SN, LC, DR)16. Many other non-DG connections that might affect the DG indirectly are also indicated (gray lines). The DG and hippocampus are highly sensitive to stress (for example, physiological, environmental) owing to direct inputs from the hypothalamic–pituitary–adrenal axis (HPA, red lines, red shading) and putative, indirect effects from the HPA to Ent (red dotted line)17–19. In turn, the HPA axis is influenced by the DG in part through hippocampal, subicular and Ent projections to the HPA axis. Working in concert, these and other brain circuits contribute to functional DG output, which are shown in schematic form to represent memory, mood, pattern separation and reward. As discussed in the main text, neuropsychiatric disorders are marked by dysregulated memory, mood, pattern separation and reward processing, symptoms that are correlated with impaired DG function and/or impaired generation or function of new DG neurons which may be affected by stress (Table 1). CA1, cornu ammon 1; CA3, cornu ammon 3; DG, dentate gyrus; DR, dorsal raphe; Ent, entorhinal cortex; HPA, hypothalamic–pituitary–adrenal; LC, locus coeruleus; NAc, nucleus accumbens septi; PFC, prefrontal cortex; PP, perforant path; PRH, perirhinal cortex; SN, substantia nigra; VTA, ventral tegmental area.
Furthermore, in humans and animal models, stressful experiences can enhance the susceptibility to and severity of these disorders and their symptoms18,22,26,27, which also suggests a role for the DG in these disorders (Table 1). Finally, successful treatment of these disorders in humans and the application of similar therapies in animal models normalize or enhance DG function (Fig. 1). For example, brain-stimulation therapy is commonly applied to brain cortical regions to ‘jump start’ or recalibrate brain circuitry in MDD28. However, stimulation of the entorhinal cortex (Ent), a brain region functionally upstream from the DG (Box 1), improves memory in both humans and mice29,30.
Table 1.
Overview of studies correlating neurogenesis in the DG and neuropsychiatric disorders. Given that more than 2,000 publications have contributed to the correlative findings summarized here, review publications are primarily cited in Table 1 to direct the reader to comprehensive and referenced tables in the literature
| Correlated with neuropsychiatric disorders | Stage of neurogenesis (human postmortem)
|
Hippocampal
|
Normalize/improve with treatment? (human/rodents) | |||
|---|---|---|---|---|---|---|
| Proliferation | Differentiation | Survival | Volume (human) | Network activity (human/rodents) | ||
| Major depressive disorder | Decreased/nc133–135 | – | Decreased*104 | Decreased136 | Decreased59,61 | ✓/nc42,97,104,133,134 |
| Bipolar disorder | nc135 | Increased125 | Decreased (trend)125 | Decreased/nc137,138 | Decreased/Increased/nc139 | ✓99,140,141 |
| Schizophrenia | Decreased132,135 | Decreased142 | – | Decreased143 | Increased/Decreased92,93,144 | ✓101 |
| Post-traumatic stress disorder | – | – | – | Decreased145 | Decreased94 | ✓100 |
| Substance-related and addictive disorder | Decreased146 | Decreased#146 | – | Decreased/nc147,148 | Increasedˆ95 | ✓41 |
Asterisk (*), fewer DG granule neurons in MDD versus MDD with medication and controls;
decreased dendritic arborization of immature neurons in heroin addicts;
carat (ˆ), increased connectivity between hippocampus and reward-related brain regions. nc, not changed;
dash (–), not studied.
Figure 1.
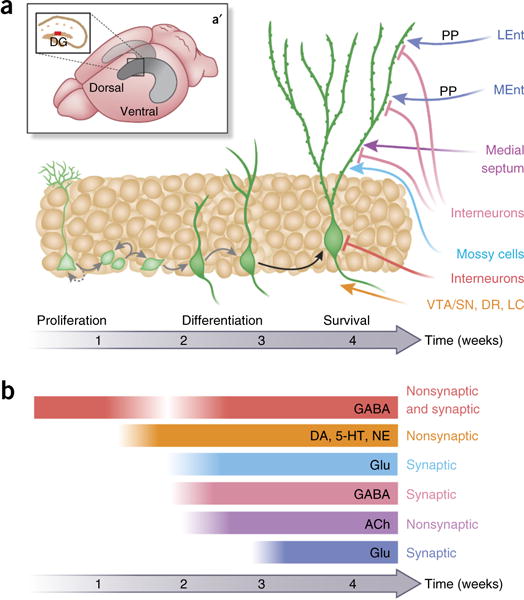
Neurogenesis in the DG and its sensitivity to neurotransmitter systems with relevance to common therapies. (a) In the adult mouse DG (a′ hippocampus in gray, dorsal DG in tan, main schematic in a is expanded view of red bar in the inset), new neurons are generated over time, depicted as green cells maturing through developmental ‘stages’ of proliferation, differentiation and survival, a process that takes ~4 weeks. Mature DG granule neurons (right, green cells) receive diverse input from other DG cells (Mossy Cells, blue; interneurons, red and pink) and other limbic (lateral and medial entorhinal cortex (LEnt, MEnt) in dark blue; medial septum in purple) and midbrain and hindbrain regions (ventral tegmental area/substantia nigra (VTA/SN), dorsal raphe (DR), locus coeruleus (LC) in orange). The main somatic input to new DG granule neurons is from inhibitory interneurons (red flathead lines; for example, DG basket cells). Late-stage differentiation and surviving cells are darker green, whereas proliferating cells are lighter green. (b) As new DG neurons develop, they are regulated by an increasingly diverse set of neurotransmitters. The first input is nonsynaptic (ambient) GABA (red bar) from DG interneurons (red input, a), which eventually transitions to include synaptic GABA as well (white gradient in red bar). The last being synaptic glutamate (bottom dark blue bar, b) from Ent neurons (dark blue arrows (a)). Treatments for neuropsychiatric disorders act on many of the neurotransmitter systems that regulate new neurons (b). Ach, acetylcholine; DA, dopamine; DG, dentate gyrus; DR, dorsal raphe; Ent, entorhinal cortex (LEnt, lateral Ent; MEnt, medial Ent); GABA, gamma-aminobutyric acid; Glu, glutamate; LC, locus coeruleus; NE, norepinephrine/noradrenergic; SN, substantia nigra; VTA, ventral tegmental area.
One aspect of DG physiology that has offered hope with regard to the normalization of DG function in neuropsychiatric disorders is its ability to give rise to new neurons throughout life (Fig. 1). These new neurons are found in the DG of mammals ranging from rodents to humans31, and work in mice shows that new neurons are able to wire and fire correctly within the DG (Fig. 1a)27,32–35.
New DG neurons are dynamically regulated by environmental and physiological factors32,36–40. Notably, the number of new neurons is increased by neuropsychiatric medications such as antidepressants and altered by drugs of abuse41,42, and this correlative link between new DG neurons and neuropsychiatric disorders is a well-established concept (Table 1)18,26,43–50.
However, in the past decade, new technologies have emerged that enable inducible manipulation of new DG neuron number, morphology or activity in mice and rats. As highlighted in Table 2 (full details in Supplementary Table 1), studies that employ these modern approaches indicate a role for new DG neurons in memory, mood, pattern separation and reward29,51–68. In addition, studies using animal models of depression coupled with these new technologies show that behavioral symptoms can be produced or ameliorated by select manipulation of new DG neurons. On the basis of this recent work in mice and rats, we posit that the connection between neuropsychiatric disorders and dysregulated DG neurogenesis is beyond correlation or epiphenomenon, and that the regulation of new DG neurons merits continued and focused attention in the ongoing effort to develop novel treatments for neuropsychiatric disorders.
Table 2.
Overview of studies linking DG neurogenesis and features of psychiatric disorders in a causative manner
| Approach to manipulate new neurons or DG
|
|||||
|---|---|---|---|---|---|
| Disrupt or inhibit | Enhance or stimulate | Animal model | Behavioral assessment of DG function | Reference | Influence of manipulation on DG function |
| MEMORY | |||||
| Disrupt | – | – | Acquisition | 51 | Impaired |
| Disrupt | – | – | Retrieval | 66 | Impaired |
| – | Enhance | – | Acquisition/retrieval | 118 | nc |
| Disrupt | – | – | Acquisition/retrieval+ | 29 | Impaired |
| Inhibit | – | – | Acquisition/retrieval | 52 | Impaired |
| Disrupt | – | – | Acquisition | 53 | Impaired |
| Inhibit | Stimulate | – | Acquisition/retrieval+ | 54 | Impaired (inhib or stim) |
| Disrupt | Enhance | – | Retrieval* | 67 | Enhanced or impaired |
| Inhibit, Disrupt | – | – | Retrieval | 55 | Impaired |
| Disrupt | – | – | Acquisition/retrievalˆ | 65 | Impaired |
| Inhibit | Stimulate | – | Acquisition/retrieval | 56 | Impaired (inhib or stim) |
| – | Enhance | – | Acquisition/retrieval+ | 57 | Enhanced |
|
| |||||
| MOOD | |||||
| Disrupt | – | Stress (chronic) | Anxiety, stress-induced despair | 58 | Increased |
| Disrupt | – | – | Despair | 59 | Increased |
| Disrupt | – | Stress (chronic) | Stress-induced despair | 60 | Increased |
| Disrupt | – | – | Anxiety, stress-induced despair | 66 | nc |
| – | Enhance | – | Anxiety, stress-induced despair | 118 | Decreased or nc |
| Disrupt | – | Stress (acute) | Anxiety, despair | 62 | Increased |
| Disrupt | – | Stress (chronic) | Anxiety | 61 | Increased |
| Disrupt | – | – | Anxiety, despair | 53 | nc |
| Inhibit | Stimulate | – | Anxiety | 54 | Decreased (stim) |
| Disrupt | – | Stress (chronic) | Stress-induced anxiety, despair# | 63 | Increased |
| – | Enhance | Stress hormone | Anxiety, stress-induced despair | 68 | Decreased |
| Disrupt | – | – | Anxiety§ | 65 | Increased |
| Disrupt | – | Stress (chronic) | Stress-induced anxiety | 108 | Increased |
| Inhibit | Stimulate | – | Anxiety | 56 | nc |
| – | Enhance | – | Anxiety, stress-induced despair | 57 | nc |
|
| |||||
| PATTERN SEPARATION | |||||
| – | Enhance | – | Pattern separation | 118 | Enhanced |
| Disrupt | – | – | Pattern separation | 53 | Impaired |
| Inhibit | – | – | Pattern separation | 56 | Impaired |
| – | Stimulate | – | Pattern separation | 57 | Enhanced |
|
| |||||
| REWARD | |||||
| Disrupt | – | Cocaine self-admin | Rat model of addiction | 64 | Increased |
| Disrupt | – | Stress (acute) | Natural reward | 62 | Decreased |
References cited target and manipulate new DG neurons or DG activity and assess a DG function (memory, mood, pattern separation, reward) relevant to neuropsychiatric disorders. Influence on DG function for memory and pattern-separation outcomes are given as enhanced, impaired or nc. Influence on DG function for mood- and reward-related are given as increased, decreased or nc. For study inclusion criteria, detailed list of results and specific figure panels that led to our conclusions in Table 2, see Supplementary Table 1.
Asterisk (*), altered neurogenesis post-learning;
carat (ˆ), dependent on type of conditioning (trace or delay);
cross (+), cognitive flexibility;
pound sign (#), only after adrenalectomy;
dollar sign (§), only after, not before, memory testing;
nc, not changed; self-admin, self-administration; —, not examined.
Adult DG neurogenesis
Adult DG neurogenesis in laboratory animals and humans shares common features69–71. For example, new DG neurons occur throughout the hippocampal DG, from the dorsal (or septal) to the ventral (or temporal) DG (mouse DG shown in Fig. 1a), albeit with distinct functional contributions along the DG longitudinal axis72–75. Also, as with the process of embryonic neurogenesis, adult DG neurogenesis in humans and mice and rats is a process, not a timepoint, with maturing cells proceeding through “stages” (Fig. 1a)27,71. In general, the proliferation, differentiation and eventual survival to maturity of new DG neurons takes 2–4 weeks in mice and rats (Fig. 1a), but weeks longer in primates and humans, as revealed by postmortem studies31,76. New DG neurons in both rodents (mice, rats) and humans do not replace existing embryonic-generated DG neurons, but rather contribute to the ongoing turnover (addition and subtraction) of newborn neurons in a restricted part of the DG (Fig. 1a)9,71,77–80.
In contrast to these shared traits, most of what is known about the specific development and afferent input of new DG neurons is understood in mice and rats. Once mature, new DG neurons become glutamatergic granule neurons that receive glutamatergic input from the upstream Ent and inhibitory GABAergic feedback from DG interneurons81 and project to the downstream hippocampal CA3 region (Box 1)16. In this sense, fully mature adult-generated DG neurons are indistinguishable from embyronic-generated DG granule neurons. However, as they mature, new DG neurons receive specialized inputs and outputs and dynamically express key intrinsic ion channels and neurotransmitter receptors (Fig. 1a,b)32,82,83. For example, younger new DG granule neurons receive direct innervation from mature DG granule neurons and downstream hippocampal cornus ammonis region 3 (CA3) pyramidal neurons84, and these inputs disappear as the new neuron matures. In addition, younger new DG granule neurons neither receive nor send feedback inhibition81, whereas older new DG neurons do. The location of inputs to new DG neurons is crucial for determining their influence on new neuron function33,35,83. For example, most glutamatergic inputs are on the dendritic tree of new neurons, whereas inhibitory inputs are on or near the soma (Fig. 1a)33,35. These inputs form gradually over the >4 weeks that it takes for new neurons to mature (Fig. 1b), with nonsynaptic, ambient GABA as the first input and synaptic glutamate from Ent as the last33,35,83. Taken together with the fact that new neurons have a lower threshold for firing relative to embryonic-generated neurons and that they are sensitive to activity-dependent regulation36,85–87, the emerging view is that immature adult-generated DG neurons are distinct from mature adult-generated and all embryonic-generated DG neurons in that they are “young and excitable”88, function independently of inhibitory GABA circuits89 and receive direct innervation from their glutamatergic “elders”84. In fact, some work suggests that adult-generated neurons remain functionally distinct from their embryonic-generated counterparts90.
Neuropsychiatric disorders and adult DG neurogenesis
It has long been known that the development of new neurons is regulated by antidepressants and drugs of abuse41,42. In fact, treatments for neuropsychiatric disorders (including antidepressants, electroconvulsive treatment, antipsychotics and mood stabilizers, all of which can improve DG functional output in humans diagnosed with or animal models of neuropsychiatric disorders) act on many of the very neurotransmitter systems that regulate new neurons (Fig. 1b)26,91. Such studies raise the idea that neuropsychiatric disorders are linked with fewer new DG neurons, and that treatment or remission is linked to more or a normalized number of new DG neurons.
Although this correlative relationship between new DG neurons and neuropsychiatric disorders relies on only a few human postmortem publications, there is much evidence in animal models that these disorders are linked to impaired generation or function of new DG neurons26,47. New DG neurons contribute to hippocampal volume and appropriate DG function and activity, and in humans, mice and rats, these disorders are also marked by impaired hippocampal volume and/or DG network activity (Table 1). For example, the relative activity between the DG and the downstream hippocampal CA1 region (Box 1) is decreased in stressed rats as compared to control rats59, which is notable, given that stress is a major precipitating factor in the exacerbation of neuropsychiatric symptoms. In animal models of bipolar disorder and schizophrenia, the stress- or novelty-induced activity of the DG is diminished92,93. Additionally, humans with PTSD have decreased hippocampus and amygdala activity relative to undiagnosed, trauma-exposed controls94. Finally, long-term cocaine users have decreased hippocampal gray matter, and abstinent heroin-dependent users have enhanced connectivity among hippocampus and reward-related brain regions relative to individuals who do not use drugs95,96.
Additional support for a correlation between the presence of fewer new DG neurons and the presence or exacerbation of neuropsychiatric symptoms comes from studies in both humans and rodents in which a decreased number of new neurons or lower DG volume is normalized or improved after treatment or during remission (Table 1). For example, postmortem studies show that chronic antidepressant treatment restores proliferation deficits in humans with MDD97. Chronic treatment with the mood stabilizer lithium increases new-neuron proliferation and differentiation in animal models, and the bipolar disorder medication valproate rescues aberrant DG activity and manic-like behavior in animal models98,99. Furthermore, anti-psychotic drugs, such as haloperidol, risperidone or clozapine, restore neurogenesis in animal models of schizophrenia, and antipsychotics can also ameliorate anxiety and restore new-neuron proliferation in an animal model of PTSD100,101.
Neuropsychiatric symptoms and neurogenesis—a causative relationship?
Recently, techniques have emerged that enable more focused exploration of the correlative–causative relationship of neurogenesis and neuropsychiatric disorders (Table 2; see also Supplementary Table 1). These include studies in mice and rats that ablate new neurons at a specific ‘stage’ of their development, manipulate the number, structure or activity of new DG neurons, or couple ‘classic’ new-neuron deletion techniques, such as irradiation, with behavioral investigations that reflect modern understanding of new neuron function29,51–65. For example, by using retroviral-mediated gene transfer to express light-sensitive channels in proliferating precursors, researchers are able to control new neuron firing remotely52. Recently developed transgenic mouse lines also enable the targeting of new neurons56 or of mature DG granule neurons54 with these light-sensitive channels, and they enable inducible gene expression or deletion selectively in new neurons53,56,62,63,65 or mature DG granule neurons54,55,57. The temporal control that researchers have over the firing of the targeted cell population in these optogenetic studies is ideal for behavioral investigations. As discussed below, most data are available for memory and mood, although studies have also confirmed a role for new DG neurons in pattern separation and reward29,51–65.
Memory
Neuropsychiatric disorders are accompanied by memory dysfunction20–22. Given that recent studies support the idea that manipulation of new neurons can alter memory, it is possible that the activation of new neurons might be useful for normalizing memory dysfunction seen in neuropsychiatric disorders. For example, studies in mice have found that the ablation or inducible silencing of new or mature DG neurons impairs aspects of memory function9,26,102, with some finding impairments in memory acquisition or the learning of a new memory52,56, and others finding impaired retrieval, or expression of the memory after it has been formed52,55. These studies, all of which manipulate adult or embryonic-generated neurons during or before learning, suggest that enhancing neurogenesis might improve memory.
Notably, understanding the timing of neurogenesis disruption is crucial to understanding the role of new neurons in memory. For example, disruption of neurogenesis after learning has taken place impairs memory retrieval, which suggests that new neurons are needed as well for ‘forgetting’66,67. Given that certain neuropsychiatric disorders (PTSD, substance-related and other addictive disorders) can be considered as being marked by aberrantly strong memories, it is feasible that postlearning enhancement of neurogenesis might help to drive the forgetting of such memories.
Also relevant to neuropsychiatric disorders is recent work showing that the ‘age’ or stage of new DG neurons determines whether the cells are involved in functional DG output52,56. For example, the stimulation of 4-week-old new DG neurons—but not of 2-week-old immature neurons or 8-week-old fully mature neurons—is required for memory retrieval52. This timing fits with that of new-neuron integration into DG-CA3 circuitry (Fig. 1a)32, and with the concept that there is a ‘critical period’ during which new neurons are needed for successful memory processing103.
Mood disorders
With regard to mood disorders, MDD, anxiety and PTSD are strongly linked to abnormal hippocampal structure and function, and impaired mood and greater anxiety are hallmarks of these human disorders18,26. Humans diagnosed with MDD often have an exacerbated or prolonged stress response and lack the typical hippocampal inhibition of the stress axis relative to individuals without depression, which again indicates that this mood disorder is linked to a dysfunctional hippocampus18. Notably, many correlative studies support the idea that rodents (mice and rats) exposed to chronic stress and humans diagnosed with MDD have decreased or dysfunctional neurogenesis, and that antidepressant treatment drives neurogenesis47,104. Techniques used to ablate new neurons led to a depressive-like phenotype in mice and rats in some studies, but not others47,105. These mixed messages as to the role that new neurons have in depression raises concerns that neurogenesis is an epiphenomenon, and thus not a reasonable target for treatment106.
However, a relatively consistent conclusion from the recent studies in rodents is that new DG neurons are required for antidepressant efficacy58–61(Table 2; Supplementary Table 1). One clue to a potential underlying mechanism is that new neurons buffer the body’s stress axis responsiveness and associated behaviors62,107. For example, a loss of new neurons results in a hyperactive stress response in mice62. In fact, it is now hypothesized that having a decreased number of DG neurons contributes to the development of depressive-like behaviors particularly under stressful conditions, such as the psychosocial stress experienced during social defeat stress63. Indeed, increased DG neurogenesis in mice is sufficient to attenuate anxiety and depressive-like behavior under stress-like conditions in which the rodents are given the stress hormone corticosterone68. In further support of the role of new DG neurons in buffering the stress response, the positive effect on stress-induced anxiety of a neurogenic-compound-induced increase in neurogenesis is blunted by the ablation of neurogenesis108.
Current studies have manipulated new DG neurons or DG activity in the absence of substantial stress to address whether mood regulation is mediated by them. Notably, direct stimulation or inhibition of new DG neurons does not change anxiety behavior under basal, nonstressed conditions56. However, direct stimulation of mature DG granule neurons in either the dorsal or ventral DG has an innate anti-anxiety effect54. This fits with prior work showing that the dorsal DG is more linked to spatial or contextual functions, whereas the ventral DG is more linked to mood and related emotional functions72,74,75, and suggests that subregional targeting of DG activity is a potential intervention for mood disorders. This functional neuroanatomical gradient is also evident in studies in which neurogenesis is manipulated. For example, whereas reversible silencing or stimulation of new neurons in the dorsal—but not ventral—DG impairs memory acquisition and retrieval, direct stimulation of ventral DG granule cells—but not of new neurons—decreases anxiety in mice, as compared to controls54,56. Thus, acute stimulation of ventral DG granule neurons could be a very useful intervention for relieving anxiety.
Pattern separation
Pattern separation is a computational term often applied to the DG to explain how cortical inputs representing spatial and contextual information converge onto the DG yet diverge into distinct outputs in the downstream CA3, a hippocampal region involved with the complementary function of pattern completion109. However, pattern separation is also a very relevant concept in neuropsychiatric disorders because overgeneralization (impaired pattern separation) is a barrier to symptom relief and is seen in many individuals with neuropsychiatric disorders22. For example, humans diagnosed with PTSD have diminished pattern separation; they generalize cues associated with a traumatic memory (crime victimization or war, for instance) to nonthreatening contexts, which thus drives fear and anxiety in these nonthreatening contexts22. Notably, this deficit is apparent in tests of pattern separation unrelated to the particular trauma: humans diagnosed with PTSD or related anxiety disorders perform poorly on neuropsychological tests of pattern separation22. By contrast, some humans diagnosed with autism spectrum disorder have enhanced pattern separation and note even slight differences in contexts or routines110. In PTSD, the severity of symptoms is correlated with a smaller and less active DG, as compared to healthy controls. These connections raise the possibility that the normalization of pattern separation will be accompanied by normalization of DG activity and diminished symptom severity.
Indeed, targeting new neurons in rodents can mimic these findings in humans: inhibition or ablation of new neurons impairs pattern separation, whereas stimulation of mature DG neurons or inducible enhancement of new neurons enhances pattern separation53,56,57,68,111,112 (Table 2 and Supplementary Table 1). In fact, when computational models of DG pattern separation account for the addition of new DG neurons, there is reduced overlap of activated GCs by previous memories, and thus improved pattern separation as compared to controls27. As with learning and memory, the ‘stage’ of the new neurons matters in regard to pattern separation. For example, pattern separation is reliant on young new neurons (just integrated into DG circuitry) but not on older mature new neurons or embyronic-generated DG neurons113. These aspects will be useful for guiding and fine-tuning potential future new-neuron-based therapies for pattern separation.
Reward
With regard to reward, the ablation of new DG neurons in rats increases cocaine drug-taking and drug-seeking, as compared to controls, which suggests that decreased adult neurogenesis is a vulnerability factor in a rat model of cocaine addiction64 (Table 2 and see also Supplementary Table 1). Given work showing that environmental-enrichment- or running-induced increase in adult neurogenesis correlates with a reduction of addictive-like behaviors in rats and mice114,115, it would be interesting to selectively and inducibly increase the number of new DG neurons or stimulate existing new DG neurons to test whether this would diminish drug-taking or drug-seeking in animal models of addiction. However, the only other study that has inducibly manipulated new neurons and examined reward-based hedonic behavior found the opposite result: the deletion of new DG neurons decreases a mouse’s preference for a rewarding substance, in this case, sucrose62. Given that it is challenging to compare an animal model of cocaine addiction with oral sucrose intake, more studies are warranted to see how the manipulation of new neurons influences reward processing.
Harnessing the DG to treat neuropsychiatric disorders
Given the above studies, it is intriguing to consider targeting new neurons to recalibrate dysfunctional DG activity to treat neuropsychiatric disorders. For example, in contrast to the healthy DG wherein Ent projections to the DG and its new neurons drive memory processes, mood regulation, pattern separation and processing of rewarding stimuli (Fig. 2a)9–16, this functional output is disrupted in neuropsychiatric disorders, which perhaps leads to or exacerbates symptoms (Fig. 2b). Regardless of whether the dysfunctional DG is due to changes in Ent input, the number of new DG neurons, regulation of DG activity, or some other parallel process, we propose two putative approaches for targeting new DG neurons as a way to normalize DG activity and functional output (Fig. 2c).
Figure 2.
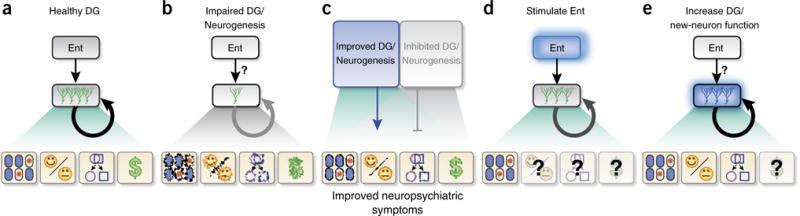
Proven and proposed approaches to targeting new DG neurons to recalibrate DG functional output in neuropsychiatric disorders. (a) In the healthy DG, input from the Ent into the DG drives new neurons and ultimately results in functional DG output, such as memory, mood, pattern separation and reward (green shaded triangle). When DG activity or function is aberrant, as is the case in many neuropsychiatric disorders (b, gray shaded triangle), new neurons are often decreased in number or function, perhaps in part owing to a dysfunctional upstream input from the Ent (indicated by a question mark (?)). (c) We propose that inducible increase in new neuron number, activity or DG activity can improve neuropsychiatric symptoms (blue box, arrow, green shaded triangle), whereas inhibition of new neuron number, activity or DG activity can block such improvement (gray box, flat head line, gray shaded triangle). Thus, targeting new neuron number, activity or DG activity might be a novel treatment for neuropsychiatric disorders. Recalibration of DG functional output is feasible via the stimulation of upstream DG regions (e.g., Ent, d) or increased number of new neurons or increased activity of new neurons or DG (e). For (d), it has already been shown in mice that Ent stimulation (blue box, bold font) enhances neurogenesis and improves learning and memory in both humans and mice (green shaded triangle, d). It remains to be tested whether this upstream stimulation and enhanced new neuron number or activity will affect mood, pattern separation or reward (indicated by question mark (?)). For (e), it has been shown in mice that direct stimulation or induction of adult neurogenesis (blue box) can improve memory, mood and pattern separation, although most of the work in this regard has shown the opposite (e.g., direct inhibition or suppression of adult neurogenesis impairs memory, mood and pattern separation). Although it has not been tested whether direct stimulation or induction of adult neurogenesis can improve reward, the converse has been shown: the suppression of adult neurogenesis enhances vulnerability in animal models of aberrant reward (e.g., addiction). DG, dentate gyrus; Ent, entorhinal cortex.
The first approach—increasing Ent input to DG and thus increasing the activity of DG neurons—might be useful for disorders that have common traits of impaired learning and memory induced by hippocampal or DG dysfunction and Ent afferent dysfunction, such as MDD, PTSD and schizophrenia. In such cases, recalibration of DG functional output is feasible via the stimulation of upstream DG regions, such as deep-brain stimulation of Ent (Fig. 2d). Indeed, it has already been shown that Ent stimulation enhances neurogenesis in mice and improves memory in both humans and mice29,116. More recently, Ent stimulation is also able to activate a DG memory in a mouse model of Alzheimer’s disease117, although the role of new neurons is not assessed in that study. It remains to be tested, however, whether this upstream stimulation and the subsequent putative enhanced new neuron number or activity will have beneficial effects on mood, pattern separation or reward (Fig. 2d). However, given that lesioning of Ent impairs pattern separation in mice84, it is reasonable to hypothesize that Ent stimulation would improve pattern separation, and in turn, improve PTSD symptoms. Notably, whereas Ent-stimulation-induced memory improvement in mice acts via increased neurogenesis29, it remains unclear whether Ent-stimulation-induced improvement in DG function is always reliant on intact neurogenesis.
The second approach—increasing new DG neuron number or activity—builds off the unique physiology and positioning of new DG neurons and the many neuropsychiatric disorders that are linked with dysfunctional DG activity (Fig. 2e). Many studies support the idea that the ablation or silencing of new neurons impairs aspects of memory, mood, pattern separation and the response to rewarding stimuli (Table 2 and Supplementary Table 1). By contrast, inducible increase in the number of new DG neurons formed, or inducible modulation of their spines, improves pattern separation and cognitive flexibility, respectively, in mice57,118, and stimulation or induction of new or mature DG neurons decreases anxiety in mice54,68. These studies suggest that a therapy based on the induction of new neurons has the potential to boost pattern separation and normalize learning and memory deficits (Fig. 2e). However, the stimulation of new neurons or mature DG neurons can also inhibit DG function54,56. Although the discrepancies between studies that stimulate new neurons might be due to technical issues, this urges caution when developing and employing such new-neuron-based therapies.
Because new neurons are highly sensitive to activity-dependent regulation, a related approach would be to stimulate DG activity to subsequently stimulate new neurons. Intriguingly, ‘neurogenic’ compounds exist that drive neurogenesis and/or improve some aspect of DG functional output in an activity-dependent manner108,119. However, so far, no neurogenic compounds act directly and solely on new DG neurons. A major challenge is identifying specific receptors or proteins on new DG neurons that could be exploited for treatment purposes. This is a particularly problematic obstacle in that new DG neurons move through so many developmental stages (Fig. 1a,b). However, the fact that new neurons develop much more slowly in primates than in rodents76, yet a larger proportion of hippocampal neurons turn over in humans than in rodents31,120, raises the possibility that identifying a new-neuron target might be more feasible in humans.
One final way to target new neurons would be to regulate their microcircuitry to normalize dysfunctional DG activity. For example, the activation of certain DG interneurons in rodents drives sparse encoding by feedback inhibition onto mature GCs81, which might improve pattern separation, as has been studied indirectly121. In addition, the stimulation of other DG interneurons in mice promotes progenitor survival and thus adult neurogenesis122, which might also enhance sparse encoding and pattern separation. Indeed, the regulation of any local mechanism that drives sparse encoding and the accompanying improvement in temporal precision of activation might be a candidate for this approach123. Such studies are not currently feasible in humans because of the limited knowledge of new-neuron microcircuitry in humans and the invasive nature of such approaches.
Conclusions and future directions
A dysfunctional DG is considered to be an endophenotype or biological correlate of most neuropsychiatric disorders22,24,59,61,124,125. The normalization or recalibration of aberrant DG function holds potential for treatment of these disorders. New DG neurons have unique connections and physiological properties that enable them to both sense and influence DG activity. Thus, we propose that targeting new DG neurons is one way of normalizing or recalibrating a dysfunctional DG (Fig. 2e). Another way of recalibrating a dysfunctional DG is upstream stimulation of the Ent (Fig. 2d). We think that both of these approaches merit consideration for therapeutic normalization of DG functional output, and we predict that—in animal models at least—this normalization occurs in part via the stimulation of new DG neurons.
There are numerous knowledge gaps that remain. First, specific DG output circuits in mice and rats need to be thoroughly defined, as has been done in regard to the ventral hippocampus–nucleus accumbens circuits for mood regulation126, because incorrect neuroanatomical or functional targeting of a circuit might impair an already disrupted network127. This is feasible, given the next generation of functional neuroanatomical techniques, which employ genetic promoter enhancers with cell-specific genetic- and viral-mediated, gene-transfer-induced labeling and lineage-specific transgenic lines128,129. Second, a broader scope of behaviors should be tested in mice and rats, particularly as new functions of the DG are recognized130. It is notable how few studies cross the ‘memory–mood’ divide, or even consider other DG functions, such as reward. Here we have highlighted papers that examine both memory and mood53,54,65, mood and reward62, or memory, mood and pattern separation56,57. Given that neuropsychiatric disorders are marked by a dysfunctional DG, and that the DG has a range of functional output, we encourage researchers to more frequently and pointedly cross the memory–mood divide and to explore more work with animal models of neuropsychiatric disorders. Third, additional human and postmortem work is warranted, particularly with regard to development, regulation, and most ambitiously, in vivo imaging31,131. Finally, although neurogenesis does exist in the postnatal primate and specifically human DG31,131, the process is presumed to take much longer than in the mouse or rat, and it is more challenging to detect cells in select stages of neurogenesis, given the limitations of current markers and the modes of assessment of new neurons in the adult human. This is relevant because postmortem human neurogenesis studies most commonly measure proliferating cells, not immature or specifically new neurons, because of technical obstacles. Should any future therapies emerge that, for example, normalize the proliferating cells that are reduced in humans diagnosed with either schizophrenia or MDD97,132, a post-treatment waiting period would be warranted to assess whether indeed the therapy has any influence on DG function. Therefore, although the common model of neurogenesis and its timeline (Fig. 1a) is a simplified representation of our current knowledge, it should continue to be rigorously tested and refined, particularly in regard to human neurogenesis.
We hope that this current view of new DG neurons and neuropsychiatric disorders will drive interest in testing the postulates put forth here. These recent studies underscore that the regulation of adult-generated DG neurogenesis merits continued and focused attention in the ongoing effort to develop novel treatments and to enable expansion of the toolkit available to treat neuropsychiatric disorders.
Supplementary Material
Acknowledgments
This work was supported by grants to A.J.E. from the US National Institutes of Health (DA023701, DA023555, MH107945) and the US National Aeronautics and Space Administration (NNX15AE09G). S.Y. was funded by a postdoctoral institutional training grant (NIMH T32-MH076690, Basic Science Training Program in the Neurobiology of Mental Illness, PI, C. Tamminga).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Murray CJ, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.Bystritsky A. Treatment-resistant anxiety disorders. Mol Psychiatry. 2006;11:805–814. doi: 10.1038/sj.mp.4001852. [DOI] [PubMed] [Google Scholar]
- 3.Mouchlianitis E, McCutcheon R, Howes OD. Brain-imaging studies of treatment-resistant schizophrenia: a systematic review. Lancet Psychiatry. 2016;3:451–463. doi: 10.1016/S2215-0366(15)00540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Culpepper L, et al. Suicidality as a possible side effect of antidepressant treatment. J Clin Psychiatry. 2004;65:742–749. doi: 10.4088/jcp.v65n0603. [DOI] [PubMed] [Google Scholar]
- 5.Player MJ, et al. Neuroplasticity in depressed individuals compared with healthy controls. Neuropsychopharmacology. 2013;38:2101–2108. doi: 10.1038/npp.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narayan VA, Manji HK. Moving from ‘diagnose and treat’ to ‘predict and pre-empt’ in neuropsychiatric disorders. Nat Rev Drug Discov. 2016;15:71–72. doi: 10.1038/nrd.2015.20. [DOI] [PubMed] [Google Scholar]
- 7.Fattore L, Diana M. Drug addiction: An affective-cognitive disorder in need of a cure. Neurosci Biobehav Rev. 2016;65:341–361. doi: 10.1016/j.neubiorev.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed AIA, et al. Neuropsychiatric adverse events of varenicline: a systematic review of published reports. J Clin Psychopharmacol. 2013;33:55–62. doi: 10.1097/JCP.0b013e31827c0117. [DOI] [PubMed] [Google Scholar]
- 9.Jonas P, Lisman J. Structure, Function, and Plasticity of Hippocampal Dentate Gyrus Microcircuits. Frontiers Media SA; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kesner RP. The Dentate Gyrus: A Comprehensive Guide to Structure, Function, and Clinical Implications. Vol. 163. Elsevier; 2007. pp. 567–576. [Google Scholar]
- 11.Scharfman HE. The Dentate Gyrus: A Comprehensive Guide to Structure, Function, and Clinical Implications. Elsevier; 2011. [Google Scholar]
- 12.Lopez-Rojas J, Kreutz MR. Mature granule cells of the dentate gyrus–Passive bystanders or principal performers in hippocampal function? Neurosci Biobehav Rev. 2016;64:167–174. doi: 10.1016/j.neubiorev.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Gilpin NW, Martin-Fardon R. Brain Reward & Stress Systems in Addiction. Frontiers Media SA; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koob GF, Le Moal M. Neurobiology of Addiction. Academic Press; 2005. [Google Scholar]
- 15.Wosiski-Kuhn M, Stranahan AM. From pattern separation to mood regulation: multiple roles for developmental signals in the adult dentate gyrus. Front Cell Neurosci. 2013;7:96. doi: 10.3389/fncel.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amaral DG, Scharfman HE, Lavenex P. The Dentate Gyrus: A Comprehensive Guide to Structure, Function, and Clinical Implications. Vol. 163. Elsevier; 2007. pp. 3–790. [Google Scholar]
- 17.Eisch AJ, Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. 2012;338:72–75. doi: 10.1126/science.1222941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoenfeld TJ, Cameron HA. Adult neurogenesis and mental illness. Neuropsychopharmacology. 2015;40:113–128. doi: 10.1038/npp.2014.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucassen PJ, et al. Stressing new neurons into depression? Mol Psychiatry. 2013;18:396–397. doi: 10.1038/mp.2012.39. [DOI] [PubMed] [Google Scholar]
- 20.Marvel CL, Paradiso S. Cognitive and neurological impairment in mood disorders. Psychiatr Clin North Am. 2004;27:19–36. doi: 10.1016/S0193-953X(03)00106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckley PF, Miller BJ, Lehrer DS, Castle DJ. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35:383–402. doi: 10.1093/schbul/sbn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci. 2012;15:1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liberzon I, Ressler K. Neurobiology of PTSD: From Brain to Mind. Oxford University Press; 2016. [Google Scholar]
- 24.Das T, Ivleva EI, Wagner AD, Stark CEL, Tamminga CA. Loss of pattern separation performance in schizophrenia suggests dentate gyrus dysfunction. Schizophr Res. 2014;159:193–197. doi: 10.1016/j.schres.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dichter GS, Damiano CA, Allen JA. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord. 2012;4:19. doi: 10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang E, Wen Z, Song H, Christian KM, Ming GL. Adult neurogenesis and psychiatric disorders. Cold Spring Harb Perspect Biol. 2016;8:a019026. doi: 10.1101/cshperspect.a019026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aimone JB, et al. Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev. 2014;94:991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morishita T, Fayad SM, Higuchi MA, Nestor KA, Foote KD. Deep brain stimulation for treatment-resistant depression: systematic review of clinical outcomes. Neurotherapeutics. 2014;11:475–484. doi: 10.1007/s13311-014-0282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone SSD, et al. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci. 2011;31:13469–13484. doi: 10.1523/JNEUROSCI.3100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suthana N, Fried I. Deep brain stimulation for enhancement of learning and memory. Neuroimage. 2014;85:996–1002. doi: 10.1016/j.neuroimage.2013.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergmann O, Spalding KL, Frisén J. Adult neurogenesis in humans. Cold Spring Harb Perspect Biol. 2015;7:a018994. doi: 10.1101/cshperspect.a018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ge S, Sailor KA, Ming GL, Song H. Synaptic integration and plasticity of new neurons in the adult hippocampus. J Physiol (Lond) 2008;586:3759–3765. doi: 10.1113/jphysiol.2008.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vivar C, van Praag H. Functional circuits of new neurons in the dentate gyrus. Front Neural Circuits. 2013;7:15. doi: 10.3389/fncir.2013.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toni N, Sultan S. Synapse formation on adult-born hippocampal neurons. Eur J Neurosci. 2011;33:1062–1068. doi: 10.1111/j.1460-9568.2011.07604.x. [DOI] [PubMed] [Google Scholar]
- 35.Song J, Olsen RHJ, Sun J, Ming GL, Song H. Neuronal circuitry mechanisms regulating adult mammalian neurogenesis. Cold Spring Harb Perspect Biol. 2016;8:a018937. doi: 10.1101/cshperspect.a018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vivar C, Potter MC, van Praag H. All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr Top Behav Neurosci. 2013;15:189–210. doi: 10.1007/7854_2012_220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bekinschtein P, Oomen CA, Saksida LM, Bussey TJ. Effects of environmental enrichment and voluntary exercise on neurogenesis, learning and memory, and pattern separation: BDNF as a critical variable? Semin Cell Dev Biol. 2011;22:536–542. doi: 10.1016/j.semcdb.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Kempermann G. Seven principles in the regulation of adult neurogenesis. Eur J Neurosci. 2011;33:1018–1024. doi: 10.1111/j.1460-9568.2011.07599.x. [DOI] [PubMed] [Google Scholar]
- 39.Cameron HA. Handbook of Contemporary Neuropharmacology. John Wiley & Sons, Inc; 2007. [Google Scholar]
- 40.Kempermann G. In: Neurogenesis in the Adult Brain I. Seki T, Sawamoto K, Parent MD, J M, Alvarez-Buylla A, editors. Springer; Japan: 2011. pp. 271–284. [Google Scholar]
- 41.Noonan MA, Choi KH, Self DW, Eisch AJ. Withdrawal from cocaine self-administration normalizes deficits in proliferation and enhances maturity of adult-generated hippocampal neurons. J Neurosci. 2008;28:2516–2526. doi: 10.1523/JNEUROSCI.4661-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown ES, Rush AJ, McEwen BS. Hippocampal remodeling and damage by corticosteroids: implications for mood disorders. Neuropsychopharmacology. 1999;21:474–484. doi: 10.1016/S0893-133X(99)00054-8. [DOI] [PubMed] [Google Scholar]
- 44.Duman RS, Malberg J, Thome J. Neural plasticity to stress and antidepressant treatment. Biol Psychiatry. 1999;46:1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- 45.Canales JJ. Comparative neuroscience of stimulant-induced memory dysfunction: role for neurogenesis in the adult hippocampus. Behav Pharmacol. 2010;21:379–393. doi: 10.1097/FBP.0b013e32833e16b6. [DOI] [PubMed] [Google Scholar]
- 46.Mandyam CD, Koob GF. The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery. Trends Neurosci. 2012;35:250–260. doi: 10.1016/j.tins.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petrik D, Lagace DC, Eisch AJ. The neurogenesis hypothesis of affective and anxiety disorders: are we mistaking the scaffolding for the building? Neuropharmacology. 2012;62:21–34. doi: 10.1016/j.neuropharm.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levone BR, Cryan JF, O’Leary OF. Role of adult hippocampal neurogenesis in stress resilience. Neurobiol Stress. 2014;1:147–155. doi: 10.1016/j.ynstr.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castilla-Ortega E, et al. A place for the hippocampus in the cocaine addiction circuit: Potential roles for adult hippocampal neurogenesis. Neurosci Biobehav Rev. 2016;66:15–32. doi: 10.1016/j.neubiorev.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 50.Solinas M, Thiriet N, Chauvet C, Jaber M. Prevention and treatment of drug addiction by environmental enrichment. Prog Neurobiol. 2010;92:572–592. doi: 10.1016/j.pneurobio.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Drew MR, Denny CA, Hen R. Arrest of adult hippocampal neurogenesis in mice impairs single- but not multiple-trial contextual fear conditioning. Behav Neurosci. 2010;124:446–454. doi: 10.1037/a0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gu Y, et al. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat Neurosci. 2012;15:1700–1706. doi: 10.1038/nn.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kheirbek MA, Tannenholz L, Hen R. NR2B-dependent plasticity of adult-born granule cells is necessary for context discrimination. J Neurosci. 2012;32:8696–8702. doi: 10.1523/JNEUROSCI.1692-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kheirbek MA, et al. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 2013;77:955–968. doi: 10.1016/j.neuron.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Denny CA, et al. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron. 2014;83:189–201. doi: 10.1016/j.neuron.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Danielson NB, et al. Distinct contribution of adult-born hippocampal granule cells to context encoding. Neuron. 2016;90:101–112. doi: 10.1016/j.neuron.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McAvoy KM, et al. Modulating neuronal competition dynamics in the dentate gyrus to rejuvenate aging memory circuits. Neuron. 2016;91:1356–1373. doi: 10.1016/j.neuron.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 59.Airan RD, et al. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- 60.Surget A, et al. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 2008;64:293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 61.Surget A, et al. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry. 2011;16:1177–1188. doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lehmann ML, Brachman RA, Martinowich K, Schloesser RJ, Herkenham M. Glucocorticoids orchestrate divergent effects on mood through adult neurogenesis. J Neurosci. 2013;33:2961–2972. doi: 10.1523/JNEUROSCI.3878-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci. 2010;30:304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seo DO, Carillo MA, Chih-Hsiung Lim S, Tanaka KF, Drew MR. Adult hippocampal neurogenesis modulates fear learning through associative and nonassociative mechanisms. J Neurosci. 2015;35:11330–11345. doi: 10.1523/JNEUROSCI.0483-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arruda-Carvalho M, Sakaguchi M, Akers KG, Josselyn SA, Frankland PW. Posttraining ablation of adult-generated neurons degrades previously acquired memories. J Neurosci. 2011;31:15113–15127. doi: 10.1523/JNEUROSCI.3432-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akers KG, et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- 68.Hill AS, Sahay A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacology. 2015;40:2368–2378. doi: 10.1038/npp.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Dijk RM, Huang SH, Slomianka L, Amrein I. Taxonomic separation of hippocampal networks: principal cell populations and adult neurogenesis. Front Neuroanat. 2016;10:22. doi: 10.3389/fnana.2016.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bédard A, Bernier PJ, Parent A. In: Neurogenesis in the Adult Brain II. Seki T, Sawamoto K, Parent JM, Alvarez-Buylla A, editors. Springer; Japan: 2011. pp. 1–21. [Google Scholar]
- 71.Kempermann G. Adult Neurogenesis: Stem Cells and Neuronal Development in the Adult Brain. Oxford University Press; 2006. [Google Scholar]
- 72.O’Leary OF, Cryan JF. A ventral view on antidepressant action: roles for adult hippocampal neurogenesis along the dorsoventral axis. Trends Pharmacol Sci. 2014;35:675–687. doi: 10.1016/j.tips.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 73.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 74.Fanselow MS, Dong HW. Arethe dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lacar B, Parylak SL, Vadodaria KC, Sarkar A, Gage FH. Increasing the resolution of the adult neurogenesis picture. F1000Prime Rep. 2014;6:8. doi: 10.12703/P6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kohler SJ, Williams NI, Stanton GB, Cameron JL, Greenough WT. Maturation time of new granule cells in the dentate gyrus of adult macaque monkeys exceeds six months. Proc Natl Acad Sci USA. 2011;108:10326–10331. doi: 10.1073/pnas.1017099108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bayer SA, Yackel JW, Puri PS. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science. 1982;216:890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- 78.Altman J, Bayer S. In: The Hippocampus. Isaacson RL, Pribram KH, editors. Springer; US: 1975. pp. 95–122. [Google Scholar]
- 79.Amrein I, Slomianka L, Lipp HP. Granule cell number, cell death and cell proliferation in the dentate gyrus of wild-living rodents. Eur J Neurosci. 2004;20:3342–3350. doi: 10.1111/j.1460-9568.2004.03795.x. [DOI] [PubMed] [Google Scholar]
- 80.West MJ, Coleman PD, Flood DG. Estimating the number of granule cells in the dentate gyrus with the disector. Brain Res. 1988;448:167–172. doi: 10.1016/0006-8993(88)91114-6. [DOI] [PubMed] [Google Scholar]
- 81.Temprana SG, et al. Delayed coupling to feedback inhibition during a critical period for the integration of adult-born granule cells. Neuron. 2015;85:116–130. doi: 10.1016/j.neuron.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lepousez G, Nissant A, Lledo PM. Adult neurogenesis and the future of the rejuvenating brain circuits. Neuron. 2015;86:387–401. doi: 10.1016/j.neuron.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 84.Vivar C, et al. Monosynaptic inputs to new neurons in the dentate gyrus. Nat Commun. 2012;3:1107. doi: 10.1038/ncomms2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Platel JC, Kelsch W. Role of NMDA receptors in adult neurogenesis: an ontogenetic (re)view on activity-dependent development. Cell Mol Life Sci. 2013;70:3591–3601. doi: 10.1007/s00018-013-1262-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma DK, Kim WR, Ming GL, Song H. Activity-dependent extrinsic regulation of adult olfactory bulb and hippocampal neurogenesis. Ann NY Acad Sci. 2009;1170:664–673. doi: 10.1111/j.1749-6632.2009.04373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mongiat LA, Schinder AF. Adult neurogenesis and the plasticity of the dentate gyrus network. Eur J Neurosci. 2011;33:1055–1061. doi: 10.1111/j.1460-9568.2011.07603.x. [DOI] [PubMed] [Google Scholar]
- 88.Doetsch F, Hen R. Young and excitable: the function of new neurons in the adult mammalian brain. Curr Opin Neurobiol. 2005;15:121–128. doi: 10.1016/j.conb.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 89.Wadiche JI, Overstreet-Wadiche L. New neurons don’t talk back. Neuron. 2015;85:3–5. doi: 10.1016/j.neuron.2014.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lemaire V, et al. Long-lasting plasticity of hippocampal adult-born neurons. J Neurosci. 2012;32:3101–3108. doi: 10.1523/JNEUROSCI.4731-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vaidya VA, Vadodaria KC, Jha S. Neurotransmitter regulation of adult neurogenesis: putative therapeutic targets. CNS Neurol Disord Drug Targets. 2007;6:358–374. doi: 10.2174/187152707783220910. [DOI] [PubMed] [Google Scholar]
- 92.Yamasaki N, et al. Alpha-CaMKII deficiency causes immature dentate gyrus, a novel candidate endophenotype of psychiatric disorders. Mol Brain. 2008;1:6. doi: 10.1186/1756-6606-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takao K, et al. Deficiency of schnurri-2, an MHC enhancer binding protein, induces mild chronic inflammation in the brain and confers molecular, neuronal, and behavioral phenotypes related to schizophrenia. Neuropsychopharmacology. 2013;38:1409–1425. doi: 10.1038/npp.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hayes JP, et al. Reduced hippocampal and amygdala activity predicts memory distortions for trauma reminders in combat-related PTSD. J Psychiatr Res. 2011;45:660–669. doi: 10.1016/j.jpsychires.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhai TY, et al. Altered intrinsic hippocmapus declarative memory network and its association with impulsivity in abstinent heroin dependent subjects. Behav Brain Res. 2014;272:209–217. doi: 10.1016/j.bbr.2014.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alia-Klein N, Parvaz MA, Woicik PA. Gene × disease interaction on orbitofrontal gray matter in cocaine addiction. Arch Gen Psychiatry. 2011;68:283–294. doi: 10.1001/archgenpsychiatry.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boldrini M, et al. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry. 2012;72:562–571. doi: 10.1016/j.biopsych.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Han K, et al. SHANK3 overexpression causes manic-like behaviour with unique pharmacogenetic properties. Nature. 2013;503:72–77. doi: 10.1038/nature12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. Enhancement of hippocampal neurogenesis by lithium. J Neurochem. 2000;75:1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- 100.Peng Z, et al. Ziprasidone ameliorates anxiety-like behaviors in a rat model of PTSD and up-regulates neurogenesis in the hippocampus and hippocampus-derived neural stem cells. Behav Brain Res. 2013;244:1–8. doi: 10.1016/j.bbr.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 101.Keilhoff G, Fusar-Poli P, Becker A. Effects of antipsychotics on dentate gyrus stem cell proliferation and survival in animal models: a critical update. Neural Plast. 2012;2012:832757. doi: 10.1155/2012/832757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tannenholz L, Jimenez JC, Kheirbek MA. Local and regional heterogeneity underlying hippocampal modulation of cognition and mood. Front Behav Neurosci. 2014;8:147. doi: 10.3389/fnbeh.2014.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kropff E, Yang SM, Schinder AF. Dynamic role of adult-born dentate granule cells in memory processing. Curr Opin Neurobiol. 2015;35:21–26. doi: 10.1016/j.conb.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boldrini M, et al. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology. 2013;38:1068–1077. doi: 10.1038/npp.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miller BR, Hen R. The current state of the neurogenic theory of depression and anxiety. Curr Opin Neurobiol. 2015;30:51–58. doi: 10.1016/j.conb.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Henn FA, Vollmayr B. Neurogenesis and depression: etiology or epiphenomenon? Biol Psychiatry. 2004;56:146–150. doi: 10.1016/j.biopsych.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 107.Tsai CY, Tsai CY, Arnold SJ, Huang G-J. Ablationof hippocampal neurogenesis in mice impairs the response to stress during the dark cycle. Nat Commun. 2015;6:8373. doi: 10.1038/ncomms9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Walker AK, et al. The P7C3 class of neuroprotective compounds exerts antidepressant efficacy in mice by increasing hippocampal neurogenesis. Mol Psychiatry. 2015;20:500–508. doi: 10.1038/mp.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Johnston ST, Shtrahman M, Parylak S, Gonçalves JT, Gage FH. Paradox of pattern separation and adult neurogenesis: A dual role for new neurons balancing memory resolution and robustness. Neurobiol Learn Mem. 2016;129:60–68. doi: 10.1016/j.nlm.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu KY, Gould RL, Coulson MC, Ward EV, Howard RJ. Tests of pattern separation and pattern completion in humans-A systematic review. Hippocampus. 2016;26:705–717. doi: 10.1002/hipo.22561. [DOI] [PubMed] [Google Scholar]
- 111.Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Niibori Y, et al. Suppression of adult neurogenesis impairs population coding of similar contexts in hippocampal CA3 region. Nat Commun. 2012;3:1253. doi: 10.1038/ncomms2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nakashiba T, et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology. 2009;34:2767–2778. doi: 10.1038/npp.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mustroph ML, Stobaugh DJ, Miller DS, DeYoung EK, Rhodes JS. Wheel running can accelerate or delay extinction of conditioned place preference for cocaine in male C57BL/6J mice, depending on timing of wheel access. Eur J Neurosci. 2011;34:1161–1169. doi: 10.1111/j.1460-9568.2011.07828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Suthana N, et al. Memory enhancement and deep-brain stimulation of the entorhinal area. N Engl J Med. 2012;366:502–510. doi: 10.1056/NEJMoa1107212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Roy DS, et al. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature. 2016;531:508–512. doi: 10.1038/nature17172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sahay A, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Petrik D, et al. Functional and mechanistic exploration of an adult neurogenesis-promoting small molecule. FASEB J. 2012;26:3148–3162. doi: 10.1096/fj.11-201426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lagace DC, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27:12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ikrar T, et al. Adult neurogenesis modifies excitability of the dentate gyrus. Front Neural Circuits. 2013;7:204. doi: 10.3389/fncir.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Song J, et al. Parvalbumin interneurons mediate neuronal circuitry-neurogenesis coupling in the adult hippocampus. Nat Neurosci. 2013;16:1728–1730. doi: 10.1038/nn.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu EP, et al. Protracted postnatal development of sparse, specific dentate granule cell activation in the mouse hippocampus. J Neurosci. 2013;33:2947–2960. doi: 10.1523/JNEUROSCI.1868-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hagihara H, Takao K, Walton NM, Matsumoto M, Miyakawa T. Immature dentate gyrus: an endophenotype of neuropsychiatric disorders. Neural Plast. 2013;2013:318596. doi: 10.1155/2013/318596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Walton NM, et al. Detection of an immature dentate gyrus feature in human schizophrenia/bipolar patients. Transl Psychiatry. 2012;2:e135. doi: 10.1038/tp.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bagot RC, et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun. 2015;6:7062. doi: 10.1038/ncomms8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cho KO, et al. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat Commun. 2015;6:6606. doi: 10.1038/ncomms7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang SM, Alvarez DD, Schinder AF. Reliable genetic labeling of adult-born dentate granule cells using Ascl1 CreERT2 and Glast CreERT2 murine Lines. J Neurosci. 2015;35:15379–15390. doi: 10.1523/JNEUROSCI.2345-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lerner TN, Ye L, Deisseroth K. Communication in neural circuits: tools, opportunities, and challenges. Cell. 2016;164:1136–1150. doi: 10.1016/j.cell.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sweeney P, Yang Y. An excitatory ventral hippocampus to lateral septum circuit that suppresses feeding. Nat Commun. 2015;6:10188. doi: 10.1038/ncomms10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Manganas LN, et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Allen KM, Fung SJ, Weickert CS. Cell proliferation is reduced in the hippocampus in schizophrenia. Aust N Z J Psychiatry. 2016;50:473–480. doi: 10.1177/0004867415589793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lucassen PJ, Stumpel MW, Wang Q, Aronica E. Decreased numbers of progenitor cells but no response to antidepressant drugs in the hippocampus of elderly depressed patients. Neuropharmacology. 2010;58:940–949. doi: 10.1016/j.neuropharm.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 134.Boldrini M, et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34:2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Reif A, et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- 136.Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Mol Psychiatry. 2005;10:160–184. doi: 10.1038/sj.mp.4001579. [DOI] [PubMed] [Google Scholar]
- 137.Elvsåshagen T, et al. Evidence for reduced dentate gyrus and fimbria volume in bipolar II disorder. Bipolar Disord. 2013;15:167–176. doi: 10.1111/bdi.12046. [DOI] [PubMed] [Google Scholar]
- 138.Frey BN, et al. The role of hippocampus in the pathophysiology of bipolar disorder. Behav Pharmacol. 2007;18:419–430. doi: 10.1097/FBP.0b013e3282df3cde. [DOI] [PubMed] [Google Scholar]
- 139.Otten M, Meeter M. Hippocampal structure and function in individuals with bipolar disorder: a systematic review. J Affect Disord. 2015;174:113–125. doi: 10.1016/j.jad.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 140.Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK. Lithium-induced increase in human brain grey matter. Lancet. 2000;356:1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- 141.Manji HK, et al. The underlying neurobiology of bipolar disorder. World Psychiatry. 2003;2:136–146. [PMC free article] [PubMed] [Google Scholar]
- 142.Barbeau D, Liang JJ, Robitalille Y, Quirion R, Srivastava LK. Decreased expression of the embryonic form of the neural cell adhesion molecule in schizophrenic brains. Proc Natl Acad Sci USA. 1995;92:2785–2789. doi: 10.1073/pnas.92.7.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 144.Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- 145.Bremner JD. Neuroimaging in posttraumatic stress disorder and other stress-related disorders. Neuroimaging Clin N Am. 2007;17:523–538. doi: 10.1016/j.nic.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bayer R, et al. Alterations of neuronal precursor cells in stages of human adult neurogenesis in heroin addicts. Drug Alcohol Depend. 2015;156:139–149. doi: 10.1016/j.drugalcdep.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 147.Mackey S, Paulus M. Are there volumetric brain differences associated with the use of cocaine and amphetamine-type stimulants? Neurosci Biobehav Rev. 2013;37:300–316. doi: 10.1016/j.neubiorev.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thompson PM, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


