Abstract
Background:
Giant cell tumor (GCT) of bone is a benign locally aggressive primary bone tumor which is risky for local recurrences and pulmonary metastasis. Till date, there are still many uncertainties in predicting the aggressiveness of GCT. We aim to investigate whether receptor activator nuclear kappa-B ligand (RANKL) expression may determine the prognosis of the lesion.
Materials and Methods:
We examined RANKL expression in 39 patients (21 males, 18 females) by immunohistochemistry. Four patients (10%) were presented with tumor recurrence, eight patients (20%) were complicated with lung metastasis, and two patients (5%) were presented with both recurrence and lung metastasis. Positive RANKL expression was assessed according to a scoring system evaluating the percentage of the immunostained epithelial area and the staining intensity. The cumulative score was calculated to determine the final score value. Data were analyzed using PASW version 18.0 and independent t-test between nonrecurrence/recurrence groups, and nonlung metastasis/lung metastasis groups. Significance was set at P < 0.05.
Results:
Thirty-two patients (82%) scored 3 in RANKL-staining percentage from whole stromal cell population (>75%), 6 patients scored 2, and 1 patient scored 1. Nine patients (23%) scored 3 in RANKL-staining intensity (most intense), 19 patients (48%) scored 2, and 11 patients (29%) scored 1. Twenty six patients (67%) had strong RANKL expression (total score of 5–6), 12 patients (31%) showed moderate score (3–4) whereas only 1 patient (2%) showed weak RANKL expression. Together, the mean value of RANKL-staining percentage was 2.79, intensity 1.95 and the total score 4.77. The mean RANKL-staining percentage between recurrence and nonrecurrence groups was statistically significant (P = 0.009). There was no significant difference in the mean staining intensity and total score between nonrecurrence and recurrence groups, and staining percentage staining intensity and a total cumulative score of RANKL expression between lung metastasis and nonlung metastasis groups.
Conclusion:
RANKL expression is generally high in Stage III GCT and is a reliable prognostic marker in predicting the risk of local recurrence however not in lung metastasis.
Keywords: Giant cell tumor of the bone, lung metastasis, receptor activator nuclear kappa-B ligand, Stage III, tumor recurrence
MeSH terms: Giant cell tumors, metastasis, tumor staging
Introduction
Stage III giant cell tumor of bone (GCT-B-III) is known to have higher risk of local recurrence and pulmonary metastasis.1,2,3 Till date, there has been no exact prognostic parameter to predict local recurrence and pulmonary metastasis of giant cell tumor (GCT).4 This study determines whether receptor activator nuclear kappa-B ligand (RANKL) may be a prognostic marker in risk prediction of local recurrence and lung metastasis in GCT of bone, hence to justify the use of denosumab as target therapy for an aggressive type of GCT.
Materials and Methods
To maintain homogeneity of study, a total of 39 patients (21 males, 18 females) of histologically proven GCT-B-III, with mean age of 36.18 years (range 11-66 years) who presented at our institution between January 1999 to December 2009 were included in the study. The GCT-B-III was defined as extraosseous lesions which break through the cortex and extended into the soft tissue, based on radiographic appearances and operative findings in conjunction with a survey of magnetic resonance imaging (MRI). All sociodemographic and clinicopathological data of the samples were evaluated. The clinical history (age, gender, ethnicity, location of tumor radiographic, MRI, and serial computed tomography [CT] thorax findings), histopathological reports, and current status of the patients were analyzed with informed consent. Ethical approval was obtained from Human Research Ethics Committee, Universiti Sains Malaysia (FWA Reg. No: 00007718; IRB Reg. No: 00004494), Malaysia.
Evaluation of receptor activator nuclear kappa-B ligand immuno-histochemical expression
Expression of RANKL was evaluated by immunohistochemical staining in representative sections from each patient. Serial sections of 5 μm thick were cut and immunohistochemical techniques were carried out using the avidin-biotin perioxidase complex method using an LSAB2 kit (Dako, Glostrup, Denmark). The primary antibody used in this study was rabbit polyclonal antibody RANKL representing the full length of human RANKL (Code: FL-317: sc-9073, Santa Cruz Biotechnology, Santa Cruz, California, USA).
RANKL expression over membrane of stromal cells was examined microscopically. Immunostainings were evaluated in five randomly chosen microscopic fields using a standard light microscope at 40 × 100 magnification by a pathologist. Positivity for RANKL expression was assessed according to a scoring system evaluating both the immunostained epithelial area and the staining intensity according to established method (Franscisco et al., 2007). The former was graded according to the score (%): 1 (<25), 2 (25–75), and 3 (>75), using an image analysis software (Olympus U-RFL-T cell F). Staining intensity was scored from 1 (least intense) to 3+ (most intense). The total of the two scores determined the final score value as follows: 0, negative; 1–2, weak; 3–4, moderate; and 5–6, strong. The highest score from five selected fields were taken for statistical analysis using PASW version 18.0 (SPSS Statistics, IBM, USA). Independent t-test was used to determine the mean difference of positive-immunostaining percentage, staining intensity and total score, comparing between nonrecurrence/recurrence groups, and nonlung metastasis/lung metastasis groups. Significance was set at P < 0.05.
Results
Twenty-one males and 18 females, with mean age of 36.18 years (range 11-66 years), were included in this study. Eighteen cases (46%) of the tumor occurred around knee region followed by 7 cases (18%) of distal radius and 4 cases (10%) of sacrum. Thirty-five out of 39 patients underwent wide resection and reconstruction whereby only three of them were treated with intralesional excision, especially for sacral GCT. There was only one patient who underwent Ray's amputation of metacarpal. Four cases (10%) had local recurrence. Two cases had soft tissue recurrence after undergoing wide resection and endoprosthesis of proximal tibia and distal femur, one case postwide resection of proximal humerus and another one case postintralesional curettage of distal radius. All patients were followed up for a minimum of 2 years duration and 20% of cases presented with lung metastasis were evaluated by serial CT of the chest taken at 6 monthly intervals for 2 years. There were two patients presented with both recurrence and lung metastasis.
Thirty two patients (82%) scored 3 in RANKL-staining percentage from whole stromal cell population (>75%) [Figure 1], 6 patients scored 2, and 1 patient scored 1. In addition, only 9 patients (23%) scored 3 in RANKL-staining intensity (most intense), 19 patients (48%) scored 2, and 11 patients (29%) were graded as 1. The total of the two scores were calculated to determine the final score value. Twenty six (67%) of the patients had shown strong RANKL expression (scored 5–6), 12 patients (31%) showed moderate score (3–4), whereby only 1 patient (2%) showed weak RANKL expression [Figure 2]. No patient showed negative RANKL expression of score 0. Together, the mean value of RANKL-staining percentage was 2.79 and mean RANKL-staining intensity was 1.95 (weak intensity). The mean total score was 4.77, showing moderate expression of RANKL among stromal cells. Expression of RANKL was also observed in multinucleated giant cells in all samples [Figure 3], whereby 67% exhibited strong positivity with mean value of 3.46 (standard deviation 0.94).
Figure 1.
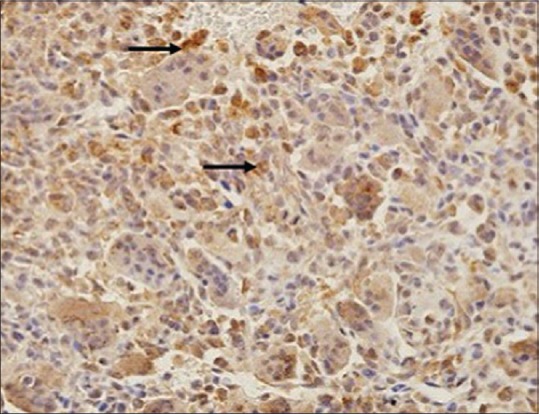
Photomicrograph of overexpressed receptor activator nuclear kappa-B ligand in stromal cells (a black arrow) (×100)
Figure 2.
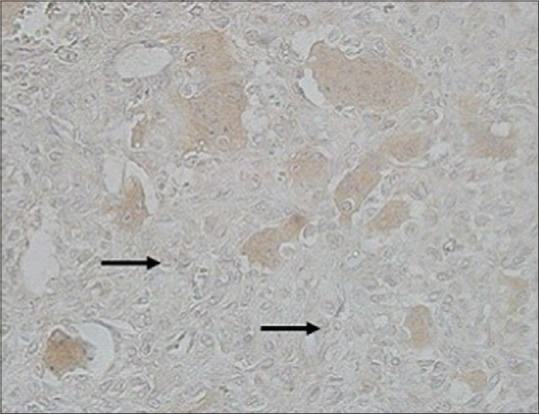
Photomicrograph of weakly staining of receptor activator nuclear kappa-B ligand (a black arrow) (×100)
Figure 3.
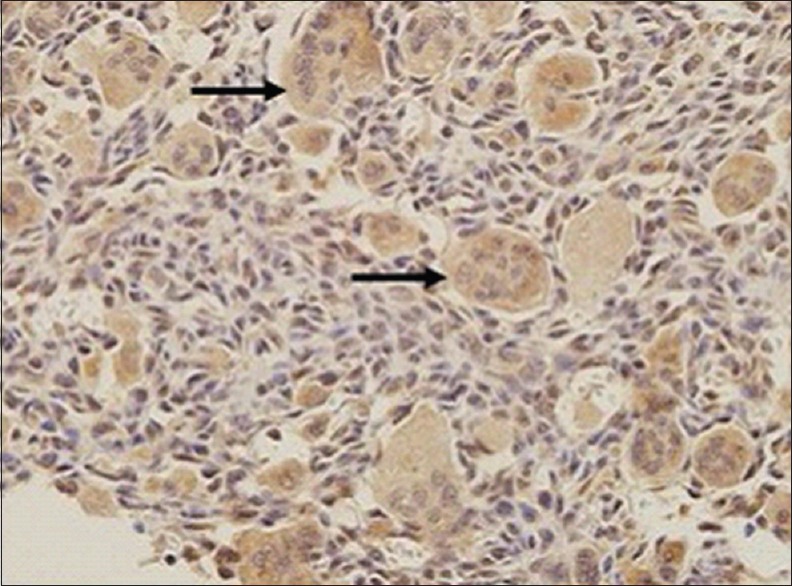
Photomicrograph of expression of receptor activator nuclear kappa-B ligand in multinucleated giant cell cytoplasm (a black arrow) (×100)
The mean percentage staining between no recurrence and recurrence group was significant (P = 0.009) with mean difference of 0.23; however, there is no significant difference between this group in terms of staining intensity (P = 0.387) and total score (P = 0.309) in the independent t-test. RANKL expression between lung metastasis group in all staining scores was not statistically significant [Tables 1 and 2].
Table 1.
Mean values of local recurrence - independent t-test (n=39)

Table 2.
Mean values of pulmonary metastasis - independent t-test (n=39)

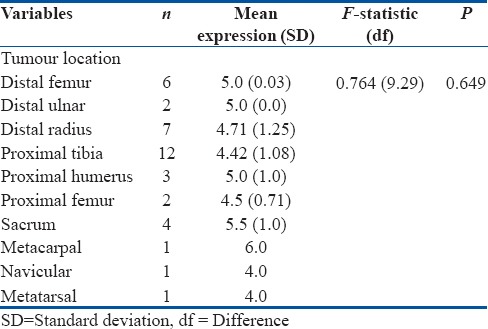
RANKL expression was also evaluated for different tumor location; however, there was no positive correlation (P = 0.649). However, GCT of the sacrum and metarcarpal had shown higher expression with mean values of 5.5 and 6, respectively [Table 3].
Table 3.
Mean values for tumor location - one-way ANOVA (n=39)
Discussion
RANKL is a Type II transmembrane protein in the tumor necrosis factor superfamily. RANKL is a key effector molecule in promoting osteoclastogenesis. Roux et al. demonstrated that RANKL is expressed in the stromal cellpopulation.5 Atkins et al. isolated stromal cells population in the GCT, using real-time reverse transcription polymerase chain reaction and detected the expression of RANKL messenger ribonucleic acid (mRNA), meanwhile Hu and Yu revealed that RANKL mRNA was enriched in GCTs, and the ratio of RANKL mRNA to glyceraldehyde-3-phosphate dehydrogenase gene in GCT is higher than that in normal bone tissues.6,7 Zhu et al. performed immune-histochemical analysis and found that RANKL was expressed in both the multinucleated giant cells and stromal cells in GCTs. Twenty-three of the 44 GCT cases had RANKL positive stromal cells and 10 cases exhibited RANKL positive multinucleated giant cells, with positive rates of 52% and 23%, respectively.8 In general, the stromal cells of osteoblast lineages are considered a neoplastic factor of GCT since the cells keep proliferating in culture.9 However, the bone resorption by osteoclast causes the osseous destruction and results in morbidity. Whether the aggressiveness of the tumor causes local recurrence and pulmonary metastasis is still debatable.
This study showed all 39 cases with aggressive GCT-B-III were positively stained with RANKL antibody with 66.6% of them had strong RANKL expression. Mean percentage of staining from overall stromal cell population was 25% to 75% with similar observation by Grimaud et al. with smaller series.10 Although RANKL is systematically localized in osteoblast and reacts to cytoplasmic domain, this study observed that most of the multinucleated giant cells exhibited a strong immunostain in the cytoplasm.
RANKL mRNA and protein were highly expressed in osteoclasts in GCT compared to stromal cells, after ex vivo generation of stromal and osteoclast-enriched cultures. The osteoclast component of GCTs is passively recruited by the production of RANKL by the stromal neoplastic component based on studies that have shown RANKL expression in GCT stromal cells.11 With the right stimulation, these cells are capable to support the formation of osteoclasts.6 The positive expression of RANKL in osteoclasts can be easily explained by an internalization of this ligand after binding to its specific receptor, RANK, located on the cell membrane. Roudier et al. identified that RANKL protein was present in the stromal component of all primary GCT and the expression was significantly higher in recurrent GCT of bone when compared to primary GCT.12 In this study, we were able to quantify the value of RANKL to achieve stronger evidence of RANKL correlation with lung metastasis and local recurrence. We found a higher level of RANKL expression for local recurrence but not pulmonary metastasis.
It was also observed that the GCT of sacrum demonstrate higher expression. These findings support the use of Denosumab therapy in sacrum GCT to minimize sacral sparring disability by total removal of the tumour.13,14
Conclusion
RANKL expression is generally high in Stage III GCT which correlates with aggressiveness of the disease. This supports the idea that RANKL may be one of the reliable prognostic markers in predicting risk of local recurrence in GCT-B-III but not lung metastasis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sung HW, Kuo DP, Shu WP, Chai YB, Liu CC, Li SM. Giant-cell tumor of bone: Analysis of two hundred and eight cases in Chinese patients. J Bone Joint Surg Am. 1982;64:755–61. [PubMed] [Google Scholar]
- 2.Takeuchi A, Tsuchiya H, Niu X, Ueda T, Jeon DG, Wang EH, et al. The prognostic factors of recurrent GCT: A cooperative study by the Eastern Asian Musculoskeletal Oncology Group. J Orthop Sci. 2011;16:196–202. doi: 10.1007/s00776-011-0030-x. [DOI] [PubMed] [Google Scholar]
- 3.Gupta R, Seethalakshmi V, Jambhekar NA, Prabhudesai S, Merchant N, Puri A, et al. Clinicopathologic profile of 470 giant cell tumors of bone from a cancer hospital in western India. Ann Diagn Pathol. 2008;12:239–48. doi: 10.1016/j.anndiagpath.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Gamberi G1, Serra M, Ragazzini P, Magagnoli G, Pazzaglia L, Ponticelli F, et al. Identification of markers of possible prognostic value in 57 giant cell tumors of bone. Oncol Rep. 2003;10:351–6. [PubMed] [Google Scholar]
- 5.Roux S, Amazit L, Meduri G, Guiochon-Mantel A, Milgrom E, Mariette X. RANK (receptor activator of nuclear factor kappa B) and RANK ligand are expressed in giant cell tumors of bone. Am J Clin Pathol. 2002;117:210–6. doi: 10.1309/BPET-F2PE-P2BD-J3P3. [DOI] [PubMed] [Google Scholar]
- 6.Atkins GJ, Haynes DR, Graves SE, Evdokiou A, Hay S, Bouralexis S, et al. Expression of osteoclast differentiation signals by stromal elements of giant cell tumors. J Bone Miner Res. 2000;15:640–9. doi: 10.1359/jbmr.2000.15.4.640. [DOI] [PubMed] [Google Scholar]
- 7.Hu Y, Yu S. Gene expression of osteoprotegerin and osteoclast differentiation factor in giant cell tumor. Chin J Pathol. 2002;3:128–31. [PubMed] [Google Scholar]
- 8.Zhu Y, Dong SK, Fan QH. Expression of osteoclast differentiation factor and its receptor and decoy receptor in giant cell tumor of bone. J Southeast Univ (Med Sci Ed) 2005;24:330–3. [Google Scholar]
- 9.Wülling M, Delling G, Kaiser E. The origin of the neoplastic stromal cell in giant cell tumor of bone. Hum Pathol. 2003;34:983–93. doi: 10.1053/s0046-8177(03)00413-1. [DOI] [PubMed] [Google Scholar]
- 10.Grimaud E, Soubigou L, Couillaud S, Coipeau P, Moreau A, Passuti N, et al. Receptor activator of nuclear factor kappaB ligand (RANKL)/osteoprotegerin (OPG) ratio is increased in severe osteolysis. Am J Pathol. 2003;163:2021–31. doi: 10.1016/s0002-9440(10)63560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang L, Xu J, Wood DJ, Zheng MH. Gene expression of osteoprotegerin ligand, osteoprotegerin, and receptor activator of NF-kappaB in giant cell tumor of bone: Possible involvement in tumor cell-induced osteoclast-like cell formation. Am J Pathol. 2000;156:761–7. doi: 10.1016/s0002-9440(10)64942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roudier MP, Kellar-Graney KL, Huang LY. Increased expression of RANKL in giant cell tumours of the bone correlates with recurrence. Seattle, WA, USA: 13th Annual Connective Tissue Oncology Society Meeting; Nov 1–3; 2007. [Google Scholar]
- 13.Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay JY, et al. Denosumab in patients with giant-cell tumour of bone: An open-label, phase 2 study. Lancet Oncol. 2010;11:275–80. doi: 10.1016/S1470-2045(10)70010-3. [DOI] [PubMed] [Google Scholar]
- 14.Thomas DM. RANKL, denosumab, and giant cell tumor of bone. Curr Opin Oncol. 2012;24:397–403. doi: 10.1097/CCO.0b013e328354c129. [DOI] [PubMed] [Google Scholar]


