Abstract
Background and Aims:
Use of lactated intravenous fluids during long surgeries could cause lactate accumulation and lactic acidosis. Acetate-based solutions could be advantageous as they are devoid of lactate. The primary aim of the study was to assess the effect of use of an acetated solution or Ringer's lactate (RL) as intraoperative fluid on lactate levels in patients without hepatic dysfunction undergoing prolonged surgeries.
Material and Methods:
This was a prospective, randomized, controlled trial involving sixty patients belonging to American Society of Anesthesiologists Physical Status I to II undergoing major head and neck surgeries with free flap reconstruction. Patients were randomly allocated into two equal groups, Group sterofundin (SF) and Group RL. Group SF was started on acetate-based crystalloid solution (sterofundin B Braun®) and Group RL received RL intravenously at the rate of 10 ml/kg/h to maintain systolic blood pressure above 90 mmHg. Blood loss >20% was replaced with packed cells. Arterial blood gas analysis was done 2nd hourly till 8 h. Chi-square test was used to compare categorical variables. Independent sample t-test was used to compare means.
Results:
Intraoperative lactate levels were significantly high in RL group at 2, 4, 6, and 8 h. The pH was comparable between groups except at 8 h where RL group had a significantly lower pH than SF group (7.42 ± 0.1 vs. 7.4 ± 0.1). Sodium, potassium, chloride, bicarbonate, and pCO2did not show any significant difference between the groups.
Conclusion:
Use of acetate-based intravenous solutions reduced levels of lactate in comparison with RL in patients undergoing free flap reconstructive surgeries.
Key words: Acetate, acid-base balance, acidosis, intravenous fluids, lactate
Introduction
The choice of the intraoperative crystalloid has gradually changed from normal saline to fluids with electrolyte composition similar to plasma. Lactated fluids could cause lactate accumulation and lactic acidosis in the presence of hypotension or impaired liver blood flow. Acetate-based solutions could be advantageous as they are devoid of lactate and since they contain maleate or gluconate in addition to acetate may actually correct any existent acidosis.
Free flap reconstructive surgeries for head and neck malignancies are long procedures requiring hemodilution with relative hypertension to improve perfusion across microanastomoses. To avoid the use of vasoconstrictors, administration of large amount of crystalloids is usually needed. Normally extraneous lactate is completely metabolized by liver, and blood levels are maintained in the range of 0.2–2 mmol/L. We evaluated the comparative effects of administration of lactated versus nonlactated solutions on serum lactate levels in patients undergoing free flap surgeries.
The primary aim of the study was to assess the effect of use of an acetated solution, Sterofundin (SF), or Ringer's lactate (RL) as replacement fluid on lactate levels in patients undergoing major head and neck free flap reconstructive surgeries. The secondary objectives included determination of arterial pH, bicarbonate, sodium, potassium, chloride, glucose levels, and the partial pressures of oxygen and carbon dioxide.
Material and Methods
This was a prospective, randomized, controlled trial conducted over a period of 2 years. Based on the previous study by Shariffuddin et al.[1] using changes in lactate levels as the objective, with an expected mean difference of 0.52, with 95% confidence, minimum estimated sample size was calculated as 17 in each group to get statistically significant results. Sixty patients belonging to American Society of Anesthesiologists (ASA) Physical Status I to II undergoing major head and neck surgeries with free flap reconstruction were recruited after obtaining consent and approval from the hospital Ethical Committee. Patients with hepatic and renal derangement, congestive cardiac failure, diabetics, and those unwilling to participate after explanation of the protocol were excluded from the study.
Patients were randomly divided into two equal groups by computer-generated sequence to Group SF and Group RL. Both the groups were kept nil per orally, 8 h for solids and 2 h for clear fluids. In the theater, an arterial cannula was introduced under local anesthesia, and a baseline arterial blood gas (ABG) sample was taken. Then, the patients of Group SF were started on acetate-based crystalloid solution (Sterofundin B Braun ®) and Group RL received RL intravenously at the rate of 10 ml/kg/h to maintain systolic blood pressure (SBP) above 90 mmHg.
All the patients received general anesthesia with endotracheal intubation and mechanical ventilation following a standardized protocol maintaining the end-tidal carbon dioxide levels between 35 and 40 mmHg. ABG samples were repeated every 2 hourly for 8 h. Arterial pH, partial pressures of carbon dioxide, bicarbonate, sodium, potassium, chloride, lactates, and glucose levels were documented. Heart rate (HR), SBP, diastolic blood pressure, and mean arterial pressure (MAP) of each patient were noted at preinduction and half hourly intraoperatively. Urine output was documented hourly and core body temperature 2 hourly. In those patients receiving RL, the fluid was changed to SF when lactate levels were >4.5 mmol/L.
Intraoperative hypotension was defined as SBP <90 mmHg and was treated initially with incremental boluses of 200 mL of the allocated crystalloid to a maximum of up to 500 ml in 30 min. Patients who did not respond to fluid boluses were treated with phenylephrine in titrated bolus doses of 50 μg intravenously, and total dose required was documented. Blood loss <20% of estimated blood volume was replaced with thrice the volume with crystalloid and loss >20% with packed blood cells.
Chi-square test was used to compare categorical variables. Independent sample t-test was used to compare means among SF and RL group. P < 0.05 was considered statistically significant.
Results
The demographic variables in both groups were comparable; mean age of the patients in years in Groups SF and RL was 52.5 ± 16.2 versus 46.5 ± 15.5 (P = 0.152) and weight in kg was 62.2 ± 9.1 vs. 58.5 ± 12.2, P = 0.185, respectively. The distribution of gender and ASA physical status (P < 0.05) were also comparable.
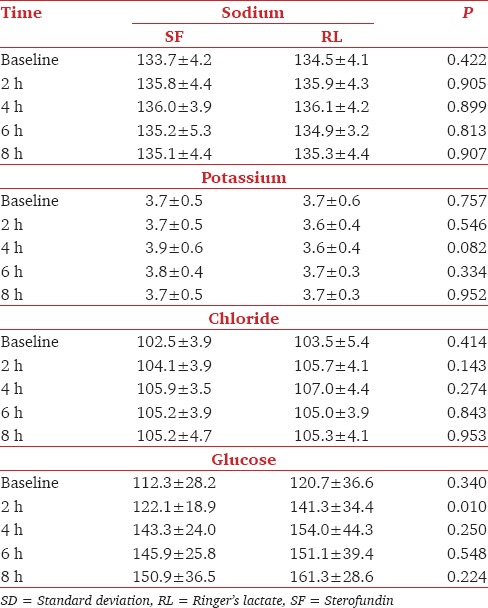
Comparison of baseline, pH, pCO2, bicarbonate, sodium, potassium, lactate, and glucose of both RL and SF groups yielded comparable results. Intraoperative pH was comparable between groups except at 8 h where RL group had a significantly lower pH than SF group Table 1. Lactate levels were significantly high in RL group at 2, 4, 6, and 8 h, but the bicarbonate levels remained comparable Table 1. Sodium, potassium, chloride, and pCO2 did not show any significant difference between the groups intraoperatively Table 1 and Table 2. At 2 h, Group B had significantly high glucose levels, but during other time points, it remained comparable with group SF Table 2.
Table 1.
Comparison of pH, lactate, bicarbonate and pCO2 (Mean±SD) in Groups SF and RL
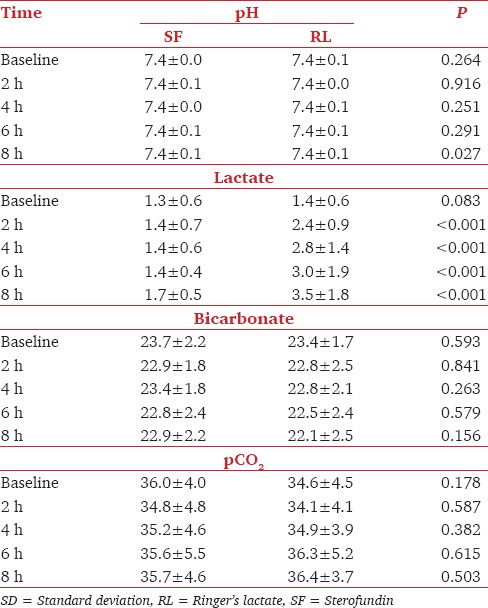
Table 2.
Comparison of electrolytes and glucose (Mean±SD) in Groups SF and RL
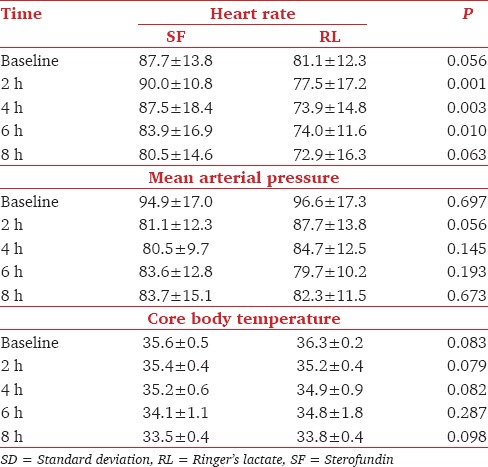
Comparison of intraoperative hemodynamics revealed a significantly high HR in Group SF at 4, 6, and 8 h with comparable MAP throughout the study period Table 3. There was a fall in core body temperature in both the groups from baseline till 8 h, but the difference between groups was not statistically significant Table 3.
Table 3.
Comparison of heart rate mean arterial pressure and temperature (Mean±SD) in Groups SF and RL
Although more patients in Group SF developed hypotension and required fluid boluses to correct hypotension as compared to RL group, the difference was insignificant (14 vs. 8, P = 0.055). Similarly, the total volume of the crystalloid used intraoperatively also did not show any significant difference between the groups [5646.7 ± 1295.0 vs. 5116.7 ± 1744, P = 0.187].
The blood loss (640.0 ± 182.6) and percentage of patients who required blood transfusion (33%) in SF group were similar to those in RL group (578.3 ± 209.1, 20%), the difference was insignificant (P = 0.183 and 0.381, respectively). Mean intraoperative urine output per hour in both the groups was comparable (76.7 ± 39.5 vs. 85.7 ± 39.0, P = 0.535). Four patients in SF group and three in RL group required intermittent bolus phenylephrine intraoperatively, and the mean requirement in these patients in both the groups did not show any statistically significant difference (129.4 ± 12.5 117.5 ± 13.8, P = 0.159).
Twenty patients (66.7%) in Group RL who had lactate levels >4.5 mmol/L at 8 h were administered acetated fluids as per protocol.
Discussion
Intraoperative fluid management continues to be a daily challenge in anesthetic practice. Head and neck free flap reconstructive procedures in particular are associated with exaggerated dehydration resulting from preoperative fasting and underlying illness. These surgeries are of long duration with major intraoperative and postoperative fluid and electrolyte loss because of extensive resections and vascular anastomoses.
The choice of fluid to administer has been investigated in numerous randomized, controlled trials and systematic reviews primarily in critically ill patients with ambiguous results and with unclear implications for fluid management in elective surgical procedures.[2,3,4,5,6] Different solutions have different effects on acid-base status, electrolyte levels, coagulation, and renal and hepatic function. Several studies have reported an increased incidence of hyperchloremic acidosis with the use of normal saline, and others an increase in blood lactate levels when large amounts of RL solutions were infused.[7]
Intraoperative fluid management comprises maintenance fluid, fasting deficits, and replacement of any losses occurring in the intraoperative period.[8] The aim of fluid therapy is to correct dehydration, maintain fluid and electrolyte balance and adequate intravascular volume, hence ensuring stable intraoperative hemodynamics, improved organ perfusion, and adequate tissue oxygenation.[9] Both fluid overload and hypovolemia may impair tissue oxygenation with negative implications for wound healing and possibly wound complications.
Majority of intraoperative fluids given are used to replace fasting deficit and third space losses which consist mainly of extracellular fluid. Hence, any intravenous fluid given during the intraoperative period should ideally have a composition as similar to plasma as possible. This makes crystalloids the ideal choice of intraoperative fluids.
Crystalloids that have almost similar composition of plasma are RL and SF. SF is a balanced isotonic solution for intravenous infusion with electrolyte composition very similar to plasma (Na - 140, k - 4.0, Ca - 2.5, Mg - 1, and Cl - 127 mmol/l). It also has a potential base excess of zero and contains acetate (24 mmol/l) and maleate (5 mmol/l) which is widely metabolized in all organs and muscles, resulting in low oxygen consumption which is 1.2 L oxygen per liter solution.[10,11]
RL contains sodium (130 mmol/L), potassium (5 mmol/L), calcium and magnesium (both at 1 mmol/l), chloride (112 mmol/L), and lactate (27 mmol/L). Its osmolarity is 276 mOsm/L which makes it slightly hypoosmotic when compared to plasma. Its base excess is 3 mmol/l, and oxygen consumption is slightly higher than that of SF at 1.8 L oxygen per liter solution. The lactate in RL was added to reduce the chloride content, thereby reducing the incidence of hyperchloremic metabolic acidosis which usually occurs after infusion of large volumes of normal saline during the intraoperative period.[10,11]
Normal lactate levels are usually <2 mmol/L. Lactate is converted to pyruvate and generates adenosine triphosphate (ATP) by oxidative phosphorylation in the mitochondria. Part of lactate is also metabolized by the Cori cycle to generate glucose with the consumption of ATP.[12] Moderate elevation in lactate levels (2–5 mmol/L) usually occurs with inadequate tissue hypoperfusion and is commonly associated with the administration of catecholamines and hyperglycemia.[13] Abnormalities of lactate metabolism are very common in patients undergoing prolonged surgery. The metabolism of lactate is dependent on the kidney and liver, and as such, when the functions of these organs are compromised, there will be lactate accumulation as well as reduction in production of bicarbonate resulting in lactic acidosis.[14] Increase in lactate levels is commonly observed if the volume of liver is inadequate following major hepatectomy.[15]
Occult hypoperfusion, defined as elevated blood lactate levels without signs of clinical shock, was associated with increased morbidity and mortality, and early correction is likely to improve the outcome. Lactate level is one of the most commonly used markers in assessing occult tissue hypoperfusion. Occult hypoperfusion is associated with an increased rate of infection and mortality. Optimization of intravascular volume resulted in a reduction in lactate levels, and it also reduced the incidence of postoperative infections and duration of Intensive Care Unit stay.[16] In patients with shock lactate levels have been referred to as the best indicator of the severity of shock.[17]
Short-term infusion of lactated Ringer's solution in normal adults who are hemodynamically stable does not falsely increase circulating lactate concentrations when 1 L of fluid was infused over a period of 1 h.[18] However, even small amount of RL- containing solutions in catheters used for blood sampling may cause false increases in the circulating lactate concentration, and small amount of non-RL crystalloid solutions in catheters used for blood sampling may falsely decrease circulating lactate values.[19]
SF was found to be safer as compared to normal saline in protecting young children undergoing major surgery against the risk of increasing plasma chlorides and the subsequent metabolic acidosis.[20]
Nakayama et al.[21] used acetated Ringer (SF) and RL solutions as an intraoperative fluid during hepatectomy and evaluated their effects on intraoperative and postoperative hemodynamics, metabolism, blood gas, and renal as well as liver functions. Intraoperative serum lactate levels increased significantly in both groups. However, the level of lactate in the RL group was significantly higher than in the SF group at the end of the operation. Although acetate level increased significantly during the operation in the SF group, it decreased to the normal range at the end of the operation. They concluded that SF may be more useful in hepatectomy than RL as an intraoperative fluid.
In contrast, Isosu et al.[22] used acetated ringer solution (AR) in his study to find its usefulness in patients with liver dysfunction and compared with RL solution. L-lactic acid increased significantly in both groups. D-lactic acid increased in LR groups, and acetic acid increased in AR groups. However, the other parameters, including the acid-base balance, electrolytes, and liver function, showed no significant changes in any group. These findings suggested that as an intraoperative fluid, SF is as useful as RL. However, there was no statistically significant difference between the data of both groups. Hence, they concluded that in patients with liver dysfunction, SF is not necessarily a better fluid when compared with RL for intraoperative infusion.
There are very few studies in literature identifying the association between intraoperative infusion of lactate or lactate precursor containing solutions and blood pH and lactate levels. Our study aimed at comparing the changes in arterial pH, lactate, and electrolytes in prolonged head and neck surgery following intraoperative infusion of RL or SF. The patients in our study were devoid of any hepatic dysfunction. The gradual rise in lactate in these patients with the prolonged use of RL indicate that under general anesthesia even in normal patients, the capacity of liver to metabolize excess lactate comes down.
In our study, following 8 h of RL administration, 66.7% patients in Group RL had lactate levels >4.5 mmol/L. Hence, serial ABG analysis for checking lactate levels should be considered mandatory in prolonged surgeries with the use of RL. A rising trend in lactate levels warrants a change of intravenous fluid to an acetate-based crystalloid solution or initiation of an infusion of dobutamine.
The baseline hemodynamic values in the present study, though comparable, had shown a higher HR and a lower blood pressure in SF group. Intraoperatively, the significantly higher HRs observed in SF group with a slightly lower blood pressure could be a continuation of the same trend or be attributed to surgical procedures and patient characteristics. The higher glucose levels seen intraoperatively in RL group could be the result of metabolism of lactate in liver through gluconeogenesis. The pH at 8 h, though significantly low in RL group when analyzed statistically, does not carry any clinical significance as it is well within the normal range.
Limitations of the study include lack of comparison of base excess, small sample size, and cessation of the study at 8 h intraoperatively. The data should have been collected up to 24 h, and the time taken for normalization of lactate documented for a better understanding of the impact of lactated solutions on the acid-base balance in prolonged surgical procedures.
Conclusion
Use of acetate-based intravenous solutions reduced levels of lactate in comparison with RL in patients undergoing free flap reconstructive surgeries.
Acknowledgment
The authors would like to thank Mrs. Seena Aravind for preparing the tables and graphs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shariffuddin II, Priya P, Adeline CS, Chinna K, Chan L. The effects of sterofundin versus Ringer's lactate on acid-base and electrolytes status in paediatric patients undergoing major surgery: A double blind randomised control trial. J Confed ASEAN Soc Anaesthesiologists. 2013;PP 09:23A. [Google Scholar]
- 2.Roberts I, Alderson P, Bunn F, Chinnock P, Ker K, Schierhout G. Colloids versus crystalloids for fluid resuscitation in critically ill patients. [Last Accessed on 2017 Jan 31];Cochrane Database Syst Rev. 2004 4:CD000567. doi: 10.1002/14651858.CD000567.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Alderson P, Bunn F, Lefebvre C, Li WP, Li L, Roberts I, et al. Human albumin solution for resuscitation and volume expansion in critically ill patients. [Last Accessed on 2017 Jan 31];Cochrane Database Syst Rev. 2004 4:CD001208. doi: 10.1002/14651858.CD001208.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Bunn F, Roberts I, Tasker R, Akpa E. Hypertonic versus near isotonic crystalloid for fluid resuscitation in critically ill patients. [Last Accessed on 2017 Jan 31];Cochrane Database Syst Rev. 2004 3:CD002045. doi: 10.1002/14651858.CD002045.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zavrakidis N. Intravenous fluids for abdominal aortic surgery. [Last Accessed on 2017 Jan 31];Cochrane Database Syst Rev. 2000 3:CD000991. doi: 10.1002/14651858.CD000991. [DOI] [PubMed] [Google Scholar]
- 6.Bunn F, Alderson P, Hawkins V. Colloid solutions for fluid resuscitation. [Last Accessed on 2017 Jan 31];Cochrane Database Syst Rev. 2003 1:CD001319. doi: 10.1002/14651858.CD001319. [DOI] [PubMed] [Google Scholar]
- 7.Orbegozo Cortés D, Rayo Bonor A, Vincent JL. Isotonic crystalloid solutions: A structured review of the literature. Br J Anaesth. 2014;112:968–81. doi: 10.1093/bja/aeu047. [DOI] [PubMed] [Google Scholar]
- 8.Bamboat ZM, Bordeianou L. Perioperative fluid management. Clin Colon Rectal Surg. 2009;22:28–33. doi: 10.1055/s-0029-1202883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grocott MP, Mythen MG, Gan TJ. Perioperative fluid management and clinical outcomes in adults. Anesth Analg. 2005;100:1093–106. doi: 10.1213/01.ANE.0000148691.33690.AC. [DOI] [PubMed] [Google Scholar]
- 10.Vennari M, Benedetto M, Agrò FE. Body Fluid Management. Italy: Springer Milan; 2013. Commercially available crystalloids and colloids; pp. 215–41. [Google Scholar]
- 11.Guidet B, Soni N, Della Rocca G, Kozek S, Vallet B, Annane D, et al. A balanced view of balanced solutions. Crit Care. 2010;14:325. doi: 10.1186/cc9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingelfinger JR. Disorders of fluids and electrolytes. Lactic acidosis. N Engl J Med. 2014;371:2309–19. doi: 10.1056/NEJMra1309483. [DOI] [PubMed] [Google Scholar]
- 13.Maillet JM, Le Besnerais P, Cantoni M, Nataf P, Ruffenach A, Lessana A, et al. Frequency, risk factors, and outcome of hyperlactatemia after cardiac surgery. Chest. 2003;123:1361–6. doi: 10.1378/chest.123.5.1361. [DOI] [PubMed] [Google Scholar]
- 14.Gladden LB. Lactate metabolism: A new paradigm for the third millennium. J Physiol. 2004;558:5–30. doi: 10.1113/jphysiol.2003.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marko P, Gabrielli A, Caruso LJ. Too much lactate or too little liver? J Clin Anesth. 2004;16:389–95. doi: 10.1016/j.jclinane.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Chytra I, Pradl R, Bosman R, Pelnár P, Kasal E, Zidková A. Esophageal Doppler-guided fluid management decreases blood lactate levels in multiple-trauma patients: A randomized controlled trial. Crit Care. 2007;11:R24. doi: 10.1186/cc5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakker J, de Lima AP. Increased blood lacate levels: An important warning signal in surgical practice. Crit Care. 2004;8:96–8. doi: 10.1186/cc2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Didwania A, Miller J, Kassel D, Jackson EV, Jr, Chernow B. Effect of intravenous lactated Ringer's solution infusion on the circulating lactate concentration: Part 3. Results of a prospective, randomized, double-blind, placebo-controlled trial. Crit Care Med. 1997;25:1851–4. doi: 10.1097/00003246-199711000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Jackson EV, Jr, Wiese J, Sigal B, Miller J, Bernstein W, Kassel D, et al. Effects of crystalloid solutions on circulating lactate concentrations: Part 1. Implications for the proper handling of blood specimens obtained from critically ill patients. Crit Care Med. 1997;25:1840–6. doi: 10.1097/00003246-199711000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Disma N, Mameli L, Pistorio A, Davidson A, Barabino P, Locatelli BG, et al. A novel balanced isotonic sodium solution vs. normal saline during major surgery in children up to 36 months: A multicenter RCT. Paediatr Anaesth. 2014;24:980–6. doi: 10.1111/pan.12439. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama M, Kawana S, Yamauchi M, Tsuchida H, Iwasaki H, Namiki A. Utility of acetated Ringer solution as intraoperative fluids during hepatectomy. Masui Jpn J Anesthesiol. 1995;44:1654–60. [PubMed] [Google Scholar]
- 22.Isosu T, Akama Y, Tase C, Fujii M, Okuaki A. Clinical examination of acetated Ringer solution in patients with normal liver function and those with liver dysfunction. Masui Jpn J Anesthesiol. 1992;41:1707–13. [PubMed] [Google Scholar]


