Abstract
Background and Aims:
Entropy monitoring entails measurement of the effect of anesthetic on its target organ rather than merely the concentration of anesthetic in the brain (indicated by alveolar concentration based on which minimum alveolar concentration [MAC] is displayed). We proposed this prospective randomised study to evaluate the effect of entropy monitoring on isoflurane consumption and anesthesia recovery period.
Material and Methods:
Sixty patients undergoing total abdominal hysterectomy under general anesthesia using an endotracheal tube were enrolled in either clinical practice (CP) or entropy (E) group. In group CP, isoflurane was titrated as per clinical parameters and MAC values, while in Group E, it was titrated to entropy values between 40 and 60. Data including demographics, vital parameters, alveolar isoflurane concentration, MAC values, entropy values, and recovery profile were recorded in both groups.
Results:
Demographic data and duration of surgery were comparable. Time to eye opening on command and time to extubation (mean ± standard deviation) were significantly shorter, in Group E (6.6 ± 3.66 and 7.27 ± 4.059 min) as compared to Group CP (9.77 ± 5.88 and 11.63 ± 6.90 min), respectively. Mean isoflurane consumption (ml/h) was 10.81 ± 2.08 in Group E and 11.45 ± 2.24 in Group CP and was not significantly different between the groups. Time to readiness to recovery room discharge and postanesthesia recovery scores were also same in both groups.
Conclusion:
Use of entropy monitoring does not change the amount of isoflurane consumed during maintenance of anesthesia or result in clinically significant faster recovery.
Key words: Anesthesia recovery period, awareness, entropy, isoflurane
Introduction
Patients suffering from major coexisting diseases with marked limitation in physiological reserves and associated end-organ damage require careful titration of inhalational anesthetic agents to minimize side-effects and simultaneously to maintain adequate depth of anesthesia (DOA). Currently, isoflurane is the most commonly used inhalational anesthetic agent to maintain DOA. Its higher concentration may cause significant morbidity because of hypotension, tachycardia, and delayed recovery, whereas lower concentration may lead to the lighter plane of anesthesia, leading to sympathetic response, intraoperative awareness or both.
Anesthesia is a balance between the amount of anesthetic drug administered and state of arousal of the patient. Patient movement in response to noxious stimulation remains an important sign of inadequate DOA, but is unreliable and is suppressed by paralysis. Traditional clinical signs such as hypertension, tachycardia, diaphoresis, and lacrimation are unreliable indicators of DOA.[1,2,3] DOA is a simplified construct of hypnosis, amnesia, antinociception, and reflex suppression. A widely used method to measure inhaled anesthetic requirement is the minimum alveolar concentration (MAC) of inhaled anesthetic at which movement in response to a nociceptive stimulus is suppressed in 50% of subjects at standard temperature and pressure.[4]
Most current proprietary DOA monitors such as Bispectral Index (BIS, Aspect Medical Systems, Newton, MA, USA) and Spectral Entropy (GE Healthcare, Helsinki, Finland) use dimensionless monotonic index as a measure of anesthetic depth, typically scaled from 100 (awake state) to 0 (deep coma). In patients who are conscious and awake, the score lies between 90 and 100, while complete cortical suppression leads to scores of 0. Score of 44-60 has been recommended for general anesthesia (GA).[5,6,7,8,9,10,11]
Entropy is a modality designed to provide information on the state of the central nervous system during GA, based on acquisition and processing of raw electroencephalography (EEG) and frontal electromyography signals using entropy algorithm. This may be useful for titration of inhalational anesthetic agents more precisely. Most importantly, it entails measurement of the effect of anesthetic on its target organ rather than merely the concentration of anesthetic in the brain.
We proposed this prospective randomised study to evaluate the effect of entropy monitoring on isoflurane consumption and recovery profile at the end of anesthesia.
Material and Methods
Institutional Ethical Committee clearance was obtained before the study and informed written consent was taken from all the patients. This study was designed to be a prospective randomized controlled trial wherein 60 patients between the age of 20 and 60 years, belonging to American Society of Anesthesiologists-Physical Status PS I and II, body mass index 18-25 kg/m2, undergoing elective abdominal hysterectomy, requiring administration of GA using endotracheal tube were included. Patients on sedatives/hypnotics preoperatively, hemodynamically unstable, with altered sensorium, hypothermic patients and with psychiatric illness/alcohol abuse were excluded from the study.
Observer 1 examined patient preoperatively and ensured that inclusion criteria were met and none of the exclusion criteria were present. Observer 2 titrated inhaled anesthetic agent intraoperatively as per the allocated group. All patients were kept nil orally as per standard guidelines and received premedication with tablet alprazolam 0.5 mg (if weighing >60 kg) and 0.25 mg (if weighing ≤60 kg) the night before surgery (HS) and in the morning of surgery. If patients had any evidence of acid peptic disease or gastrointestinal reflux disease, they also received tablet metoclopramide 10 mg and tablet ranitidine 150 mg HS and on the morning of surgery. Patients were randomized using computer generated numbers to one of two groups: Group clinical practice (CP) or Group entropy (E).
In operating room, intravenous (IV) access was secured, and standard monitoring consisting of noninvasive blood pressure (BP), pulse-oximetry, and 5-electrode electrocardiogram were established. Skin of forehead was carefully wiped with an alcohol swab and allowed to dry. A self-adhesive EEG electrode strip used to monitor entropy was positioned on the forehead as indicated on the strip. The monitor was set such that all patients were monitored using MAC and entropy. However, in Group CP, MAC was displayed on monitor whereas entropy values were recorded but not displayed. In Group E, entropy values were displayed but MAC values were recorded but not displayed.
Anesthesia was induced with IV propofol 1.5-2 mg/kg and IV fentanyl 2 μg/kg followed by neuromuscular blockade using IV vecuronium 0.1 mg/kg of body weight. The patient was ventilated with 2% isoflurane in 6 L/min of oxygen (O2). After endotracheal intubation, anesthesia was maintained with nitrous oxide (N2O) and O2 in ratio of 1:1, along with isoflurane with the total fresh gas flow (FGF) of 3 L/min. Isoflurane was delivered through TEC7 vaporizer by regulating its concentration through dial setting in percentage. The ventilatory rate was adjusted to maintain end-tidal carbon dioxide concentration 30-35 mm Hg. Analgesia was provided with IV morphine 0.1 mg/kg bolus given slowly over 5 min and subsequent infusion at 20 μg/kg/h was started.
After induction of anesthesia, additional monitoring included capnography, anesthesia gas analysis, entropy and nasopharyngeal temperature. Patients were kept normothermic using heat and moisture exchanger and surface body warmer. Intermittent boluses of IV vecuronium 1 mg were administered on the visual appearance of second twitch using train of four (TOF) stimuli. Isoflurane was used to maintain DOA in all the patients and use of other sedative/hypnotic drugs such as midazolam or propofol was avoided.
The amount of isoflurane required was titrated according to the allocated group of patient. In Group CP, a blinded person titrated isoflurane using clinical parameters such as heart rate (HR) and BP. Inhaled anesthetic concentration was titrated to maintain MAC of 1-1.3. Minimum MAC of 0.6 was maintained, even if the patient became hemodynamically unstable, as it might increase the possibility of intraoperative awareness in the absence of any sedative-hypnotic. The person was blinded to entropy values (which were recorded by monitor but not displayed on the front screen, and were retrieved at the end of the procedure and noted in proforma). On the other hand, in Group E, isoflurane was titrated keeping the response entropy (RE) score between 40 and 60 and person titrating was blinded to MAC values (which were recorded by the monitor but not displayed on the front screen, and were retrieved at the end of the procedure and noted down in proforma). After changing set isoflurane concentration, no subsequent changes were made for at least 2 min. All monitoring data were recorded manually by blinded person on proforma at 5 min interval, beginning from the start of controlled ventilation after induction.
Intraoperative hypertension or hypotension was defined as systolic BP 25% above or below the baseline value, and tachycardia or bradycardia as HR 25% above or below the baseline value, respectively. Intraoperative hypotension was treated with boluses of IV fluid, IV mephentermine 3 mg, or IV phenylephrine 50 μg as appropriate. Isoflurane concentration was reduced if necessary to a minimum MAC of 0.6 and increased back to MAC of 1–1.3 as soon as possible. Intraoperative hypertension was treated by increasing the set isoflurane concentration by 0.5%, administration of IV vecuronium 1 mg or IV morphine 1 mg, as appropriate in both groups. In Group E, if the difference between RE and state entropy (SE) was >10 for >2 min, additional IV morphine 1 mg was given and at the same time if RE values were above 60, isoflurane concentration was increased. Measurement of intraoperative consumption of isoflurane in milliliters was calculated as described by Dion.[12]

where, set % is a concentration of the anesthetic set on the vaporizer and FGF is in L/min.
Isoflurane was continued until closure of skin incision in both groups. Morphine infusion was discontinued 20-30 min before the expected time of completion of the procedure. Isoflurane was cut off at the end of skin suturing, and FGF was increased to 4 L/min maintaining O2 and N2O in 1:1 ratio. Neuromuscular blockade was reversed with neostigmine 50 μg/kg body weight and glycopyrrolate 10 μg/kg body weight when four responses were seen with TOF stimulus at 40 mA. N2O was discontinued after giving reversal agent, and FGF increased to 6 L/min. Trachea of patients in both groups were extubated when they fulfilled subjective and objective criteria of extubation (following commands, intact gag reflex, ability to generate tidal volume of 6 ml/kg body weight and T1/T4 ratio of 0.9 on TOF using TOF guard or no fade detected with TOF stimulus at 40 mA using peripheral nerve stimulator.
From discontinuation of isoflurane, the time taken by the patient to open eyes on verbal command, time to extubation and time to readiness for shifting to recovery room was recorded in both groups. Finally, on arrival to postanesthesia care unit (PACU), the immediate PAR score[13] was recorded in all patients by a blinded person. Patients with PAR score of ≥8 were considered fit to be shifted out of PACU. IV infusion of morphine was continued for postoperative pain management. Finally, all patients were visited on the 1st postoperative day and interviewed about the intraoperative recall.
Statistical data analysis
The sample size was calculated to be 60 patients on the basis of a study done by Wong et al. in elderly western population.[14] After power analysis, it was suggested that 28 patients in each group would be adequate to detect 20% reduction in time to get orientation after discontinuation of anesthesia. Sixty patients were studied in randomized controlled study, with 30 patients in each group. The demographic data, duration of surgery, amount of isoflurane used, time taken to open eyes and to extubation were analyzed using Student's t-test. The PAR score was analyzed using Chi-square test. P value <0.05 was considered as statistically significant. SPSS version 16.0, (IBM Software Group's Business Analytics, IBM Corporation) software was used to conduct these tests.
Results
Demographic data (age, height and weight) of patients in both groups were comparable [Table 1]. Mean (±standard deviation) duration of surgery was similar in both the groups. The amount of isoflurane used was also similar in both groups [Table 2]. Time taken to open eyes on commands as well as time to extubation were faster in Group E as compared to Group CP [Table 3]. There was a statistically significant difference of 3 min of recovery time in group E compared to Group CP. The PAR score was also similar between the two groups [Table 4]. All patients had a score of 8 by the time they were extubated. None of the patients had recall of intraoperative events.
Table 1.
Demographic data (all values are expressed as mean ± SD)
Table 2.
Amount of isoflurane consumed (all values expressed in mean ± SD)

Table 3.
Recovery times in minutes (mean ± SD) after discontinuation of isoflurane
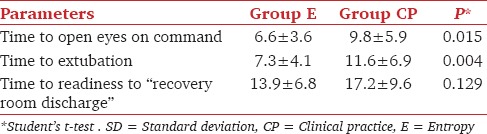
Table 4.
Number of patients with PAR score of 10, 9 and 8 on arrival in PACU

Discussion
DOA is widely assessed by using clinical parameters such as HR, BP, sweating and tearing (PRST score) in day to day clinical practice, especially in developing countries, where more sophisticated monitoring equipment to assess DOA are not easily available or affordable.[15] In this study, entropy monitoring was compared to clinical practice for titration of isoflurane during maintenance of anesthesia and subsequent anesthesia recovery period. Our results suggest that use of entropy monitoring does not result in a decrease in isoflurane consumption. Furthermore, though anesthesia recovery period was shorter with entropy use, time to discharge from recovery room was not influenced.
Bannister et al. concluded that, in older (age >6 years) children undergoing tonsillectomy and/or adenoidectomy, BIS-guided anesthetic management was associated with significant reduction in anesthetic use, earlier emergence, and shorter recovery. In patients of 0-6 months of age undergoing inguinal herniotomy, sevoflurane consumption was lesser in BIS group, whereas, emergence and recovery measures were unaffected by BIS titration. In children of 6 months to 3 years of age, there were no significant differences between standard practice and BIS groups in anesthetic use or recovery profile.[16] In this study, sevoflurane was used as inhaled anesthetic agent, whereas, we used isoflurane. Moreover, this study was conducted in infants and children, whereas, our study was done in adults. In this study, there is no mention about the duration of procedure and use of other analgesics intraoperatively or sedative-hypnotics preoperatively, all of which can affect anesthetic consumption. Recart et al. concluded that cerebral monitoring with either BIS or auditory evoked potential devices reduced maintenance anesthetic (desflurane) requirement, resulting in a shorter length of stay in PACU and improved quality of recovery after laparoscopic surgery.[17] In this study, desflurane was used as inhaled anesthetic agent, in contrast to isoflurane used in our study, which has very low blood: gas coefficient and thereby, faster induction and recovery.
Wheeler et al. showed that pain induced elevated RE was related to the lighter plane of anesthesia rather than recovery of myodynamia. The peri-intubation reaction did exist because there were significant elevations of HR and BP. However, no obvious peri-intubation changes of RE, SE, and BIS were detected.[18] In our study, the difference between RE and SE scores of more than 10 was considered as the need for additional analgesia, even if RE and SE scores were between 40 and 60. We did not have any such patients who needed additional analgesics with the adequate plane of anesthesia as suggested by SE score of 40-60.
Aimé et al. proved that use of BIS or spectral entropy monitoring consistently reduced sevoflurane use by 29%, compared with standard clinical practice, in patients undergoing anesthetic procedures longer than 1 h and receiving sufentanil infusion.[8] In this study, sevoflurane was used as inhaled anesthetic and sufentanil as an analgesic, in contrast to our study, where isoflurane and morphine were used, respectively. In this study authors have not specified dose of sufentanil; utilized in both the groups and age of the patients was also not specified.
Shafiq et al. concluded that use of BIS in elderly Asian population resulted in 40% reduction of isoflurane and significant shorter recovery times in study group at the end of anesthesia.[19] In contrast to our study, elderly population was studied, and duration of surgery or surgical procedures were not standardized. Gao et al. confirmed values of RE and SE as approximating BIS value. At the onset of unconsciousness during propofol target controlled infusion anesthesia and after elimination of myoelectrical activation, all values of RE, SE, and BIS decreased significantly and the three indices were less sensitive to noxious stimuli than cardiovascular responses.[20] El Hor et al. concluded that monitoring with entropy was associated with a reduction in sevoflurane uptake by 30% and a faster extubation.[21] In contrast to our study, sevoflurane was used in this study. Review of literature by Lobo and Schraag concluded that clinicians should be aware of limitations of commercial devices intending to monitor DOA, which may not reflect the real underlying level of unconsciousness. Processed EEG indices can be altered by nonanesthetic influences ranging from artifacts, that affect signal quality and signal processing, adverse effects of some anesthetic and nonanesthetic drugs, neuromuscular blocking agents, to conditions inherent to the patient such as cerebral tumors, brain ischemia, and temperature.[22]
Dion's method to calculate the usage of inhaled anesthetic vapor consumption is based on a logical assumption that all inhaled anesthetic vapors behave as ideal gases, which in actual practice is not true. The formula is, thus, likely to underestimate the usage of anesthetic vapor. It can be still used to measure usage of inhaled anesthetic vapors due to its simplicity and usability in a routine operating room setting. It is solely dependent on accuracy in noting time duration, set dial concentration and FGF.
We conclude that there is no evidence-based reason for anesthesiologists to change their current practice of titrating anesthetics based on MAC values and clinical parameters.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Smajic J, Praso M, Hodzic M, Hodzic S, Srabovic-Okanovic A, Smajic N, et al. Assessment of depth of anesthesia: PRST score versus bispectral index. Med Arh. 2011;65:216–20. doi: 10.5455/medarh.2011.65.216-220. [DOI] [PubMed] [Google Scholar]
- 2.Rani DD, Harsoor S. Depth of general anaesthesia monitors. Indian J Anaesth. 2012;56:437–41. doi: 10.4103/0019-5049.103956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drummond JC. Monitoring depth of anesthesia: With emphasis on the application of the bispectral index and the middle latency auditory evoked response to the prevention of recall. Anesthesiology. 2000;93:876–82. doi: 10.1097/00000542-200009000-00039. [DOI] [PubMed] [Google Scholar]
- 4.Stoelting RK, Hillier SC, editors. Stoelting's Pharmacology and Physiology in Anaesthetic Practice. New York: Lippincott Williams & Wilkins; 2006. Pharmacokinetics and pharmacodynamics of injected and inhaled drugs; pp. 23–82. [Google Scholar]
- 5.American Society of Anesthesiologists Task Force on Intraoperative Awareness. Practice advisory for intraoperative awareness and brain function monitoring: A report by the American Society of Anesthesiologists task force on intraoperative awareness. Anesthesiology. 2006;104:847–64. doi: 10.1097/00000542-200604000-00031. [DOI] [PubMed] [Google Scholar]
- 6.Monk TG, Weldon BC. Does depth of anesthesia monitoring improve postoperative outcomes? Curr Opin Anaesthesiol. 2011;24:665–9. doi: 10.1097/ACO.0b013e32834c7acf. [DOI] [PubMed] [Google Scholar]
- 7.Fritz BA, Rao P, Mashour GA, Abdallah AB, Burnside BA, Jacobsohn E, et al. Postoperative recovery with bispectral index versus anesthetic concentration-guided protocols. Anesthesiology. 2013;118:1113–22. doi: 10.1097/ALN.0b013e31828604ab. [DOI] [PubMed] [Google Scholar]
- 8.Aimé I, Verroust N, Masson-Lefoll C, Taylor G, Laloë PA, Liu N, et al. Does monitoring bispectral index or spectral entropy reduce sevoflurane use? Anesth Analg. 2006;103:1469–77. doi: 10.1213/01.ane.0000246838.93153.23. [DOI] [PubMed] [Google Scholar]
- 9.Lefoll-Masson C, Fermanian C, Aimé I, Verroust N, Taylor G, Laloë PA, et al. The comparability of bispectral index and state entropy index during maintenance of sufentanil-sevoflurane-nitrous oxide anesthesia. Anesth Analg. 2007;105:1319–25. doi: 10.1213/01.ane.0000287247.30810.aa. [DOI] [PubMed] [Google Scholar]
- 10.Balci C, Karabekir H, Sivaci R. Determining entropy values equivalent to the bispectral index values during sevoflurane anaesthesia. Arch Med Sci. 2010;6:370–4. doi: 10.5114/aoms.2010.14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansen JW, Sebel PS. Development and clinical application of electroencephalographic bispectrum monitoring. Anesthesiology. 2000;93:1336–44. doi: 10.1097/00000542-200011000-00029. [DOI] [PubMed] [Google Scholar]
- 12.Dion P. The cost of anaesthetic vapours. Can J Anaesth. 1992;39:633. doi: 10.1007/BF03008331. [DOI] [PubMed] [Google Scholar]
- 13.Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesth Analg. 1970;49:924–34. [PubMed] [Google Scholar]
- 14.Wong J, Song D, Blanshard H, Grady D, Chung F. Titration of isoflurane using BIS index improves early recovery of elderly patients undergoing orthopedic surgeries. Can J Anaesth. 2002;49:13–8. doi: 10.1007/BF03020413. [DOI] [PubMed] [Google Scholar]
- 15.Evan JM, Davis WL. Monitoring anaesthesia. Clin Anaesth. 1984;2:243–62. [Google Scholar]
- 16.Bannister CF, Brosius KK, Sigl JC, Meyer BJ, Sebel PS. The effect of bispectral index monitoring on anesthetic use and recovery in children anesthetized with sevoflurane in nitrous oxide. Anesth Analg. 2001;92:877–81. doi: 10.1097/00000539-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Recart A, Gasanova I, White PF, Thomas T, Ogunnaike B, Hamza M, et al. The effect of cerebral monitoring on recovery after general anesthesia: A comparison of the auditory evoked potential and bispectral index devices with standard clinical practice. Anesth Analg. 2003;97:1667–74. doi: 10.1213/01.ANE.0000087041.63034.8C. [DOI] [PubMed] [Google Scholar]
- 18.Wheeler P, Hoffman WE, Baughman VL, Koenig H. Response Entropy Increases During Painful Stimulation. J Neurosurg Anaesthesiol. 2005;17:86–90. doi: 10.1097/01.ana.0000151408.62650.b5. [DOI] [PubMed] [Google Scholar]
- 19.Shafiq F, Naqvi HI, Ahmed A. Effects of bispectral index monitoring on isoflurane consumption and recovery profiles for anesthesia in an elderly Asian population. J Anaesthesiol Clin Pharmacol. 2012;28:348–52. doi: 10.4103/0970-9185.98335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao JD, Zhao YJ, Xu CS, Zhao J, Huang YG, Wang TL, et al. Evaluation of entropy for monitoring the depth of anesthesia compared with bispectral index: A multicenter clinical trial. Chin Med J (Engl) 2012;125:1389–92. [PubMed] [Google Scholar]
- 21.El Hor T, Van Der Linden P, De Hert S, Mélot C, Bidgoli J. Impact of entropy monitoring on volatile anesthetic uptake. Anesthesiology. 2013;118:868–73. doi: 10.1097/ALN.0b013e3182850c36. [DOI] [PubMed] [Google Scholar]
- 22.Lobo FA, Schraag S. Limitations of anaesthesia depth monitoring. Curr Opin Anaesthesiol. 2011;24:657–64. doi: 10.1097/ACO.0b013e32834c7aba. [DOI] [PubMed] [Google Scholar]


