Abstract
Background and Aims:
The effect of stellate ganglion blocks (SGBs) was examined in complex regional pain syndromes (CRPS) of the upper body.
Material and Methods:
A total of 287 SGB were given to patients with documented CRPS on medications. Spontaneous and provoked pain assessment was done with numeric pain rating scale (NPRS). The Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire and range of motion (ROM) was recorded before and after each blockade. Difference between a 15-point “global rating of change” scale determined the minimal clinically important difference of the DASH score.
Results:
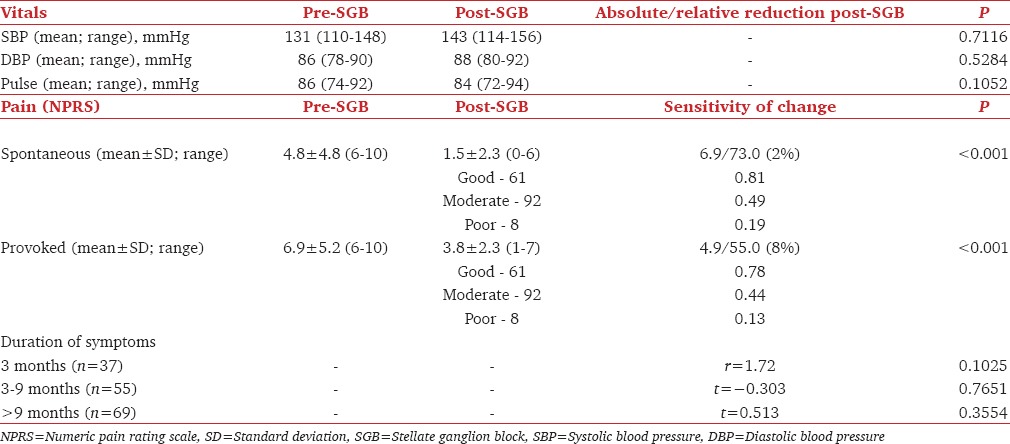
The overall mean pain reduction was 73.2% (r = 0.83, P < 0.001) considering spontaneous and 55.8% (r = 0.77, P < 0.001) on provoked pain. Mean DASH score decreased from 53 (range 36–63; P = 0.14) to 10.4 (range 10–49.2; P = 0.005). The sensitivity to change was 6.9 for spontaneous and 4.9 for provoked pain. Increase in ipsilateral limb temperature has a good correlation with Horner's syndrome (HS) and sympathetic blockade. Minor, self-limiting complications, such as hoarseness, dysphagia, local hematoma, and ipsilateral brachial plexus block occurred in 11.5%. A rare complication of contralateral HS was documented. One patient developed a small pneumothorax, but it did not require intervention.
Conclusions:
SGB are relatively safe and effective management in patients with neuropathic conditions already on pharmacotherapy. Serial blocks attained an average reduction in pain by >3 NPRS points from the baseline for both spontaneous and provoked pain with a decrease in mean DASH score and improvement in ROM.
Keywords: Complex regional pain syndromes, stellate ganglion blocks, upper limb
Introduction
Complex regional pain syndromes (CRPSs) Type I (reflex sympathetic dystrophy) and Type II (causalgia) have been considered as a dysfunction of the autonomic nervous system, partially sympathetically-maintained in view of the trophic changes, increased sweating and lower temperature commonly observed.[1,2] First-line treatment includes pharmacological therapy, physical rehabilitation, and occupational therapy. Stellate ganglion blocks (SGBs) had been reserved for therapy-refractory cases with a 2B+ recommendation (benefits closely balanced with risk and burdens) only.[3,4,5] However, recent studies suggest a higher efficacy of SGB if given within 12–17 weeks of onset of symptoms.[2,6,7]
Image-guided SGBs have increased safety and accuracy as compared with blind, landmark-based as well as fluoroscopic techniques.[2,4,7,8] Ipsilateral Horner's syndrome (HS) almost always develops after SGB and is taken as evidence of a successful sympathetic blockade of the upper limb. However, development of HS itself does not ensure a complete sympathetic blockade. An ipsilateral warming of 1.5°C–7.5°C of the arm has been variously reported as being a more reliable indicator of sympathectomy.[2,3,6]
A series of SGB at the Pain Clinic in a Tertiary Hospital of the Armed Forces were evaluated with the hypothesis that SGB can achieve at least 3 point reduction in numeric pain rating scale (NPRS) as well as a >10 point reduction in Disabilities of the Arm, Shoulder and Hand questionnaire (DASH)[9,10] from the baseline spontaneous and provoked values. The primary objective was to measure the extent of pain relief following SGB. The secondary objective was to see the percentage of patients with the ipsilateral warming of the upper extremity with or without HS who had a reduction in pain relief and symptoms of CRPS.
Material and Methods
After the approval of the Hospital Ethics Committee (reference number: 03/11/Mar/BHDC-14 of Base Hospital, Delhi Cantt, Indraprasth University on 11 Mar 2014), all diagnosed patients as per the Budapest Criteria for CRPS referred to the Pain Clinic were enrolled into the study. Inclusion criteria included patients more than 18 years of age, on prior medications for at least 3 months continuously, which then would not be altered during study. In the case of a <3 point reduction in NPRS or a <10 point reduction in the DASH score at the time of review after 3-weeks, a repeat SGB was considered. All injections were included in the study. All patients were subjected to a Short Portable Mental Status Questionnaire (SPMSQ) indicating intact or mildly impaired cognitive functioning.[11] Those who refused inclusion, had exclusion comorbidities, an SPMSQ score of <6 and change in medication during the study were excluded from the study.
Written informed explained consent for the procedure, its possible complications and study participation was obtained from all patients.
Technique
Patients were positioned supine after securing a peripheral intravenous line and applying standard five parameters non-invasive monitoring. Head was rotated to contralateral side and neck slightly extended. Patients were instructed not to speak during the procedure.
The longus coli muscle in the neck was identified ultrasonically by placing the transducer (phased array transducer, frequency 4–15 MHz; Terason, U Smart 3200T, Teratech Corporation, Burlington, USA) transversely on the ventromedial aspect of the neck, 1 cm inferior to the cricoid cartilage on the side to be blocked. A 22 Gauge single-shot needle (Stimuplex ® Ultra, B. Braun, India) was inserted up to the prevertebral fascia on the longus coli following skin infiltration. The tip was repositioned if any paresthesia of the arm or hand was elicited. After negative aspiration, 6 ml of a local anesthesia mixture (LAM) including 5 ml 0.3% bupivacaine and 4 mg dexamethasone was injected. The spread of LAM around the longus coli muscle was observed. After needle withdrawal, patients were positioned in a 30° upright position to ensure proper spread.
Development of ipsilateral HS (ptosis, miosis, enophthalmos, facial anhidrosis, and conjuctival injection) within 10 min of the first injection was taken as the primary end-point for inclusion into the study.
Data were collected on four occasions following each injection: Initially, before the injection, followed by 1 h postinjection. Two follow-up assessments were done-1 and 3 weeks later and depending on relief of symptoms, the block was repeated after 3 weeks. To increase inter-rater reliability, a second rater conducted a second assessment.
Documented observations included distal skin surface temperature of both upper extremities pre- and postblock using fever scan (FirstCry India). An increase in local temperature of >2.0°C in the ipsilateral arm along with the HS was taken as complete sympathetic nervous system blockade. Other signs of sympathetic blockade were noted (flushing of face, nasal stuffiness, and lacrimation). Complications were recorded.
On all four occasions, patients were instructed for the use of NPRS (0 = no pain; 10 = worst pain imaginable) and values for spontaneous and provoked pain were documented. The range of motion (ROM) in all the joints of the affected upper extremity was recorded. Functional assessment was done by the DASH questionnaire.[9] The score was calculated as ([sum of n responses]/n)−1) where n represented the number of completed items and expressed as a percentage. If up to three items were missing, patients were included as intention-to-treat. Those who had a repetition of up to three items of the DASH score missing were excluded from the analysis. A 15-point “global rating of change” scale was used as an anchor of the DASH score. Participants were asked to answer “Since your last visit, how much change has there been in the function of your arm?”. Scoring options ranged from “−7 a very great deal worse” through “0 no change” to “+7 a very great deal better.” Interpretation of the scores were: 0 and ±1 were considered “no change,” ±2 and ±3 a “small change” and equivalent to the minimal clinically important difference (MCID), ±4 and ±5 a “moderate change,” and ±6 and ±7 a “large change.”
The outcome measures were a minimal detectable change (MDC) and a MCID of 2 points of the NPRS and the MDC of 11 points and an MCID of 15 points of the DASH questionnaire. Participants who demonstrated a change beyond the MDC and MCID of the NPRS and DASH were considered to have achieved a successful outcome. For comparability of pain reduction levels, patients were classified as good (>50%) pain reduction, moderate (30%–50%), and poor (<30%) pain reduction responders, based on their individual degree of pain reduction.[6]
Statistical analysis was performed by the SPSS program for Windows, version 22.0 (SPSS, Chicago, Illinois, USA) and Microsoft Excel (Microsoft, Redmond, WA, USA). Descriptive statistics were used to describe sample characteristics. Continuous variables are presented as the mean ± standard deviation (SD), and categorical variables are presented as absolute numbers and percentage. Data were tested for and fulfilled assumptions for parametric calculations (Shapiro–Wilk test). Change scores of the outcome measures between initial assessment and follow-up were calculated and evaluated with paired t-tests for significance testing of change and evaluated with the Cohen's d effect size for head-to-head comparison of change measured using the outcome measures. The sensitivity to change was calculated by the change divided by SD of change,[10] whereas the Wilcoxon signed-rank test was used for those variables that were not normally distributed (pain scores). Categorical variables were analyzed using either the Chi-square test or Fisher's exact test. For all statistical tests, a P < 0.005 was taken to indicate a significant difference.
Results
A total of 208 patients reported to the Pain Clinic over 2 years. The study group received a total of 334 SGB. Thirty-one patients did not develop HS and were not included in the study. Sixteen patients were excluded as 11 changed their initial pharmacotherapy and 05 were lost to follow-up.
A total of 287 injections to 161 patients were subjected to statistical analysis. The demographic data are given in Table 1. The pharmacotherapy was noted but not analyzed [Table 1].
Table 1.
Demographic data
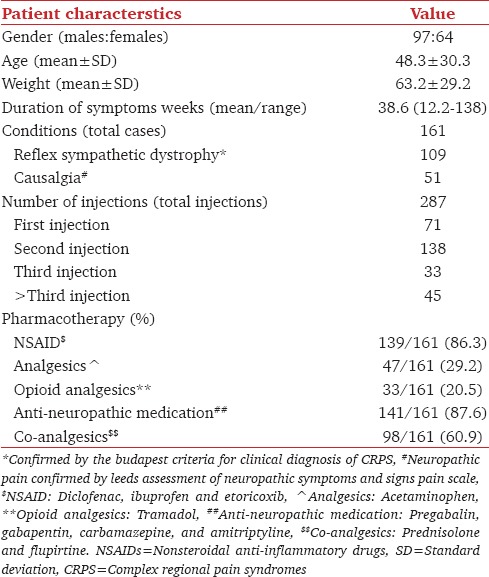
All patients developed ipsilateral HS. Ninety patients (55.9%) needed more than one SGB due to persistent symptoms with a majority requiring two injections 3 weeks apart (69/161 patients; 42.9% and 138/287 injections; 48.1%). Only 57.1% of patients (92/161) were moderately satisfied with the outcome of their treatment (179/287 injections; 62.8%). Increase in ipsilateral temperature of >2.0°C was seen following 68.6% injections, ranging between 7.3% and 30.3% as per the number of injections (197/287) [Tables 1 and 2].
Table 2.
Observations (sympathetic blockade and level of satisfaction)
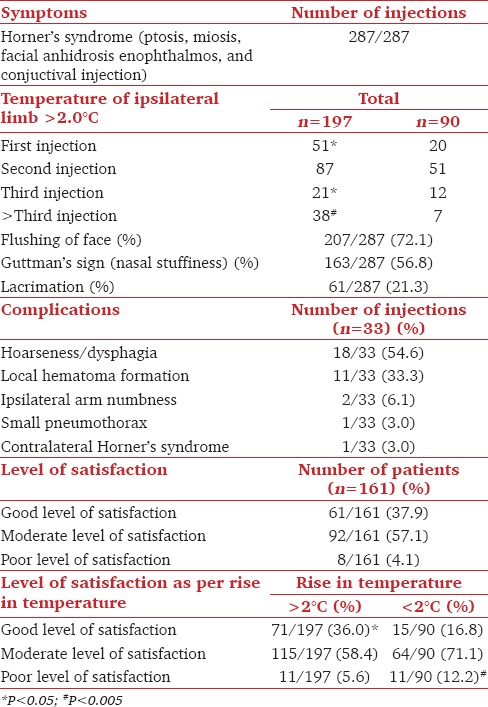
Satisfaction levels were significantly lower (P < 0.005) following those injections in which an increase in ipsilateral limb temperature of >2.0°C was not observed (12.2% vs. 5.6%) [Figures 1 and 2]. Facial flushing was observed in 72.1%, nasal stuffiness in 56.1%, and lacrimation in 21.6% of injections [Table 2].
Figure 1.
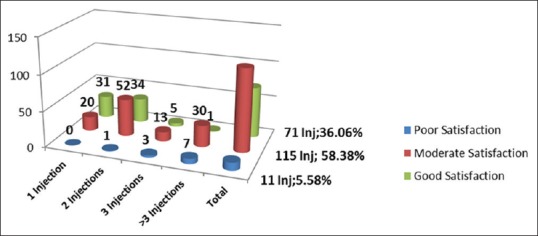
Patient satisfaction in patients with increase in ipsilateral limb temperature of >2.0°C
Figure 2.
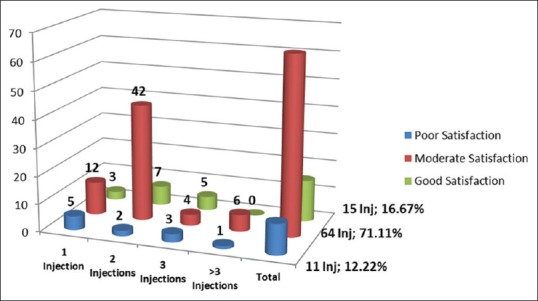
Patient satisfaction in patients with increase in ipsilateral limb temperature of <2.0°C
Of the 8 patients who reported being dissatisfied with their outcome before giving their unwillingness for further blocks, 5 patients received 3 injection, 2 received 4 injections, and one patient received four SGB. Increase in temperature was demonstrated only in 6 of these 8 patients.
Evaluation of the self-reported questionnaire revealed a mean pain reduction of 73.2% (r = 54.86, P < 0.001) regarding spontaneous and 55.8% (r = 55.5, P < 0.001) regarding provoked pain [Table 3] with 79 patients reporting any ongoing pain. The sensitivity to change was 6.9 for spontaneous and 4.9 for provoked pain. The mean DASH score was 53 (range 36–63; r = 0.13; P = 0.14) which decreased to 10.4 (range 10–49.2; P = 0.005). The ROM was compared with the contralateral extremity on shoulder forward flexion and abduction, elbow flexion and extension, and forearm pronation and supination, wrist movements. Sixty-two patients (38.5%) had mild residual loss of ROM, with 41 demonstrating decreased forearm pronation and supination of 10° and 15°, respectively, and four demonstrating decreased shoulder internal rotation of 10°. Seventeen patients reported functional limitations, both related to overhead activities.
Table 3.
Observations
The 62 patients with a decrease in ROM were sub-analyzed. None had an MCID of <2 points of the NPRS. Five patients had an MDC of <11 points and an MCID of 15 points of the DASH questionnaire Seven received only one injection; 41 received two; nine received three and five received > three injections. Thirty-nine among these (59.7%) did not demonstrate a rise in temperature >2°C. This was, however, not statistically significant.
Recorded blood pressure and the pulse showed an increase postprocedure but were not statistically significant. No electrocardiogram abnormalities were observed during the period of observation in any SGB.
A total complication rate of 11.1% (33 out of 287 injections) was observed. All complications were mild and self-limiting [Table 2]. None complained of breathing difficulty. On indirect laryngoscopy, ipsilateral vocal cord paresis was observed in all these patients.
One patient had a contralateral HS. 10 min after administration of third left SGB, right sided HS with mild right-sided facial flushing was observed, with no signs of sympathetic denervation on left side of the face. Both right and left arms demonstrated a rise in temperature with pain relief (pre-block NPRS 6 improved to NPRS 3 postblock) in the left elbow and left hand. An ultrasound scan showed bilateral drug spread, with spread on the right side more than the left.
There were no reported cases of inadvertent intravascular injection, spinal or epidural block.
Discussion
CRPS encompass a variety of painful conditions following injury, which are disproportionate to the inciting event. Repeated SGB has been shown to improve outcomes, decrease pain and increase ROM in upper limb CRPS.[1,2,5] A systematic review of literature included 29 studies that evaluated 1,144 patients on the role of sympathetic blocks in CRPS patients. The authors reported that 29% of patients had full pain relief, 41% had partial relief, and 32% had absent relief.[12] Most of our patients (57.1%) responded to two injections with moderate pain relief while 4.1% had minimal pain relief even after >3 injections. It is possible that our results differ as these patients were referred by physicians early in the course of CRPS.
Ultrasound guided SGB is now the standard of care. Chassaignac tubercle is only a surrogate marker as the cervical sympathetic chain lies on the prevertebral fascia.[4] Structures that are at risk (vertebral artery, nerve roots, trachea, and esophagus) remain unseen under fluoroscopy.[7] Transient side effects, (hoarseness, dysphagia), are commonly observed by fluoroscopic techniques[13,14] though severe complications such as intravascular injections, esophageal injury, and airway obstruction due to hematoma formation described with landmark techniques are avoided.[3,13,14,15,16] Real-time visualization of the spread of the Local anesthetic depot yields a higher accuracy during placement of the depot.[14] Feigl et al. investigated the distribution of three different volumes of solution in a total of 42 cadavers (84 halves) and concluded that 5 ml resulted in an almost ideal vertical distribution, whereas higher volumes of 10–20 ml were at risk of spreading both vertically as well as to other regions of the neck.[17] A total volume of 6 ml was used in this study.
Stellate ganglion is formed by the fusion of the inferior cervical ganglion and the first thoracic (T1) ganglion. Other efferent sympathetic pathways described include extra-ganglionic sympathetic pathways and intermediate ganglions in the spinal roots of C8–T2, but these are not clinically significant. Resection of the second thoracic (T2) sympathetic ganglion is also required for near total sympathetic denervation of the upper limb. Pupillary fibers originate from T1 ganglion, pass through the stellate ganglion and synapse in the superior cervical ganglion. HS may develop without sympathectomy if only the upper half of the ganglion is affected. To confirm sympathectomy, an ipsilateral warming of 1.5°C–7.5°C of the arm has been variously reported,[2,3,6] although an increase of 2.0°C is generally accepted as a definite sign for the same.[18,19] In this series, the increase in ipsilateral arm skin temperature was seen in 68.6% of the cases, with greater percentages seen with an increase in the number of injections [Figures 1 and 2]. It is postulated that in the presence of existing vasoconstriction, as in late stages of CRPS, the skin temperatures show a greater rise, whereas large temperature differences are usually not seen in early stages.[7,8,19,20] The larger number of patients demonstrating an increase in temperature in this study (197 injections/287) could be due to the cut-off of 2.0°C even though there was a clinical improvement in CRPS with repeated injections.
In this study, the incidence of facial flushing, nasal stuffiness, and lacrimation observed correlates well with other studies as signs of sympathetic blockade.[2,3,6,7,19,21] Murakawa et al. reported a drop in ipsilateral tympanic temperature in 30 patients following SGB and opined that this is a better sign of sympathetic blockade.[22] Matsukawa et al. however noted a slight, insignificant increase in ipsilateral tympanic membrane temperature 10 min after SGB and subsequently.[23] Tympanic temperatures were not noted in this study.
Both subjective and objective measures were used as the former provides a different insight into the patient's problem than objective or performance-based measures. The DASH score has documented limitations inspite of widespread use which includes a combination of multiple measurement dimensions into a single outcome score, combined subjective and objective measures and lack of standardization.[24] Hence, a 15-point “global rating of change” scale was used as an anchor of the DASH score.
Severe and possibly fatal consequences of SGB have been reported in the literature.[3,7,12,16] Direct spread of the LA can produce hoarseness, an elevated hemidiaphragm and dyspnea as a result of blockade of the recurrent laryngeal and phrenic nerve, respectively.[3,20] Transient ipsilateral vocal cord palsy was observed in 18 patients, which recovered without intervention by 2 h. Hence, SGB should be avoided in patients with contra-or bi-lateral vocal cord paralysis or palsy. Moreover, since the incidence of hoarseness of voice ranges from <10% with volumes <10 ml and up to 80% with 20 ml,[4,7,18,25] it is prudent to advise to advise a “Nil per os” status of a minimum 4 h duration. Injury to adjacent vascular structures, including the vertebral artery, carotid artery and internal jugular vein, can result in hematoma formation.[3,7,26] There were 11 cases of local hematoma formation with no incidence of airway compromise. Pneumothorax, esophageal perforation, and chylothorax from thoracic duct injury have been reported due to the proximity of these structures to the stellate ganglion.[3,4,7,16,20,27] These are uncommon when the block is done under image guidance. There was only one case of pneumothorax which was small, not warranting intervention.
Neuraxial migration of LA into the epidural space, intrathecally or brachial plexus can occur.[3,4,7,22,26] Two patients had transient ipsilateral brachial plexus involvement in this series evident by the presence of ipsilateral upper limb weakness.
Three cases of bilateral and four cases of contralateral HS have been previously reported in the literature.[27] Postulated causes are LA migration to the contralateral side, cross-variations in stellate ganglion innervations or ipsilateral ciliospinal center inhibition. The LA can migrate to the opposite side if there is a medial angulation of the needle, injection given behind the longus colli muscle, or a large LA volume injected. The ciliospinal center of Budge lies in the spinal cord and may be inhibited by LA migration. This results in an ipsilateral pupillary dilation, facial hyperhidrosis, and lid lift which may be confused as contralateral HS.[4,27] In addition, it is also postulated that the cervical sympathetic trunk can be blocked independent of upper limb thoracic sympathetic fibers, especially when the drug is injected at the level of C6 tubercle.[4] Similar findings can be seen in upper spinal cord lesions and Harlequin syndrome where hemifacial cutaneous sympathetic denervation is caused by the failure of the upper thoracic sympathetic chain with sparing of T1 segment. These suggest that bilateral sympathetic blockade occurs commonly, ranging from functional changes (such as increased hand warming and facial skin blood flow) without gross anatomic changes (such as HS) to a full spectrum.[26,27,28,29] In our series, we injected 6 ml LA, with the needle apparently perpendicular to the plane, although slight medial angulation of the needle cannot be ruled out. It is theorized that in this patient, the contralateral HS developed due to blockade of contralateral cervical and bilateral thoracic sympathetic chain.
Intravascular/intrathecal injection of LA can result in arrhythmia, seizures, bradycardia, hypotension, and cardiovascular collapse due to inhibition of sympathetic fibers. In addition, soft tissue infection, osteitis, and neuraxial infection (meningitis) may be seen.[2,7,13,14,16] There were no such complications in this series.
Conclusion
Serial SGBs attained an average reduction in pain by 3 NPRS points from patient's baseline for both spontaneous and provoked pain, in patients with CRPS already on pharmacotherapy. SGB's should be administered early in the course of CRPS and pharmacotherapy can be reduced subsequently. Development of HS does not ensure successful upper limb sympathetic blockade after SGB; demonstration of an ipsilateral increase in skin temperature should also be documented. Due to adjacent important neurovascular structures and underlying anatomical variations, various potential life-threatening complications can be associated with this seemingly simple procedure. Thorough anatomical knowledge, vigilant postprocedure monitoring and emergency preparedness are warranted.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Yucel I, Demiraran Y, Ozturan K, Degirmenci E. Complex regional pain syndrome type I: Efficacy of stellate ganglion blockade. J Orthop Traumatol. 2009;10:179–83. doi: 10.1007/s10195-009-0071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Eijs F, Stanton-Hicks M, Van Zundert J, Faber CG, Lubenow TR, Mekhail N, et al. Evidence-based interventional pain medicine according to clinical diagnoses 16.Complex regional pain syndrome. Pain Pract. 2011;11:70–87. doi: 10.1111/j.1533-2500.2010.00388.x. [DOI] [PubMed] [Google Scholar]
- 3.Day M. Sympathetic blocks: The evidence. Pain Pract. 2008;8:98–109. doi: 10.1111/j.1533-2500.2008.00177.x. [DOI] [PubMed] [Google Scholar]
- 4.Elias M. Cervical sympathetic and stellate ganglion blocks. Pain Physician. 2000;3:294–304. [PubMed] [Google Scholar]
- 5.Ackerman WE, Zhang JM. Efficacy of stellate ganglion blockade for the management of type 1 complex regional pain syndrome. South Med J. 2006;99:1084–8. doi: 10.1097/01.smj.0000233257.76957.b2. [DOI] [PubMed] [Google Scholar]
- 6.van Eijs F, Geurts J, van Kleef M, Faber CG, Perez RS, Kessels AG, et al. Predictors of pain relieving response to sympathetic blockade in complex regional pain syndrome type 1. Anesthesiology. 2012;116:113–21. doi: 10.1097/ALN.0b013e31823da45f. [DOI] [PubMed] [Google Scholar]
- 7.Schürmann M, Gradl G, Wizgal I, Tutic M, Moser C, Azad S, et al. Clinical and physiologic evaluation of stellate ganglion blockade for complex regional pain syndrome type I. Clin J Pain. 2001;17:94–100. doi: 10.1097/00002508-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Imani F, Hemati K, Rahimzadeh P, Kazemi MR, Hejazian K. Effectiveness of stellate ganglion block under fuoroscopy or ultrasound guidance in upper extremity CRPS. J Clin Diagn Res. 2016;10:UC09–12. doi: 10.7860/JCDR/2016/14476.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 10.Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: The DASH (disabilities of the arm, shoulder and hand) [corrected].The Upper Extremity Collaborative Group (UECG) Am J Ind Med. 1996;29:602–8. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 11.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol. 1994;47:81–7. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 12.Cepeda MS, Lau J, Carr DB. Defining the therapeutic role of local anesthetic sympathetic blockade in complex regional pain syndrome: A narrative and systematic review. Clin J Pain. 2002;18:216–33. doi: 10.1097/00002508-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Jadon A. Revalidation of a modified and safe approach of stellate ganglion block. Indian J Anaesth. 2011;55:52–6. doi: 10.4103/0019-5049.76601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapral S, Krafft P, Gosch M, Fleischmann D, Weinstabl C. Ultrasound imaging for stellate ganglion block: Direct visualization of puncture site and local anesthetic spread. A pilot study. Reg Anesth. 1995;20:323–8. [PubMed] [Google Scholar]
- 15.Peng PW, Narouze S. Ultrasound-guided interventional procedures in pain medicine: A review of anatomy, sonoanatomy, and procedures: Part I: Nonaxial structures. Reg Anesth Pain Med. 2009;34:458–74. doi: 10.1097/AAP.0b013e3181aea16f. [DOI] [PubMed] [Google Scholar]
- 16.Wei K, Feldmann RE, Jr, Brascher AK, Benrath J. Ultrasound-guided stellate ganglion blocks combined with pharmacological and occupational therapy in Complex Regional Pain Syndrome (CRPS): A pilot case series ad interim. Pain Med. 2014;15:2120–7. doi: 10.1111/pme.12473. [DOI] [PubMed] [Google Scholar]
- 17.Feigl GC, Rosmarin W, Stelzl A, Weninger B, Likar R. Comparison of different injectate volumes for stellate ganglion block: An anatomic and radiologic study. Reg Anesth Pain Med. 2007;32:203–8. doi: 10.1016/j.rapm.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Stevens RA, Stotz A, Kao TC, Powar M, Burgess S, Kleinman B. The relative increase in skin temperature after stellate ganglion block is predictive of a complete sympathectomy of the hand. Reg Anesth Pain Med. 1998;23:266–70. doi: 10.1016/s1098-7339(98)90053-0. [DOI] [PubMed] [Google Scholar]
- 19.Hogan QH, Taylor ML, Goldstein M, Stevens R, Kettler R. Success rates in producing sympathetic blockade by paratracheal injection. Clin J Pain. 1994;10:139–45. doi: 10.1097/00002508-199406000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Kakuyama M, Toda H, Osawa M, Fukuda K. The bilateral effect of stellate ganglion block on the facial skin blood flow. Reg Anesth Pain Med. 2000;25:389–92. doi: 10.1053/rapm.2000.6450. [DOI] [PubMed] [Google Scholar]
- 21.Peng PW, Castano ED. Survey of chronic pain practice by anesthesiologists in Canada. Can J Anaesth. 2005;52:383–9. doi: 10.1007/BF03016281. [DOI] [PubMed] [Google Scholar]
- 22.Murakawa K, Noma K, Ishida K, Matsuda M, Maeda S, Nishimura M, et al. Changes of tympanic temperature by stellate ganglion block. Masui. 1995;44:824–7. [PubMed] [Google Scholar]
- 23.Matsukawa T, Ozaki M, Nishiyama T, Yamaguchi T, Imamura M, Kumazawa T. Stellate ganglion block does not change the tympanic membrane temperatures of either block or unblock sides in male volunteers. J Clin Anesth. 1998;10:619–22. doi: 10.1016/s0952-8180(98)00092-0. [DOI] [PubMed] [Google Scholar]
- 24.Franchignoni F, Giordano A, Sartorio F, Vercelli S, Pascariello B, Ferriero G. Suggestions for refinement of the Disabilities of the Arm, Shoulder and Hand Outcome Measure (DASH): A factor analysis and Rasch validation study. Arch Phys Med Rehabil. 2010;91:1370–7. doi: 10.1016/j.apmr.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 25.Jung G, Kim BS, Shin KB, Park KB, Kim SY, Song SO. The optimal volume of 0.2% ropivacaine required for an ultrasound-guided stellate ganglion block. Korean J Anesthesiol. 2011;60:179–84. doi: 10.4097/kjae.2011.60.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shankar H, Simhan S. Transient neuronal injury followed by intravascular injection during an ultrasound guided stellate ganglion block. Anesth Pain Med. 2013;2:134–7. doi: 10.5812/aapm.7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amhaz HH, Manders L, Chidiac EJ, Pallekonda V, Chakrabortty S. Unusual case of contralateral Horner's syndrome following stellate-ganglion block: A case report and review of the literature. Local Reg Anesth. 2013;6:31–3. doi: 10.2147/LRA.S49580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagasaka Y, Wasner G, Sharma B, Fleischmann K. Harlequin syndrome after thoracic paravertebral block. A A Case Rep. 2016;6:48–51. doi: 10.1213/XAA.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 29.Mohindra A, Herd MK, Roszkowski N, Downie IP. Concurrent Horner's and Harlequin syndromes. Int J Oral Maxillofac Surg. 2015;44:710–2. doi: 10.1016/j.ijom.2015.01.008. [DOI] [PubMed] [Google Scholar]


