Abstract
Background
Cutaneous squamous cell carcinoma (cSCC) is the second most widespread cancer in humans and its incidence is rising. Novel therapy with better efficacy is needed for clinical treatment of cSCC. Many studies have shown the importance of DNA repair pathways during the development of cancer. A key nucleotide excision repair (NER) protein, xeroderma pigmentosum group D (XPD), is responsible for the excision of a large variety of bulky DNA lesions.
Material/Methods
To explore the role of XPD in A431 cells, we overexpressed XPD in A431 cells and performed MTT assay, flow cytometry, and Western blot analysis to examine cell proliferation, cell apoptosis, and genes expression.
Results
We found that the overexpression of XPD suppressed cell viability, induced cell cycle arrest at G1 phase, and promoted cell apoptosis. Additionally, XPD blocked the expression of c-myc, cdc25A, and cdk2, and improved the levels of HIPK2 and p53.
Conclusions
These results provide new evidence to reveal the role of XPD in cSCC A431 cells and suggest that XPD may serve as an anti-oncogene during cSCC development.
MeSH Keywords: Apoptosis; Carcinoma, Squamous Cell; Cell Cycle; Cell Proliferation
Background
Cutaneous squamous cell carcinoma (cSCC) originates from stem/progenitor cells of the basal cell layer of the epidermis and cutaneous basal cell carcinoma [1], which is mostly common in skin epidermis or appendages [2]. cSCC is a serious threat to human health. Clinicians have developed some common treatments according to individual characteristics of patients, including radiotherapy, surgery, and chemotherapy [3]. However, these therapies have several drawbacks and limited treatment efficacy [4]. Therefore, novel therapy with better efficacy is needed for clinical treatment of cSCC.
DNA repair pathways play an essential role in cancer susceptibility by maintaining genomic integrity [5]. Studies have demonstrated that defects in DNA damage repair lead to accumulated DNA damage [6], which confers a higher risk of cSCC cancer [7]. As a research focus and problem in recent studies, molecular targeting treatment is a potential therapy for cSCC, but its efficacy largely depends on the selection of highly specific molecular targets [8]. The most important DNA damage repair mechanism in humans is nucleotide excision repair (NER). A key NER protein, Xeroderma pigmentosum group D (XPD), as a subunit of transcription factor IIH, has ATP-dependent 5′-3′ helicase activity and is involved in gene transcription with the action of RNA polymerase II [9,10]. Some reports revealed that genetic polymorphisms of XPD in the nucleotide excision repair pathway affected the capacity for DNA repair to influence susceptibility to many cancers [6], such as hepatocellular carcinoma [11], esophageal cancer [6], and squamous cell carcinoma [12]. Also, ectopic expression of XPD was associated with the occurrence of angioderma pigmentosum and Cockayne’s syndrome [13]. However, a few studies have explored the relationship between XPD and cSCC. Furthermore, the studies of XPD were mainly focused on the association between XPD polymorphisms and various cancers [14,15]. The mechanism by which XPD is involved in cancer development is unclear.
In the present study, we used cSCC A431 via the expression of XPD to investigate the role of XPD in cSCC development. We found that the overexpression of XPD repressed cell proliferation, blocked cell cycle, and enhanced cell apoptosis in A431 cells. Furthermore, ectopic expression of XPD regulated the expression of cell proliferation-related, cell cycle-related, and cell apoptosis-related genes. We conclude that XPD regulates expression of cell proliferation-related, cell cycle-related, and cell apoptosis-related genes to modulate the development of cSCC.
Material and Methods
Cell culture and transfections
Human cutaneous squamous cell carcinoma A431 cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). A431 cells were seeded into 6-well plates incubated with Dulbecco’s modified Eagle’s medium (DMEM, Hyclone, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone, USA) and incubated in a 5% CO2 cell culture incubator at 37°C for 48 h. When the density reached 90%, cell transfection was carried out with Lipofectamine 2000 (Invitrogen, USA). Cells were divided into 4 groups and the control group (Control group) was administered the same amount of medium. The other 3 groups were transfected with only Lipofectamine (LF group), pEGFP-N2 (empty vector) + Lipofectamine (EM+LF group), or pEGFP-N2-XPD + Lipofectamine (XPD+LF group) in FBS-free medium. After 6 h, the old media were removed and cells were incubated with fresh DMEM containing 10% FBS for 40 h for further experiments.
MTT assay for cell proliferation
After cell transfection, A431 cells were incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphe-nyltetrazolium bromide (MTT, 5 mg/mL) with fresh culture medium at the concentration of 100 μg/well for 4 h. After removing the supernatant, formazan crystal formed were dissolved in 100 μL of dimethyl sulfoxide and the absorbance was measured at 492 nm using a multifunction microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). Six-well replication was used to calculate the cell relative viability. Data were analyzed from 3 independent experiments.
Determination of cell apoptosis by flow cytometry with Annexin V-FITC/PI reagent
After different treatments, A431 cells were digested with trypsin (Sigma, USA) and washed with phosphate-buffered saline (PBS) twice. Then, cells were incubated with 100 μL of binding buffer and 10 μL of FITC-labeled Annexin V. After being incubated at room temperature for 5 min in the dark, cells were analyzed by fluorescence-activated cell sorting (FACS) by flow cytometry (BD FACSCalibur, USA). Cells that stained positive for early apoptosis markers (annexin V-FITC stained only) and for late apoptosis markers (annexin V-FITC and PI stained) were combined for analysis.
Cell cycle distribution measured using flow cytometry assay
To analyze the cell cycle distribution, the transfected A431 cells were digested with trypsin and fixed with 70% cold ethanol overnight at 4°C. After being washed with PBS twice, the cells were subsequently stained with propidium iodide (PI, 50 μg/mL) and RNase A (100 μg/mL). One hour later, cells were subjected to flow cytometry analysis with flow cytometry (BD FACSCalibur, USA).
Quantitative real-time PCR (qRT-PCR)
After cell transfection, cells were collected and total RNA was isolated with an RNA Extraction Kit (Cwbiotech, Beijing, China). The quality and quantity of RNA were detected by measuring the absorbance at 260 nm and 280 nm using a Nano Drop-2000 ultramicrospectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). One μg of RNA was reverse transcribed into cDNA, and qRT-PCR was carried out with SYBR Green qPCR SuperMix (Invitrogen, USA) with the Real-Time PCR system (Bio-Rad, USA). The relative expression of genes was normalized to β-actin with the method of 2−ΔΔCT. The primers for qRT-PCR are listed in Table 1.
Table 1.
The primers for qRT-PCR.
| Genes | Forward primers(5′-3′) | Reverse primers (5′-3′) |
|---|---|---|
| XPD | gcccgctctggattatacg | ctatcatctcctggccccc |
| c-myc | aacccttgccgcatccac | cctcctcgtcgcagtagaaa |
| cdc25A | tgatgaggatgatggcttcg | ctgcacccttgat-gtggc |
| cdK2 | actggcattcctcttccc | ctggcttggtcacatcctg |
| p53 | ctacaagcagtcacagcacatga | tcattcagctctcggaacatctcg |
| HIPK2 | cctaccttacgagcagaccatc | acgcttgagtccacatttttg |
| β-actin | gggcacgaaggctcatcatt | agcgagcatcccccaaagtt |
Western blot
Proteins were extracted by using RIPA lysis buffer (Santa Cruz Biotechnology, USA) and centrifuged at 10 000 rpm at 4°C for 20 min. The concentration of protein was detected by bicinchoninic acid kit (Pierce, Germany) according to the manufacturer’s protocol. Equal amounts of protein were subjected to SDS-PAGE and transferred to PVDF membranes (Millipore, Bedford, MA, USA). After being blocked with 5% non-fat milk in Tris-buffered saline containing 0.05% Tween-20 (TBST), the membrane was incubated with primary anti-c-myc mouse monoclonal antibody (D199941, 1: 5000 dilution), anti-cdc25A rabbit polyclonal antibody (D120394, 1: 1000 dilution), anti-cdk2 mouse monoclonal antibody (D199431, 1: 1000 dilution), anti-HIPK2 (D161742, 1: 1000 dilution), anti-p53 rabbit polyclonal antibody (D220082, 1: 1000 dilution), or anti-XPD mouse monoclonal antibody (D198536, 1: 5000 dilution) (Sangon, Beijing, China) at 4°C overnight. The next morning, the membrane was probed with the corresponding secondary antibody at room temperature for 1 h. After being washed with TBST 3 times, the blot was developed with electrochemiluminescence solution (Pierce, Germany). The target bands were normalized to β-actin using Quantity One software v4.1 (Bio-Rad, USA).
Statistical analysis
Statistical analysis was conducted using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). All experiments were carried out at least 3 times using independent samples. All analytical data are expressed in mean values ± standard deviation, and differences were analyzed using the t test. P<0.05 was considered to indicate a statistically significant difference.
Results
The overexpression of XPD suppressed cell proliferation
Firstly, we transfected A431 cells with recombined vector targeting XPD to explore the role of XPD. The transfection efficiency in A431 cells was examined by the signals of green fluorescence intensity. In Figure 1A, in pEGF-N2-transfected (EM+LF) or pEGF-N2-XPD-transfected (XPD+LF) groups, more than 70% of total cells were green. However, no signal was observed in the control group (Control) or Lipofectamine only transfected group (LF group). Figure 1B and 1C show there was no marked difference among Control, LF, and EM+LF groups; however, the mRNA and protein levels of XPD in the XPD+LF group were significantly enhanced compared to those in the EM+LF group (P<0.01). These data show that the recombined pEGF-N2-XPD was successfully overexpressed in A431 cells for further experiments.
Figure 1.
XPD repressed cell proliferation. A431 cells were divided into 4 groups, and the control group was administered the same amount of medium (Control group). The other 3 groups were transfected with Lipofectamine (LF group), pEGFP-N2 (empty vector) + Lipofectamine (EM+LF group), or pEGFP-N2-XPD + Lipofectamine (XPD+LF group). (A) The signals of XPD were detected after pEGFP-N2-XPD transfection. After cell transfection, A431 cells were observed with an inverted fluorescence microscope. Scale bar=100 μm. (B) The mRNA level of XPD was increased in the XPD+LF group. Total RNA was isolated from 4 groups for QRT-PCR analysis. * P<0.05, ** P<0.01. (C) The protein level of XPD was enhanced in the XPD+LF group. After cell transfection, proteins were extracted for Western blot analysis. * P<0.05, ** P<0.01. (D) The overexpression of XPD blocked cell proliferation. After cell transfection, cell proliferation was examined by MTT assay. * P<0.05, ** P<0.01.
To examine the impact of XPD overexpression on cell proliferation, we transfected cells with pEGF-N2-XPD and performed MTT assay. In Figure 1D, there was no significant difference among Control, LF, and EM+LF groups. Compared with the EM+LF group, cell viability in the XPD+LF group was markedly suppressed (P<0.01). These results reveal that XPD obviously repressed cell proliferation in A431 cells.
XPD induced cell cycle arrest in G1 phase
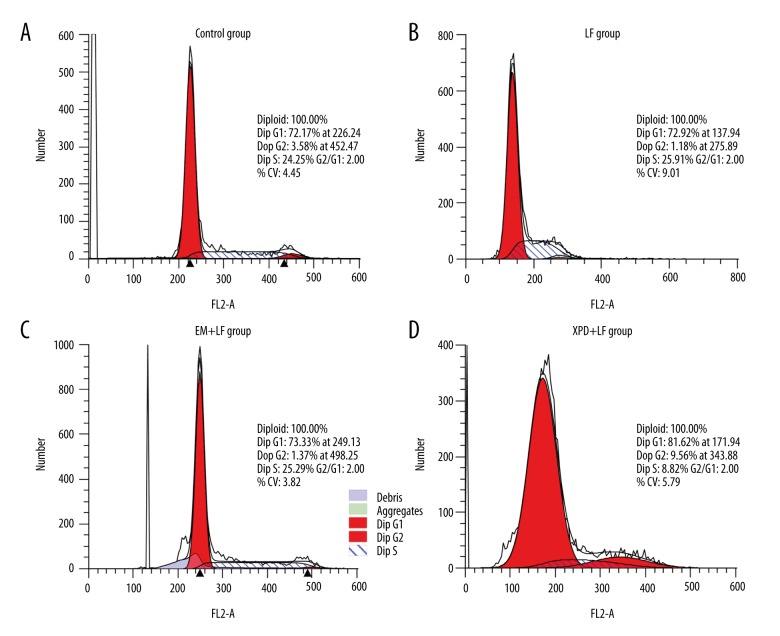
To explore the role of XPD on cell cycle, we incubated cells with pEGF-N2-XPD and examined cell cycle distribution by PI staining and flow cytometry. There was no significant difference in the percentages of A431 cells at G1, S, or G2 phases among the Control group, LF group, or EM+LF group (P>0.05, Figure 2A–2C), suggesting that LF only had no significant effect on cell cycle. However, compared with the EM+LF group, the percentages in the XPD+LF group at G1 phase were markedly increased, and the percentages at S phase were significantly decreased (Figure 2D), indicating that overexpression of XPD led to the G1 arrest of A431 cells. Table 2 shows the percentages of A431 cells in G1, S, and G2 phases from different groups as detected by flow cytometry. These data reveal that XPD induced cell cycle arrest at G1 phase in A431 cells.
Figure 2.
XPD induced cell cycle arrest in G1 phase. A431 cells were transfected with the same amount of medium (Control, A), Lipofectamine (LF, B), pEGFP-N2 (EM) + Lipofectamine (EM+LF group, C), or pEGFP-N2-XPD + Lipofectamine (XPD+LF group, D). Cell cycle distribution was measured using flow cytometry assay.
Table 2.
Percentage of A431 cells in each cell cycle phase detected by flow cytometry.
| Groups | G1 | S | G2 |
|---|---|---|---|
| Control | 72.17±3.42 | 24.25±4.51 | 3.58±2.42 |
| LF | 73.00±4.61 | 26.92±5.48 | 0.08±1.26 |
| EM+LF | 73.83±3.63 | 26.04±4.97 | 0.14±1.76 |
| XPD+LF | 81.62±5.75* | 8.82±4.58** | 9.56±2.1** |
Compared with EM+LF group,
P<0.05,
P<0.01.
XPD enhanced cell apoptosis
We analyzed cell apoptosis in A431 cells after pEGF-N2-XPD transfection to detect the effect of XPD on cell apoptosis. As accessed by flow cytometry with Annexin V-FITC/PI reagent, very few cells were apoptotic in Control, LF, and EM+LF groups (Figure 3A, 3B). However, the cell apoptosis in the XPD+LF group was nearly 4-fold greater than that in the EM+LF group (P<0.01, Figure 3C, 3D). These data indicate that the overexpression of XPD promotes cell apoptosis in A431 cells.
Figure 3.
The overexpression of XPD promoted cell apoptosis. A431 cells were transfected with the same amount of medium (Control, A), Lipofectamine (LF, B), pEGFP-N2 (EM) + Lipofectamine (EM+LF, C), or pEGFP-N2-XPD + Lipofectamine (XPD+LF, D). Cells that stained positive for early apoptosis markers (annexin V-FITC stained only) and for late apoptosis markers (annexin V-FITC and PI stained) were combined for analysis.
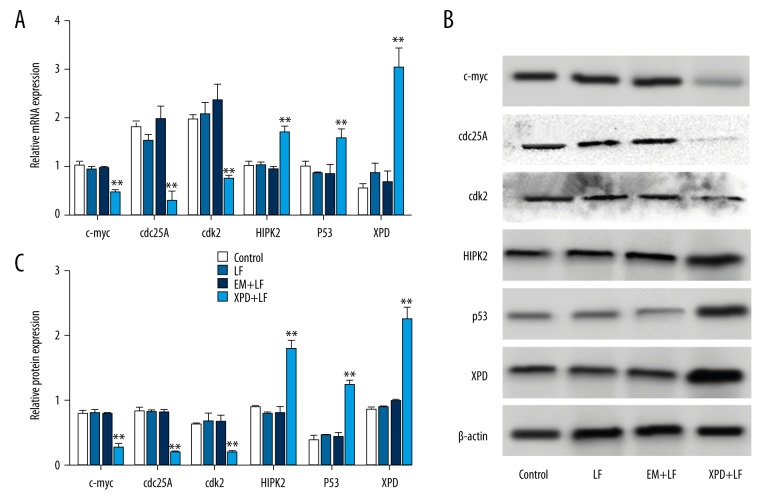
XPD regulated relates genes expression for cell proliferation and cell apoptosis
To determine how XPD modulates cell proliferation, cycle, and apoptosis in A431 cells, we detected the effect of XPD overexpression on cell proliferation-, cell cycle-, and cell apoptosis-related genes expression. In Figure 4A, no significant differences were observed on the mRNA expression of c-myc, cdc25A, cdk2, homeodomain-interacting protein kinase 2 (HIPK2), p53, and XPD among Control, LF, and EM+LF groups (P>0.05). However, the overexpression of XPD suppressed the mRNA levels of c-myc, cdc25A, and cdk2, but promoted the expression of HIPK2, p53, and XPD. A similar trend in the protein levels is shown in Figure 4B and 4C. Taken together, XPD monitored the expression of cell proliferation-related, cell cycle-related, and cell apoptosis-related genes.
Figure 4.
XPD monitored the related genes expression. A431 cells were transfected with the same amount of medium (Control), Lipofectamine (LF), pEGFP-N2 (EM) + Lipofectamine (EM+LF), or pEGFP-N2-XPD + Lipofectamine (XPD+LF). (A) The overexpression of XPD affected the mRNA levels of related genes for cell proliferation and cell apoptosis. After cell transfection, cells were collected and total RNA was isolated for qRT-PCR. (B) The effect of XPD overexpression on related proteins expression was analyzed by Western blot. (C) The target bands were normalized to β-actin using Quantity One software. * P<0.05, ** P<0.01.
Discussion
cSCC is the second most widespread metastatic skin cancer, with increasing incidence, which has a more invasive growth pattern and higher potential to metastasize [16]. Although great research efforts have been made, the outcome of cSCC remains poor because of a lack of knowledge regarding molecular markers. Some evidence indicates the importance of DNA repair pathways in cancer genetic processes, such as in prostate cancer [17]. Indeed, deficient DNA repair may lead to deregulation of cell growth, imbalance of cell cycle control, and development of many diseases, including cancers [18]. The highly conserved NER pathway is responsible for the excision of a large variety of bulky DNA lesions [19]. XPD, encoded by the ERCC2 gene, is an ATP-dependent helicase enzyme involved in the NER pathway [20]. In our study, we found that the overexpression of XPD in A431 cells plays an important role in the progression of cSCC through the cell cycle, cell proliferation, and cell apoptosis pathways. XPD may potentially act as an anti-oncogene in cSCC and have clinical value as a prognostic marker for SCC.
In our subjects, we found that the overexpression of XPD was involved in cell proliferation, cell cycle, and cell apoptosis. C-myc is the master transcription factor for cell proliferation and is involved in numerous hematological and solid cancers [21]. In hepatoma cells, XPD functions in cell proliferation and apoptosis by suppressing the expression of c-myc and cdk2 [22]. The mutation of XPD affected proper regulation of c-myc expression and is involved in the development of malignancy [23]. Combined with our results, we conclude that XPD participates in cell proliferation via monitoring the expression of c-myc. In normal cells, progression through G1, S, and G2 phases of the cell cycle is dependent on temporal activation of cyclin-dependent kinases CDK1/2. The subsequent dephosphorylation and activation of CDK1/2 is mediated by 1 of 3 CDC25 phosphatases (A, B, or C) [24]. CDC25A regulation is complex, with input from multiple other kinases, resulting in either degradation or sequestration, depending on cell cycle phase [25]. CDK2 [26] and CDC25A [27] are important regulators of the G1/S phase transition. CDK2 is usually considered essential for progression through S phase [28]. Some reports indicated that overexpression of XPD leads to cell cycle arrest at G1 phase via suppression of CDK2 and CDC25A, which contributes to cell growth inhibition [29]. In our study, we observed that the overexpression of XPD increased the percentages of A431 cells at G1 phase and suppressed the mRNA and protein levels of CDK2 and CDC25A, which is in agreement with previous studies. In A431 cells, the overexpression of XPD induced cell cycle arrest at G1 phase by the blockage of CDK2 and CDC25A.
HIPK2 is a member of the nuclear Ser/Thr kinase family, originally identified as a DNA damage-responsive cell fate regulator, which is negatively regulated by oncogenic signaling [30]. HIPK2 phosphorylates and activates the apoptotic program through interaction with diverse downstream targets [31]. HIPK2 activates tumor suppressor p53-dependent transcription and apoptosis by phosphorylating p53 at Ser46 [32]. P53 is stabilized, binds to specific target gene promoters, and regulates gene sets for cell fate towards apoptosis or DNA repairs [33]. In our study, the high expression of XPD significantly improved cell apoptosis and enhanced the expression of HIPK2 and p53. In hepatoma cells, XPD functions in cell apoptosis by inducing the expression of p53 [22]. A previous study reported that XPD interacts with p53 to regulate cell apoptosis [34] and the p53/HIPK2 connection by showing the role of HIPK2 in repressing activity in severe DNA damage response [35]. Our data were in agreement with a previous study reporting that XPD was involved in cell apoptosis by regulating the expression of HIPK2 and p53.
Conclusions
Taken together, XPD repressed cell proliferation by arresting cell cycle in G1 phase and induced cell apoptosis by suppressing the levels of c-myc, cdc25A, and cdk2 and enhancing the expression of HIPK2 and p53. These data provide new evidence that XPD serves as an anti-oncogene in cSCC. However, there also exist some limitations in our study. First, our study was mainly focused on the role of XPD in A431 cells in vitro. Further examinations need to be performed on the role of XPD in vivo, which would more comprehensively explain the function of XPD in cSSC. Second, in our study, we actually found that XPD affected the expression of c-myc, cdc25A, cdk2, HIPK2, and p53; however, the molecular mechanism of XPD regulating expression of these genes was unclear. Thus, more efforts need to be made for molecular basis exploration on gene regulation.
Abbreviations
- cSCC
cutaneous squamous cell carcinoma
- HIPK2
homeodomain-interacting protein kinase 2
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphe-nyltetrazolium bromide
- NER
nucleotide excision repair
- PBS
phosphate-buffered saline
- PI
propidium iodide
- XPD
xeroderma pigmentosum group D
Footnotes
Conflicts of interest
None.
Source of support: The study was supported by the Jiangxi Province Natural Science Fund Project (20142BAB205051)
References
- 1.Youssef KK, Van Keymeulen A, Lapouge G, et al. Identification of the cell lineage at the origin of basal cell carcinoma. Nat Cell Biol. 2010;12:299–305. doi: 10.1038/ncb2031. [DOI] [PubMed] [Google Scholar]
- 2.Zhang SH, Li ZY, Liu ZJ, et al. MicroRNA15b regulates apoptosis of cutaneous squamous cell carcinoma SCL-1 cell line: A mechanism study. Eur Rev Med Pharmacol Sci. 2017;21:227–33. [PubMed] [Google Scholar]
- 3.Li X, Huang K, Yu J. Inhibition of microRNA-21 upregulates the expression of programmed cell death 4 and phosphatase tensin homologue in the A431 squamous cell carcinoma cell line. Oncol Lett. 2014;8:203–7. doi: 10.3892/ol.2014.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertero T, Bourget-Ponzio I, Puissant A, et al. Tumor suppressor function of miR-483-3p on squamous cell carcinomas due to its pro-apoptotic properties. Cell Cycle. 2013;12:2183–93. doi: 10.4161/cc.25330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravegnini G, Nannini M, Simeon V, et al. Polymorphisms in DNA repair genes in gastrointestinal stromal tumours: Susceptibility and correlation with tumour characteristics and clinical outcome. Tumour Biol. 2016;37:13413–23. doi: 10.1007/s13277-016-5276-7. [DOI] [PubMed] [Google Scholar]
- 6.Li RZ, Sun J. Association between XPD gene polymorphisms and esophageal squamous cell carcinoma. Mol Med Rep. 2013;7:674–78. doi: 10.3892/mmr.2012.1215. [DOI] [PubMed] [Google Scholar]
- 7.Feller L, Khammissa RA, Kramer B, et al. Basal cell carcinoma, squamous cell carcinoma and melanoma of the head and face. Head Face Med. 2016;12:11. doi: 10.1186/s13005-016-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neel VA, Todorova K, Wang J, et al. Sustained Akt activity is required to maintain cell viability in seborrheic keratosis, a benign epithelial tumor. J Invest Dermatol. 2016;136:696–705. doi: 10.1016/j.jid.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 9.Egly JM. The 14th Datta Lecture. TFIIH: From transcription to clinic. FEBS Lett. 2001;498:124–28. doi: 10.1016/s0014-5793(01)02458-9. [DOI] [PubMed] [Google Scholar]
- 10.Lehmann AR. The xeroderma pigmentosum group D (XPD) gene: One gene, two functions, three diseases. Genes Dev. 2001;15:15–23. doi: 10.1101/gad.859501. [DOI] [PubMed] [Google Scholar]
- 11.Wang XC, Wang F, Quan QQ. Roles of XRCC1/XPD/ERCC1 polymorphisms in predicting prognosis of hepatocellular carcinoma in patients receiving transcatheter arterial chemoembolization. Genet Test Mol Biomarkers. 2016;20:176–84. doi: 10.1089/gtmb.2015.0267. [DOI] [PubMed] [Google Scholar]
- 12.Farnebo L, Stjernstrom A, Fredrikson M, et al. DNA repair genes XPC, XPD, XRCC1, and XRCC3 are associated with risk and survival of squamous cell carcinoma of the head and neck. DNA Repair (Amst) 2015;31:64–72. doi: 10.1016/j.dnarep.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 13.de Vries-van der Weij J, Toet K, Zadelaar S, et al. Anti-inflammatory salicylate beneficially modulates pre-existing atherosclerosis through quenching of NF-kappaB activity and lowering of cholesterol. Atherosclerosis. 2010;213:241–46. doi: 10.1016/j.atherosclerosis.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Shkarupa VM, Mishcheniuk OY, Henyk-Berezovska SO, et al. Polymorphism of DNA repair gene XPD Lys751Gln and chromosome aberrations in lymphocytes of thyroid cancer patients exposed to ionizing radiation due to the Chornobyl accident. Exp Oncol. 2016;38:257–60. [PubMed] [Google Scholar]
- 15.Wang M, Li Q, Gu C, et al. Polymorphisms in nucleotide excision repair genes and risk of primary prostate cancer in Chinese Han populations. Oncotarget. 2017;8:24362–71. doi: 10.18632/oncotarget.13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riihila P, Nissinen L, Farshchian M, et al. Complement component C3 and complement factor B promote growth of cutaneous squamous cell carcinoma. Am J Pathol. 2017;187:1186–97. doi: 10.1016/j.ajpath.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Banks P, Xu W, Murphy D, et al. Relevance of DNA damage repair in the management of prostate cancer. Curr Probl Cancer. 2017 doi: 10.1016/j.currproblcancer.2017.06.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Szkandera J, Absenger G, Liegl-Atzwanger B, et al. Common gene variants in RAD51, XRCC2 and XPD are not associated with clinical outcome in soft-tissue sarcoma patients. Cancer Epidemiol. 2013;37:1003–9. doi: 10.1016/j.canep.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–18. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altinkilic EM, Isbir S, Gormus U, et al. RRM1, RRM2 and ERCC2 gene polymorphisms in coronary artery disease. In Vivo. 2016;30:611–15. [PubMed] [Google Scholar]
- 21.Wierstra I, Alves J. The c-myc promoter: Still MysterY and challenge. Adv Cancer Res. 2008;99:113–333. doi: 10.1016/S0065-230X(07)99004-1. [DOI] [PubMed] [Google Scholar]
- 22.Wang HY, Xiong GF, Zhang JX, et al. The role of XPD in cell apoptosis and viability and its relationship with p53 and cdk2 in hepatoma cells. Med Oncol. 2012;29:161–67. doi: 10.1007/s12032-011-9818-y. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Akoulitchev S, Weber A, et al. Defective interplay of activators and repressors with TFIH in xeroderma pigmentosum. Cell. 2001;104:353–63. doi: 10.1016/s0092-8674(01)00223-9. [DOI] [PubMed] [Google Scholar]
- 24.Boutros R, Lobjois V, Ducommun B. CDC25 phosphatases in cancer cells: Key players? Good targets? Nat Rev Cancer. 2007;7:495–507. doi: 10.1038/nrc2169. [DOI] [PubMed] [Google Scholar]
- 25.Honaker Y, Piwnica-Worms H. Casein kinase 1 functions as both penultimate and ultimate kinase in regulating Cdc25A destruction. Oncogene. 2010;29:3324–34. doi: 10.1038/onc.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawana H, Tamaru J, Tanaka T, et al. Role of p27Kip1 and cyclin-dependent kinase 2 in the proliferation of non-small cell lung cancer. Am J Pathol. 1998;153:505–13. doi: 10.1016/s0002-9440(10)65593-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blomberg I, Hoffmann I. Ectopic expression of Cdc25A accelerates the G(1)/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases. Mol Cell Biol. 1999;19:6183–94. doi: 10.1128/mcb.19.9.6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones RM, Petermann E. Replication fork dynamics and the DNA damage response. Biochem J. 2012;443:13–26. doi: 10.1042/BJ20112100. [DOI] [PubMed] [Google Scholar]
- 29.Zou J, Zhang W, Li XL. Effects of SOST gene silencing on proliferation, apoptosis, invasion, and migration of human osteosarcoma cells through the Wnt/beta-catenin signaling pathway. Calcif Tissue Int. 2017;100(6):551–64. doi: 10.1007/s00223-016-0231-6. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann TG, Glas C, Bitomsky N. HIPK2: A tumour suppressor that controls DNA damage-induced cell fate and cytokinesis. Bioessays. 2013;35:55–64. doi: 10.1002/bies.201200060. [DOI] [PubMed] [Google Scholar]
- 31.Kuwano Y, Nishida K, Akaike Y, et al. Homeodomain-interacting protein kinase-2: A critical regulator of the DNA damage response and the epigenome. Int J Mol Sci. 2016;17(10) doi: 10.3390/ijms17101638. pii: E1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nardinocchi L, Puca R, D’Orazi G. HIF-1alpha antagonizes p53-mediated apoptosis by triggering HIPK2 degradation. Aging (Albany NY) 2011;3:33–43. doi: 10.18632/aging.100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vousden KH, Prives C. Blinded by the light: The growing complexity of p53. Cell. 2009;137:413–31. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 34.Williams AB, Schumacher B. p53 in the DNA-damage-repair process. Cold Spring Harb Perspect Med. 2016;6(5) doi: 10.1101/cshperspect.a026070. pii: a026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mancini F, Pieroni L, Monteleone V, et al. MDM4/HIPK2/p53 cytoplasmic assembly uncovers coordinated repression of molecules with anti-apoptotic activity during early DNA damage response. Oncogene. 2016;35:228–40. doi: 10.1038/onc.2015.76. [DOI] [PMC free article] [PubMed] [Google Scholar]