Abstract
Introduction
It has been shown that CD47 is an important diagnostic and prognostic marker in many cancer types. However, the relationship between CD47 and bladder tumor stage has not been shown in previous studies. To the best of our knowledge, this is the first study investigating the association of CD47 with stages of bladder cancer.
Material and methods
Surgical specimens of 175 patients were included in the study. The CD47 staining assessment was performed in the following categories; none, focal, moderate and diffuse. The statistics of the study were tested using t-test and analysis of variance.
Results
We demonstrated much less CD47 staining extent in Ta tumor pathology compared to T1 and T1+T2+T3+T4 tumor pathology (p = 0.034 and p = 0.016, respectively). We also showed that the average value of CD47 staining extent with CIS+ was significantly higher compared to CIS– among NMIBC (p = 0.0248). However, no significant differences in CD47 staining pattern were observed in the following study groups: high vs. low-grade tumors in non-muscle invasive bladder cancer (NMIBC); MIBC (T2–T4) vs. NMIBC; lymph node involvement (N1–N3) vs. non-lymph node involvement (N0) in MIBC (T2–T4).
Conclusions
Our study demonstrated that CD47 might have a critical role in the progression of Ta to T1 stage. Furthermore, we showed that CD47 is highly expressed in CIS+ NMIBC compared to CIS- NMIBC. Thus, differentiating stages with the help of this new potential marker may help clinicians treat bladder tumors better. Future studies to determine the role of CD47 on pathophysiology, diagnosis and prognosis of bladder tumor are warranted.
Keywords: CD47, bladder tumor, immunohistochemical stain
INTRODUCTION
Bladder cancer is the seventh most common malignancy in males and seventeenth in females worldwide, whereas, in developed countries, it is the fourth and ninth, respectively [1, 2]. This high incidence and the tendency to recuring result has a major impact on health care and increases treatment costs significantly [3]. Cluster of Differentiation 47 (CD47) is a 50 kDa transmembrane glycoprotein, which is coded by the CD47 gene in humans, formerly known as Integrin Associated Protein (IAP), it is extracellular, has a N-terminal IgV area, five transmembrane areas and a intracellular area consisting of a C-terminal [4, 5]. It serves as a receptor for Thrombospondin-1(TSP-1) and counter-receptor for Signal Regulatory Protein-α (SIRP-α). These two interactions of CD47 take part in many processes such as hemostasis, cardiovascular physiology, ischemic damage, inflammation, radiation injury and cancer.
In its relationship with SIRP-α, CD47 acts as a ligand. SIRP-α is an inhibitor transmembrane receptor and takes part in the regulation of the bi-directional signaling pathway, which allows intercellular communication. In some studies, it has been found that CD47 served as a ligand for this receptor and sent an ‘anti-phagocytic message’ to phagocytic cells and its expression increased in tumour cells [6, 7]. It has been shown that increased CD47 mRNA expression levels in some solid tumors correlated with a decreased probability of patient survival [8]. Also, it has been seen in experimental studies that the effectiveness of treatments such as chemotherapy and radiotherapy increased in CD47+ tumors treated with CD47 antibody [9].
In this retrospective study, in which the immunohistochemical staining extent of CD47 was compared to the tumor stage and its histopathological features in patients who underwent surgery due to bladder tumor, the aim was to find whether CD47 could be used as an indicator in either one of diagnosis, follow-up, or treatment processes. We believe that our study will contribute to the literature; to our knowledge, there have been no studies on the relationship between bladder tumor stage and CD47 until today.
MATERIAL AND METHODS
In this study, the pathology specimens of 175 patients were retrospectively examined. One hundred and eighteen (118) of these patients underwent transurethral resection of bladder tumor (TUR-B) between December 2011 and December 2013; 57 patients underwent radical cystectomy between December 2008 and December 2013 in the Urology Clinic of Türkiye Yüksek İhtisas Training and Research Hospital, Turkey. In this study, we investigated the relationship between immunohistochemical staining extent of CD47 signal regulation protein with tumor stage and histopathologic features. For this purpose, the pathology specimens were immunohistochemically stained with CD47. Clear information about the past treatment of the patients in this study could not be obtained. In this study, the 2009 World Health Organization (WHO) TNM classification was used for the determination of T stage and the 2004 WHO grading system was used for grading of tumor.
Immunohistochemical study
For the immunohistochemical study, 3-micron thick sections were taken from paraffin blocks using a rotary microtome and these were examined using Poly-L-lysine coated slides. ‘The Avidin-biotin method’ was used as the immunohistochemical staining system. All pathology preparations were incubated for 30 minutes with CD47 antibody, stained manually and counterstained with hematoxylin. CD47 immunohistochemical staining was done using mouse monoclonal [B6H12.2] antibody (Abcam CD47 antibody ab3283, Cambridge, UK).
For each case, CD47 staining extent was assessed using an Olympus CX31 microscope and slides prepared with immunohistochemical method and graded according to method mentioned below. One pathologist evaluated the pathology slides in terms of staining extent with CD47. Brown staining in the cytoplasm and membrane of tumor cells was used as criteria for CD47 antigen positivity.
The staining assessment was performed arbitrarily in four categories;
0: No staining in tumor cells (None)
1+: Approximately less than 1/3 staining in total tumor area (Focal)
2+: Approximately between 1/3–2/3 staining in total tumor area (Moderate)
3+: Approximately more than 2/3 staining in total tumor area (Diffuse)
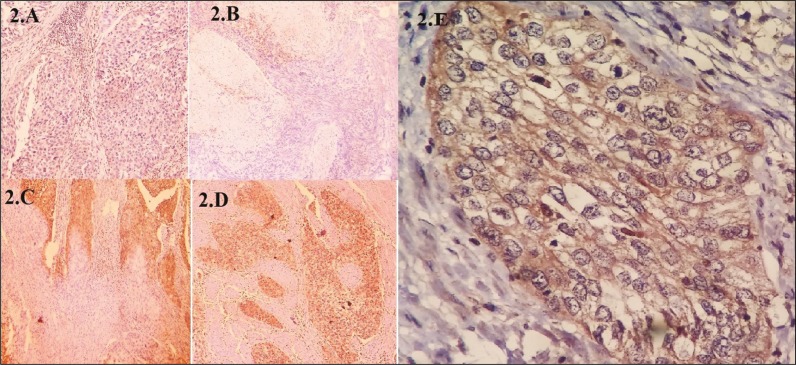
In this evaluation, cells with staining were accepted as negative (category 0); and cells with a staining extent of 1 to 3 were considered positive (Figure 1).
Figure 1.
Staining of tumor cells with CD47. (2A) No staining in tumor cells (none), (2B) Approximately less than 1/3 staining in total tumor area (focal), (2C) Approximately between 1/3–2/3 staining in total tumor area (moderate), (2D) Approximately more than 2/3 staining in total tumor area (diffuse), (2E) Membranous staining of tumor cells.
Group formation
As shown in Table 1, the patients were grouped according to histopathological diagnosis of tumor.
Table 1.
Groups according to the pathology
| Group | Pathology |
|---|---|
| 1 | Ta low-grade TCC |
| 2 | Ta high-grade TCC |
| 3 | T₁low-grade TCC |
| 4 | T₁high-grade TCC |
| 5 | T₂ TCC |
| 6 | T3 TCC |
| 7 | T4 TCC |
| 8 | Inflammation |
| 9 | Adenocarcinoma |
| 10 | Giant cell sarcoma |
| 11 | Neuroendocrine tumor |
Statistics
In this study, first of all, the age, gender and pathology data of the patients were determined as descriptive statistics. Then, the hypothesis that there was no statistical difference between CD47 staining extents of two groups formed according to histopathological features of bladder tumor was tested using two sample t-test and analysis of variance. In cases where statistical hypothesis was rejected, the hypothesis of the CD47 staining relationship direction of groups was tested with one sample t-test. The results were summarized in related tables. SPSS 22.0 was used for statistical analysis and the statistical significance was accepted as p <0.05.
RESULTS
A total of 175 (100%) patients were included in the study. One hundred sixty-one (161) of these patients were male (92%) and 14 were female (8%). The average age of the patients included in the study was 66.2; the median age was found to be 67. It was found that 14 patients had carcinoma in situ (CIS)+ in their pathologies (8%) and 161 patients had CIS- in their pathologies (92%). According to TNM staging system, there were 156 N0 patients (89.1%), 12 N1 patients (6.8%) and 1 N3 patient (0.57%). Twenty-nine (29) of the patients included in our study were diagnosed with TCC Ta low-grade (16.57%), 28 (16%) with TCC Ta high-grade, 2 (1.14%) with TCC T1 low-grade, 32 (18.29%) with TCC T1 high-grade, 30 (17.14%) with TCC T2, 25 (14.29%) with TCC T3, 10 (5.71%) with TCC T4, 13 (7.43%) inflammation, 2 (1.14%) with adenocarcinoma, 1 (0.57%) giant cell sarcoma, 3 (1.71%) with neuroendocrine tumors (Table 2).
Table 2.
Clinicopathological parameters of patients
| Operation | (n) | (%) |
|---|---|---|
| TUR-B | 118 | 67 |
| Cystectomy | 57 | 33 |
| Sex | ||
| Male Female |
161 14 |
92 8 |
| Age | ||
| Mean Median |
66.2 67 |
|
| Carsinoma in situ | ||
| Carsinoma in situ + Carsinoma in situ - |
14 161 |
8 92 |
| Lymph node | ||
| N0
N1 N2 N3 |
156 12 6 1 |
89.1 6.8 3.4 0.57 |
| Pathology | ||
| Ta low-grade TCC Ta high-grade TCC T₁ low-grade TCC T₁high-grade TCC T₂ TCC T3 TCC T4 TCC Inflammation Adenocarcinoma Giant cell sarcoma neuroendocrine tumor |
29 28 2 32 30 25 10 13 2 1 3 |
16.57 16 1.14 18.29 17.14 14.29 5.71 7.43 1.14 0.57 1.71 |
| Total | 175 | 100 |
There was a statistically significant difference between Ta (Group 1+2) tumor pathology (1.52 +/0.88) and T1 (Group 3+4) tumor pathology (1.91 ±0.71), in terms of average value of CD47 staining extent (p = 0.034). The direction of this difference was found that T1 tumors were stained significantly more compared to Ta tumors (p = 0.00001) (Table 3).
Table 3.
The relationship between clinicopathological para-meters of patients with CD47 staining extent
| n | Mean CD47 Value | P | |
|---|---|---|---|
| Ta(Group 1+2) T1 (Group 3+4) |
57 34 |
(1.52 ±0.88) (1.91 ±0.71) |
p = 0.034 |
| Ta (Group 1+2) T1+T2+T3+T4 (Group 3+4+5+6+7) |
57 76 |
(1.52 ±0.88) (1.86 ±0.73) |
P = 0.016 |
| Ta low-grade+T1 low-grade (Group 1+3) Ta high-grade+T1 high-grade (Group 2+4) |
31 60 |
(1.67±0.79) (1.66 ±0.87) |
p = 0.9545 |
| NMIBC (Group 1+2+3+4) MIBC (Group 5+6+7) |
91 65 |
(1.67 ±0.84) (1.76 ±0.87) |
p = 0.4794 |
| TCC (Group 1+2+3+4+5+6+7) İnflammation (Group 8) |
156 13 |
(1.71 ± 0.85) (0.23 ±0.59) |
p <0.001 |
| Carcinoma in situ+ in NMIBC Carcinoma in situ- in NMIBC |
13 78 |
(2.15 ±0.55) (1.58 ±0.85) |
p = 0.0248 |
| N0 in MIBC N1+N2+N3 in MIBC |
47 15 |
(1.74 ±0.84) (1.83 ±0.98) |
p = 0.71 |
| TUR-B Cystectomy |
118 57 |
(1.55 ±0.93) (1.71 ±0.92) |
p = 0.26 |
There was a statistically significant difference between Ta (Group 1+2) tumor pathology (1.52 +/0.88) and T1+T2+T3+T4 (Group 3+4+5+6+7) tumor pathology (1.86 ±0.73), in terms of average value of CD47 staining extent. T1+T2+T3+T4 tumors were stained significantly more compared to Ta tumors (p = 0.016) (Table 3).
There was no statistically significant difference between the average value of CD47 staining extent with Ta low-grade+T1 low-grade (Group 1+3) tumor pathology (1.67 ±0,79) and the average value of CD47 staining extent with Ta high-grade+T1 high-grade (Group 2+4) tumor pathology (1.66 ±0.87) (p = 0.9545). In short, there was no statistically significant difference between high-grade and low-grade tumors in terms of CD47 staining extent.
There was no statistically significant difference between the average value of CD47 staining extent with non-muscle invasive bladder cancer (NMIBC) (Ta+T1) pathology (1.67 ±0.84) and the average value of CD47 staining extent with muscle invasive bladder cancer (MIBC) (T2-T4) pathology (1.76 ±0.87) (p= 0.4794).
There was a statistically significant difference between transitional cell cancer (TCC) tumor pathology (1.71 ±0.85) and inflammation (Group 8) pathology (0.23 +/-0.59) in terms of average value of CD47 staining extent (p <0.001). Tumor tissues were stained significantly more compared to inflammation (p <0.0001).
There was a statistically significant difference between the average value of CD47 staining extent with CIS+ (2.15 ±0.55) and the average value of CD47 staining extent CIS- (1.58 ±0.85) in NMIBC (Ta+T1) (p = 0.0248). CIS+ were stained significantly more compared to CIS- tumors in NMIBC (p = 0.0002).
Among patients with MIBC (T2–T4), there was no statistically significant difference between the average value of CD47 staining extent of the group consisting of 47 patients belonging to N0 category according to TNM classification (1.74 ±0.84) and the average value of CD47 staining of the group consisting of 15 patients belonging to (N1–N3) category (1.80 ±0.98) (p = 0.71).
There was no statistically significant difference between patients who underwent TUR-B (1.55 ±0.93) and patients who underwent cystectomy (1.71 ±0.92), in terms of the average value of CD47 staining extent (p = 0.26). We found a correlation coefficient of 0.13 between CD47 staining extent and tumor stage and concluded that there was no significant relationship between them.
DISCUSSION
There are intensive studies conducted on various markers in order to determine the diagnosis and prognosis of bladder tumor. There have been studies on markers associated with oncogenes, cell cycle regulators, and apoptosis. Although some are promising, no markers have been found to be suitable for routine clinical use [10].
The functions of CD47 cell membrane receptors are neutrophil traffic, T cell co-simulation and neuronal regeneration [11]. CD47 interacts with SIRP-α, which is the specific receptor on macrophages, and down-regulates the phagocytic functions of macrophages [12].
There is an increase in the expression of CD47 in many malignancies seen in humans. There are studies that indicate that high CD47 expression is a diagnostic marker and an unfavorable prognostic factor in patients with leukemia and ovarian cancer [6, 13, 14]. In the most extensive and multicenter study conducted by Willingham et al., it has been found that CD47 expression increased in solid cancers such as bladder, prostate, lung, kidney, stomach cancers, hepatocellular carcinoma, glioblastoma multiforme, and sarcoma compared to normal tissue and that it was an unfavorable prognostic factor [8].
It has been demonstrated that cancer cells circulated in peripheral blood in many cancer types (Circulating Tumor Cell, CTC) [15]. A sub-population that has the ability to regenerate itself was defined among these CTCs. These cells, which are called metastasis initiating cells (MIC) and cancer stem cells (CSC), have been identified in breast, prostate, lung and colorectal cancers [16, 17, 18]. It has been shown that CSCs are CD44+, CD47+, CD123+, EpCAM+, CD133+, IGF-1 receptor positive. It has been proven that CSCs play an important role in resistance to the modern cancer treatment and it has been predicted that targeting the receptors mentioned above shall increase the rate of success in cancer treatment [19, 20, 21]. It has been shown that in hepatocellular carcinoma patients, CD47 expression of tumor initiating cells detected in patients who were resistant to chemotherapy were upregulated compared to chemosensitive patients [22].
In 2009, Chan et al. have defined the tumor initiating cell in bladder cancer, reporting CD47 as highly expressed [23]. They have also proven that these cells were associated with poor prognosis.
Zhao et al. opposed the argument put forward by Wilingham et al. that tumor cells avoided phagocytosis due to CD47's interaction with SIRP-α and claimed that CD47 antibodies bound CD47+ tumor cells and tumor cells were killed by neutrophils and Natural Killer cells with antibody dependent cell death method [7, 24]. Whatever the causative mechanism is, CD47 is indeed expressed in numerous tumors and can be targeted for treatment.
There are no studies in the literature investigating the relationship between bladder tumor stage and CD47 staining level. We have found a statistically significant difference in terms of CD47 staining levels between Ta stage bladder tumor and T1 stage bladder tumor (p = 0.034). Also there was a statistically significant difference in terms of CD47 staining levels between Ta stage bladder tumor and T1+T2+T3+T4 bladder tumor (p = 0.016). The fact that this difference could not be observed between MIBC and NMIBC brings up the question whether or not CD47 is critical in the progression from Ta stage to T1. The abundance of CD47 antigens in the T1 stage will lead to the invasion of tumor cells and will likely increase risks of unfavorable progression. The question that needs to be asked is whether or not CD47 can aid urologists in the management of Ta disease, functioning as a marker for progression to T1, since prognosis and treatment options are significantly different in these scenarios.
In this study, we have shown that CD47 cannot be used to predict tumor grade and that there was not a significant difference in terms of CD47 staining levels between low-grade and high-grade NMIBC (p = 0.9545).
Investigating whether CD47 could predict CIS+ in NMIBC, we found that CD47 staining levels of CIS+ group and CIS- group were significantly different in NMIBC (p = 0.0248). Thus we propose the idea that CD47 could be a new marker in CIS+ patients. These observations should be interpreted with caution, since much of our cohort was submitted to TUR-B, where the mucosa adjacent to the tumor is not always accessible, and therefore, carcinoma in situ incidence might be underestimated. It is recommended by the European Association of Urology(EAU) Guidelines to combine conventional cystoscopy with cytology since the risk of overlooking is high in carcinoma in situ. If CD47+ can be shown immunohistochemically in patients whose specimen pathology result is Ta or T1, it will be possible to achieve a more successful photodynamic cystoscopy or random biopsy to show CIS+. Considering that CIS+ tumors can turn into invasive bladder cancer with a probability of 50–55% in 5 years, if they do not receive any treatment, the importance of this diagnosis can be understood once again.
We could not find a statistically significant difference between CD47 staining levels of NMIBC (Ta+T1) and MIBC (T2–T4). Therefore, CD47 is insufficient to demonstrate muscle invasion in bladder tumors.
There was no difference in MIBC (T2–T4) CD47 staining levels between the lymph node involvement group and the non-lymph node involvement group (p = 0.71). Therefore, CD47 was insufficient in terms of predicting lymph node involvement, which is considered to be the first step of systemic spread in invasive tumors.
A comparison between CD47 staining levels of the patient group with inflammation pathology and the group with TCC pathology has shown that there was a statistically significant difference in favor of the TCC group (P <0.001). However, the lack of normal bladder epithelium in the control group and the fact that number of patients in the group with inflammation pathology was relatively low compared to the group with tumor (13 vs. 156), make this finding debatable. Therefore, the idea that CD47 expression level is higher in tumor tissue than non-tumor tissue remains a prediction.
It is obvious that results regarding CD47 are promising, however there is a need for prospective studies on a larger scale.
CONCLUSIONS
We have validated the overexpression of CD47 in bladder cancer and have shown important differences between its expression in Ta and T1 lesions. This finding is exciting since markers for progression from tumour to invasive disease are lacking in clinical practice. Although our findings remain to be reproduced by studies with a prospective design on a larger scale, we believe CD47 is also promising as a marker for therapy, since this protein can be targeted and because bladder cancer chemosensitivity has currently been linked to tumor and host immune responses.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Burger M, Catto W, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, et al. Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 10. Lyon, France: International Agency for Research on Cancer; 2010. GLOBOCAN 2008. [Google Scholar]
- 3.Sievert KD, Amend B, Nagele U, et al. Economic aspects of bladder cancer: what are the benefits and costs? World J Urol. 2009;27:295–300. doi: 10.1007/s00345-009-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller YE, Daniels GL, Jones C, Palmer DK. Identification of a cell-surface antigen produced by a gene on human chromosome 3 (cen-q22) and not expressed by Rhnull cells. Am J Hum Genet. 1987;41:1061. [PMC free article] [PubMed] [Google Scholar]
- 5.Isenberg JS, Frazier WA, Roberts DD. Thrombospondin-1: a physiological regulator of nitric oxide signaling. Cell Mol Life Sci. 2008;65:728–742. doi: 10.1007/s00018-007-7488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majeti R, Chao MP, Alizadeh AA, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kershaw MH, Smyth MJ. Immunology. Making macrophages eat cancer. Science. 2013;341:41–42. doi: 10.1126/science.1241716. [DOI] [PubMed] [Google Scholar]
- 8.Willingham SB, Volkmer J P, Gentles AJ, Sahoo D, Dalerba P, Mitra SS. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dotsika G, Konowalchuk T, Major PP, et al. Cellular heterogeneity in normal and neoplastic human urothelium: a study using murine monoclonal antibodies. Br J Cancer. 1987;56:439. doi: 10.1038/bjc.1987.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xylinas E, Kluth LA, Lotan Y, et al. Blood-and tissue-based biomarkers for prediction of outcomes in urothelial carcinoma of the bladder. In: Urologic Oncology: Seminars and Original Investigations. Elsevier. 2014;32(3):230–242. doi: 10.1016/j.urolonc.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47–SIRPα signalling pathway. Trends Cell Biol. 2009;19:72–80. doi: 10.1016/j.tcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 12.van den Berg TK, van der Schoot CE. Innate immune'self'recognition: a role for CD47–SIRPα interactions in hematopoietic stem cell transplantation. Trends Immunol. 2008;29:203–206. doi: 10.1016/j.it.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Van Niekerk CC, Ramaekers FC, Hanselaar AG, Aldeweireldt J, Poels LG. Changes in expression of differentiation markers between normal ovarian cells and derived tumors. Am J Pathol. 1993;142:157. [PMC free article] [PubMed] [Google Scholar]
- 14.Buist MR, Kenemans P, Molthoff CF, et al. Tumor uptake of intravenously administered radiolabeled antibodies in ovarian carcinoma patients in relation to antigen expression and other tumor characteristics. Int J Cancer. 1995;64:92–98. doi: 10.1002/ijc.2910640204. [DOI] [PubMed] [Google Scholar]
- 15.Naoe M, Ogawa Y, Morita J, et al. Detection of circulating urothelial cancer cells in the blood using the Cell Search System. Cancer. 2007;109:1439–1445. doi: 10.1002/cncr.22543. [DOI] [PubMed] [Google Scholar]
- 16.Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non–smallcell lung cancer. J Clin Oncol. 2011;29:1556–1563. doi: 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- 17.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castrationresistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 18.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. NEngl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen LV, Vanner R, Dirks P, et al. Cancer stem cells:an evolving. Nat Rev Cancer. 2012;12:133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 20.Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21:283–296. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valent P, Bonnet D, De Maria, et al. Cancer stem cell definitions and terminology: the devil is in the details. Nat Rev Cancer. 2012;12:767–775. doi: 10.1038/nrc3368. [DOI] [PubMed] [Google Scholar]
- 22.Lee TK, Cheung VC, Lu P, et al. Blockade of CD47-mediated cathepsin S/protease-activated receptor signaling provides a therapeutic target for hepatocellular carcinoma. Hepatology. 2014;60:179–191. doi: 10.1002/hep.27070. [DOI] [PubMed] [Google Scholar]
- 23.Chan KS, Espinosa I, Chao M, et al. Identification, molecular characterization,clinical prognosis, and therapeutic targeting of human bladder tumor initiating cells. Proc Natl Acad Sci USA. 2009;106:14016–14021. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chao MP, Majeti R, Weissman I. Response: mechanisms of targeting CD47-SIRPα in hematologic malignancies. Blood. 2012;119:4334–4335. [Google Scholar]