Abstract
Introduction
The aim of our study was to determine the contemporary practice in the utilization of life expectancy (LE) calculations among urological clinicians.
Material and methods
Members of the Irish Society of Urology (ISU) and the British Association of Urological Surgeons (BAUS) completed a questionnaire on LE utilization in urological practice.
Results
The survey was delivered to 1251 clinicians and the response rate was 17% (n = 208/1251). The majority (61%, n = 127) of urologists were aware of methods available for estimated LE calculation.
Seventy-one percent (n = 148) had never utilized LE analysis in clinical practice and 81% (n = 170) routinely used 'eyeballing' (empiric prediction) for estimating LE. Life expectancy tables were utilized infrequently (12%, n = 25) in making the decision for treatment in the setting of multi-disciplinary meetings.
Conclusions
LE is poorly integrated into treatment decision-making; not only for the management of urological patients but also in the multidisciplinary setting. Further education and awareness regarding the importance of LE is vital.
Keywords: cancer, intuition, survey, multidisciplinary, life-expectancy tables
INTRODUCTION
Life expectancy (LE) is defined as the number of years one is expected to live as determined by statistics [1]. The LE of our population has improved in the 20th century with an estimated rise in the population of those ≥65 years of age from 524 million in 2010 to approximately 1.5 billion in 2050 [2]. The global number of centenarians is projected to increase 10-fold between 2010 and 2050. This poses a challenge on national infrastructures and healthcare systems in particular. Global ageing is likely to result in an acceleration in cancer incidence in the coming decades. Clinicians are often challenged by managing elderly patients with increased life expectancy, and decisions made based on chronological age are no longer sound in the face of this exponential increase in life expectancy.
Prostate cancer is the most common non-cutaneous male malignancy. Accurate LE estimation is especially relevant in the screening and treatment of prostate cancer because it is a relatively indolent malignancy that usually affects older men [3]. Men with a LE >10 years are more likely to have a survival benefit with treatment as they are likely to live long enough to achieve the delayed benefit of active treatment. The American Urological Association (AUA), European Association of Urology (EAU) and the National Comprehensive Cancer Network (NCCN) guidelines recommend that patients benefit from radical prostatectomy if their LE is more than 10 years and from radical radiotherapy with different LE depending on clinical factors [4]. LE estimation is a critical part of a urologist's practice considering the management of one of the most commonly encountered cancers in our practice is heavily influenced by it. We sought to determine whether urologists have integrated LE estimation and calculation into contemporary uro-oncology practice.
MATERIAL AND METHODS
Overview of study design
An anonymous online questionnaire (Surveymonkey©) was emailed to all consultant urologists and trainees who had a documented email address on the British Association of Urological Surgeones (BAUS) and Irish Society of Urology (ISU) registries between March 2016 and October 2016. Respondents were asked a series of questions relating to LE estimation in urological practice as demonstrated in Table 1. This was a yes/no survey with only two options for each question. The survey was anonymous, hence it was not possible to assess if the respondent was a trainee or a consultant. Two follow-up emails were sent after 2 weeks to encourage non-responders. After 6 weeks all responses were analyzed and compared.
Table 1.
Survey circulated among urologists in the United Kingdom and Republic of Ireland
| Question | Response |
|---|---|
| Are you aware of the different methods available for measuring life expectancy? | Yes/No |
| Have you ever used one of the methods of life expectancy calculation in practice? | Yes/No |
| Do you use intuition or eyeballing as a general method to estimate life expectancy in the clinical setting? | Yes/No |
| Does your unit utilise life expectancy tables/questionnaires for formal analysis of life expectancy in clinical practice? | Yes/No |
| Are decisions made in MDT setting in your unit based on an accurate life expectancy calculation? | Yes/No |
This was a 5-part questionnaire requiring Yes/ No in the response section.
Statistical analysis
Statistical analysis was performed with SPSS version 24.0 (Armonk, USA) Comparative analyses were performed using chi-square tests and a p-value <0.05 was considered statistically significant.
RESULTS
Respondents
The survey was delivered to 1200 urological clinicians and the response rate was 17% (208/1251); of whom 29 were from the Republic of Ireland (RoI) and 179 were from the United Kingdom (UK). The majority (61%; n = 127) of clinicians in both countries were aware of the methods available for estimated LE calculation. A higher percentage of urologists in the UK were familiar with LE estimation methods as compared to the Republic of Ireland (62%, n = 112 versus 52% n = 15 respectively, p = 0.27).
Utilisation of life expectancy models in clinical practice
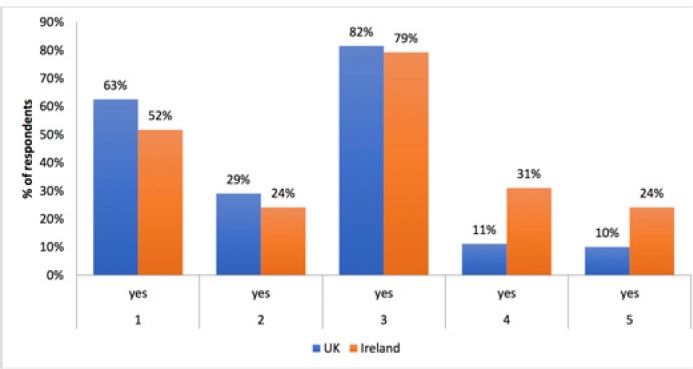
Figure 1 summarizes the responses to the survey. The majority of urologists (71%, n = 147) had no experience with LE analysis in clinical practice. Twenty-nine percent (n = 52) had experience with LE estimation methods in the UK and 24% (n = 7) in the RoI (p = 0.73). Overall, 14% (n = 29) reported that their urological institution routinely utilized LE models in clinical practice (31%, n = 9 versus 11%, n = 20 in the RoI and UK respectively; p = 0.002). Intuition or ‘eyeballing’ was the most common method for estimating LE in clinical practice with 82% respondents overall (n = 170) and 82% (n=146) of respondents from the UK regularly ‘eyeballing’ patients to calculate LE as compared to 79% (n = 23) in the RoI (p = 0.63). LE models were routinely utilized in treatment decision-making in the multi-disciplinary meetings (MDM) setting by 12% of respondents (n = 25, 10% [n = 18] in the UK versus 24% [n =7] in the RoI, P = 0.002).
Figure 1.
Summary of responses to questions 1–5. The majority of respondents (82% in the UK and 79% in the RoI) use personal intuition or eyeballing for estimating LE. *See Table 1 for details of questions 1–5.
DISCUSSION
Eyeballing or empirical estimation of LE based on intuition was the most common method among respondents with approximately 82% of clinicians routinely using this technique in clinical practice. Even when relevant information is available including age, family history and comorbidities, gross LE estimation based on intuition can be inaccurate. One study involving 18 urologists and oncologists examined 70 patient cases (including duplicate scenarios) and found that the mean underestimation of 10-year LE was 10.8% (range 10.1–11.5%) [5]. Junior clinicians were less accurate than their senior counterparts for estimating LE. These variations can have significant implications on patient care as underestimating LE can deny the best available treatment to patients while overestimating LE can lead to aggressive management with treatment associated comorbidities. Hence, LE estimation utilizing the best available tools becomes imperative to allow rational treatment of urology patients, and a lack of emphasis on the same in clinical urological practice in the British Isles can have significant implications on service delivery.
Walz et al. investigated LE prediction among urologists, trainees and medicals students in prostate cancer patients and noted a poor predictive accuracy of 0.68 for LE among the group [6]. Conversely, Krahn et al. [7] demonstrated a 31% accuracy of LE based only on prostate cancer scenarios within 1 year of the Markov model projections, 67% accuracy within 3 years and 82% accuracy within 10-years among urologists and radiation oncologists. A two-staged Markov model (living, dead) was created to model life expectancy in this study based on age and comorbidity. The authors concluded that clinicians can use the 10-year rule with sufficient accuracy. Sammon et al. assessed different methods for LE estimation in prostate cancer and found that clinician predicted LE estimation was inaccurate with a discrimination of 0.67 (0.60–0.72) among attendings (consultants), 0.69 (0.64–0.74) among residents and 0.67 (0.58–0.76) among medical students. A subsequent analysis of various models for LE-estimation from 5 different studies (1996–2013) found that their model predicted LE calculations for prostate cancer with a c-index ranging from 0.65 to 0.84 [8]. The c-index or the concordance index validates the predictive ability of a survival model. These studies clearly reveal that the ‘eyeballing’ or ‘intuitive' estimation of LE is largely inaccurate.
Methods currently available for estimating LE in urology include a specific model predicted LE, and general LE estimation methods such as government life tables. Despite widely available tools for LE in urology and 62% of our respondents being aware of these methods, only 29% of respondents use them in clinical practice. The model with the highest c-index was Walz et al. (0.84) who investigated non-cancer mortality within 10 years of receiving definitive therapy [6]). The authors also analyzed the accuracy Social Security Administration (SSA) tables and found that SSA life tables provide consistent LE estimation and should perhaps be utilized more frequently in clinical practice. EAU guidelines [9] suggest that the Cumulative Illness Score Rating-Geriatrics (CISR-G) is the optimal LE tool for assessing mortality risk unrelated to prostate cancer. Compared to the Charlson Comorbidity index (CCI) (which only grades potentially lethal comorbid conditions), the CISR-G also grades non-lethal conditions in accordance to their severity and level of control. The International Society of Geriatric Oncology (SIOG) Prostate Cancer Working Group (PCWG) recommend that treatment for senior adults is based on systematic evaluation of health status. The G8 (Geriatric 8) health status screening tool is a discriminant tool recommended in all senior adults with localized prostate cancer by the EAU. If the patient scores >14 they are considered fit to be treated as young men, if they score <14 they will undergo a full geriatric evaluation based on comorbidities, nutritional status, physical and cognitive status using a tool like CISR-G. This would further enable the clinician to divide these patients into four categories: ‘healthy’, ‘vulnerable’, ‘frail’ and ‘too sick’ in order to make treatment decisions [9]. Data on LE estimation in the treatment of other urological cancers is limited. Daskivich et al. [10] demonstrated that kidney cancer patients with a LE <10 years (26%, n = 2569) and a minority of patients with a LE <5 years (1%, n = 145) were being treated with surgery or ablation for stage T1a kidney cancer. Kyung et al. [11] investigated elderly patients (70 years and older) with localized T1 stage renal masses and identified a need to incorporate underlying morbidities and risk-benefits in each individual case before surgical treatment. Finally, Abousassalyy et al. [12] evaluated optimal treatment strategies for small renal masses (<4 cm in elderly patients) and noted that treatment protocols were most commonly based on a patient’s age due to a paucity of data on treatment outcomes based on comorbidities. This limitation emphasizes the importance of LE estimation to facilitate appropriate treatment decisions in patients with suspicious renal masses. Kulkarni et al. assessed [13] optimal treatment strategies for high- risk bladder cancer by introducing the decision-analytic Markov LE model for selecting patients that were suitable for radical cystectomy. The authors pioneered their model in bladder cancer in order to estimate the LE gained as well as the Quality-adjusted life expectancy (QALE) obtained from each of the two treatment strategies for bladder cancer: immediate cystectomy versus initial conservative treatment with intravesical BCG followed by delayed cystectomy. The authors found that co-morbid status was most relevant for the age groups 70–80 years and 60–70 years and had little effect in the younger or older patients for accurately estimating mean LE and Quality adjusted LE (QALE). Hence decisions for treatment in these age groups should be based on comorbid status rather than chronological age to provide the most appropriate management. Our survey highlighted that although urologists are aware of the various tools used to estimate LE in urology, there is low clinical application by individual clinicians (29%), but the institutional application of these tools is even lower at 14% with only 12% of respondents claiming the use of these LE tools in multi-disciplinary meeting decisions in their center. One cannot be certain of the reasons behind this reluctance to use LE tools in practice, it could be time constraints or clinicians’ notions that ‘eyeballing’ can provide an accurate LE estimation.
There are some limitations to the present study and our results should be viewed with caution. The response rate of the survey was low. Despite reminders it was difficult to obtain comments about this important issue from urologists across the British Isles, which in itself is a point to be noted. The results are respondent dependent. An exact estimation of the application of LE estimation in urological practice by auditing urological centers would be more accurate. However, as the questionnaire was online, a wider number of urological clinicians were contactable to obtain data as opposed to focusing on a single centre or deanery.
CONCLUSIONS
Treatment decisions for relatively indolent urological malignancies such as prostate cancer should consider objective LE estimation and patient health status as opposed to chronological age. The present study demonstrates limited application of LE models in contemporary urological practice, for reasons that are unclear. This study emphasizes the need to introduce LE estimation models across urological centers to allow adherence to international guidelines on LE estimation in uro-oncology.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1. http://www.dictionary.com/browse/life-expectancy.
- 2.Organisation WH. Global Health and Aging. http://www.who.int/ageing/publications/global_health.pdfAccessed 13/12/16. NIH Publication no. 11-7737. 2011.
- 3.Daskivich TJ. The Importance of accurate life expectancy prediction in men with prostate cancer. Eur Urol. 2015;68:766–767. doi: 10.1016/j.eururo.2015.03.040. [DOI] [PubMed] [Google Scholar]
- 4.McGuire BM, Fitzpatrick JM. Prostate Cancer: Localized Disease. In: Jean-Pierre Droz RAA, editor. Management of Urological Cancers in Older People. edn. 2012. pp. 96–97. [Google Scholar]
- 5.Wilson JR, Clarke MG, Ewings P, Graham JD, MacDonagh R. The assessment of patient life-expectancy: how accurate are urologists and oncologists? BJU Int. 2005;95:794–798. doi: 10.1111/j.1464-410X.2005.05403.x. [DOI] [PubMed] [Google Scholar]
- 6.Walz J, Gallina A, Perrotte P, et al. Clinicians are poor raters of life- expectancy before radical prostatectomy or definitive radiotherapy for localized prostate cancer. BJU Int. 2007;100:1254–1258. doi: 10.1111/j.1464-410X.2007.07130.x. [DOI] [PubMed] [Google Scholar]
- 7.Krahn MD, Bremner KE, Asaria J, et al. The ten-year rule revisited: accuracy of clinicians' estimates of life expectancy in patients with localized prostate cancer. Urology. 2002;60:258–263. doi: 10.1016/s0090-4295(02)01712-0. [DOI] [PubMed] [Google Scholar]
- 8.Sammon JD, Abdollah F, D'Amico A, et al. Predicting life expectancy in men diagnosed with prostate cancer. Eur Urol. 2015;68:756–765. doi: 10.1016/j.eururo.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolla M. Guidelines on Prostate Cancer - European Association of Urology. https://uroweb.org/wpcontent/uploads/1607-Prostate-CancerAccessed 02/01/17. 2015.
- 10.Daskivich TJ, Tan HJ, Litwin MS, Hu JC. Life expectancy and variation in treatment for early stage kidney cancer. J Urol. 2016;196:672–677. doi: 10.1016/j.juro.2016.03.133. [DOI] [PubMed] [Google Scholar]
- 11.Kyung YS, You D, Kwon T, et al. The type of nephrectomy has little effect on overall survival or cardiac events in patients of 70 years and older with localized clinical t1 stage renal masses. Korean J Urol. 2014;55:446–452. doi: 10.4111/kju.2014.55.7.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abouassaly R, Yang S, Finelli A, Kulkarni GS, Alibhai SM. What is the best treatment strategy for incidentally detected small renal masses? A decision analysis. BJU Int. 2011;108:E223–231. doi: 10.1111/j.1464-410X.2011.10115.x. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni GS, Finelli A, Fleshner NE, Jewett MA, Lopushinsky SR, Alibhai SM. Optimal management of high-risk T1G3 bladder cancer: a decision analysis. PLoS Med. 2007;4:e284. doi: 10.1371/journal.pmed.0040284. [DOI] [PMC free article] [PubMed] [Google Scholar]