Abstract
Background
The aim of this study was to investigate the expression of long non-coding RNAs (lncRNA) cancer susceptibility candidate 2a (CASC2a) in patients with urothelial carcinoma of the bladder (UCB) and its predictive value in the recurrence of UCB after radical cystectomy (RC).
Material/Methods
Tumor and paired adjacent normal tissues were obtained from 112 patients with UCB who underwent RC in our hospital from March 2010 to March 2012. The expression of CASC2a was evaluated by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) and fluorescence in situ hybridization (FISH).
Results
CASC2a was down-regulated in UCB tissues, and was highly negatively correlated with the pT, pN, tumor size, and lymphovascular invasion (LVI). The sensitivities of CASC2a for diagnosing UCB and its recurrence after RC were 89.30% and 81.55%, respectively, and the specificities were 71.43% and 58.21%, respectively. Patients with a high expression of CASC2a had a higher 5-year recurrence-free survival rate than those with low expression of CASC2a. Kaplan-Meier survival analysis demonstrated that the pT, pN, tumor grade, tumor size, concomitant carcinoma in situ (CIS), LVI, soft tissue surgical margin (STSM), and CASC2a expression were related to the recurrence in patients undergoing RC for UCB. Cox proportional hazard model analysis showed that CASC2 expression, pT4, lymph node metastasis, and CIS were independent risk factors.
Conclusions
CASC2a was down-regulated in patients with UCB, and was associated with the risk of recurrence among patients undergoing RC, indicating that lncRNAs could act as predictive biomarkers and potential therapeutic targets in bladder cancer, including CASC2a.
MeSH Keywords: Early Diagnosis; Neoplasm Recurrence, Local; RNA, Long Noncoding; Urinary Bladder Neoplasms
Background
Urothelial carcinoma of the bladder (UCB) is the most common tumor of the urinary system, with an estimated 74 690 new cases and 15 580 deaths in 2014 worldwide [1], which can be classified into muscle-invasive bladder cancer (MIBC) and non-MIBC (NMIBC) [2]. A proportion of patients with initially confirmed NMIBC will progress to muscle invasion, which could increase the risk of cancer-specific death [3]. The mainstay of therapy for MIBC patients has been radical cystectomy (RC) with extended lymphadenectomy [4]; however, owing to stage differences, the outcomes following RC are not as good as expected [5]. Therefore, it is particularly urgent to explore the molecular mechanism to find reliable biomarkers for treating UCB.
Long non-coding RNAs (lncRNAs) with a length of >200 nucleotides, which do not encode proteins, are involved in multiple biological processes [6,7]. Recently, it has been found that lncRNA may become a new marker for tumor diagnosis and prognosis evaluation. For example, Zhou et al. revealed that 12 differentially expressed lncRNAs had an important role in the tumor recurrence of patients with breast cancer [8]. Additionally, the study by Duan et al. demonstrated that the AUC values of a 3-lncRNA (MEG3, SNHG16, and MALAT1) panel were 0.865 and 0.828 for the training and validation sets, respectively, and the expression of MEG3 can affect the recurrence-free survival of bladder cancer patients [9]. CASC2, a newly discovered lncRNA, generates 3 different mRNAs: CASC2a, CASC2b, and CASC2c, and has a significant impact on the occurrence and progression of various human cancers, including colorectal cancer [10], lung cancer [11], renal cell carcinoma [12], gastric cancer [13], and glioma [14]. Baldinu et al. demonstrated CASC2a may act as a tumor suppressor gene, providing a growth advantage in EC cells [15]. In addition, although Pei et al. reported that CASC2 can affect the biological features of bladder cancer cells by inhibiting the Wnt/β-catenin signaling pathway [16]. It has not been clarified whether CASC2a can be used for early diagnosis and prognosis evaluation of bladder cancer. Therefore, our study collected tumor and adjacent normal tissues from 112 patients with UCB who underwent RC. qRT-PCR and FISH were performed to detect the expression of CASC2a, aiming to investigate the value of CASC2a for diagnosis and recurrence prediction in UCB patients following RC, thus representing a promising therapeutic option for suppressing bladder cancer progression.
Material and Methods
Ethics statement
This study obtained approval of the Clinical Research Ethics Committee of the Affiliated Hospital of Hangzhou Normal University and was performed according to the Declaration of Helsinki [17]. Informed consent was obtained from all subjects prior to study enrollment.
Study subjects
Tumor tissues and paired adjacent normal tissues (2 cm to tumor edge) were collected from 112 patients with pathologically confirmed UCB. All patients were treated with RC in our hospital from March 2010 to March 2012. The general clinical pathological information of patients is displayed in Table 1. Of the 112 patients, 23 were treated with chemotherapy, 9 with neoadjuvant chemotherapy, 13 with adjuvant chemotherapy, and 1 with a combination of neoadjuvant chemotherapy and adjuvant chemotherapy. The inclusion criteria were as follows: patients pathologically diagnosed with UCB; diagnosis fully supported by imaging and clinical examinations; and followed up or reviewed at least once after discharge. Patients were excluded if they were not cleared to undergo transurethral resection of bladder tumor; suffered from other neoplastic diseases; or already had distant metastasis of UCB before treatment.
Table 1.
General information of 112 UCUB patients.
| Mean ±SD/N | |
|---|---|
| Age (years) | 61.71±11.08 |
| BMI | 23.12±3.58 |
| Gender | |
| Male | 89 |
| Female | 23 |
| pT classification | |
| pT0–1 | 36 |
| pT2 | 28 |
| pT3 | 35 |
| pT4 | 13 |
| pN classification | |
| pN0 | 85 |
| pN+ | 27 |
| Tumor grade | |
| Low grade (grade 1, 2) | 9 |
| High grade (grade 3) | 103 |
| Tumor size | |
| <3 cm | 100 |
| ≥3 cm | 12 |
| Concomitant CIS | |
| Absent | 61 |
| Present | 51 |
| LVI | |
| Absent | 76 |
| Present | 36 |
| STSM | |
| Negative | 106 |
| Positive | 6 |
| Adjuvant chemotherapy | |
| No | 89 |
| Yes | 23 |
The expression of CASC2a detected by qRT-PCR in UCB tissues and paired adjacent normal tissues
The frozen tissue samples were stored in a −80°C freezer until used in RNA extractions. The total RNA was extracted with the miRNeasy Total RNA Extraction Kit (Qiagen, Hilden, Germany), and submitted immediately upon isolation for RNA-sequencing. The remaining RNAs were stored at −80°C after the confirmation of RNA-sequencing results. The A260/A280 ratio was determined with a nanodrop ultraviolet spectrophotometer (Thermo, Waltham, MA, USA). Based on the gene sequences in the GenBank database, primers were designed with Primer5.0 and synthesized by Sangon Biotech Co., Ltd (Shanghai, China). The primer sequences are shown in Table 2. The sample RNA was reverse-transcribed into cDNA and stored at −20°C in accordance with the instructions of the Reverse Transcription Reagent Kit (6210A, Takara Biotechnology (Dalian) Co., Ltd., China). The reaction conditions were 37°C for 30 min for the RT reaction and 85°C for 5 s for reverse transcriptase inactivation reaction. The cDNA obtained by reverse transcription was mixed, and the PCR amplification reaction system (20 μl) was prepared according to the instructions on the Fluorescence Quantitative PCR Kit (RR82LR, Takara Biotechnology (Dalian) Co., Ltd., China), which consists of 10 μl of SYBR Green, 0.8 μl of upstream primer (10 μM), 0.8 μl of downstream primer (10 μM), 2 μl of cDNA (reverse transcription reaction system), and 6.4 μl of distilled water. qRT-PCR was performed with a ABI9700 quantitative PCR instrument (ABI Company, USA). The reaction conditions were as follows: pre-denaturation at 95°C for 30 s and a total of 40 cycles of denaturation at 95°C for 5 s and 60°C for 45 s. With GAPDH as an internal reference, the relative expression levels of the target gene were calculated using the 2−ΔΔCt method, and the calculation formulas were as follows:
Table 2.
Primer sequences for qRT-PCR.
| Genes | Sequences | |
|---|---|---|
| CASC2a | Forward | 5′-GCACATTGGACGGTGTTTCC-3′ |
| Reverse | 5′-CCCAGTCCTTCACAGGTCAC-3′ | |
| GAPDH | Forward | 5′-AGAAGGCTGGGGCRCATTTG-3′ |
| Reverse | 5′-AGGGGCCATCCACAGTCTTC-3′ |
Fluorescence in situ hybridization (FISH) in UCB tissues and paired adjacent normal tissues
Tumor and normal tissues were all detect using QuantiGene® ViewRNA ISH tissue assay (Panomics Srl, Vignate-Milano, Italy) for RNA-ISH in line with the manufacturer’s instructions. After deparaffinized, sections were boiled for 5 min in pretreatment solution and digested for 10 min with proteinase K. Next, the sections were hybridized with a custom-designed QuantiGene ViewRNA probe against human CASC2a for 3 h at 40°C. A no-probe sample was used as a control. Slides were stored overnight in storage buffer after the probe hybridization. The next day, hybridized probes were amplified from Affymetrix using PreAmp and Amp molecules. Alkaline phosphatase-conjugated oligonucleotide probes were added and Fast Red was used as substrate to produce signal (red dots) (Pierce, Rockford, IL, Staffs, USA). Slides were counterstained with Hematoxylin and scanned using a Zeiss Mirax Midi Slide Scanner with fluorescence scanner (Centre for Microscopy and Image analysis, UZH, Irchel). Tissue sections were analyzed with Panoramic Viewer software (v. 1.15.2, 3DHISTECH Ltd, Budapest, Hungary,).
Follow-up
We followed up all patients and their families by telephone and outpatient visits to verify they were still alive. The recurrence, death, or loss to follow-up indicated the completion of follow-up. The recurrence of bladder cancer was defined as a newly growing focus based on cystoscopy or imaging findings. The recurrence-free survival time was the specific time period from the date of surgery to the date of recurrence or death [18]. The last follow-up was conducted in March 2017, and the 5-year recurrence-free survival rate was calculated accordingly. In our study, all patients were followed up.
ROC curves
The results of the clinical experiment are displayed through a receiver operating characteristic (ROC) curve, and the area under the curve (AUC) and 95% confidence interval (95% CI) were calculated to evaluate the efficiency of the clinical diagnosis as well as recurrence prediction for UCB. Youden Index [Maximum (Sensitivity + Specificity − 1)] was calculated and corresponding diagnostics test result values were the cut-off threshold point for CASC2a [19].
Statistical analysis
All data were processed with SPSS 21.0 software (SPSS Inc, Chicago, IL, USA), and measurement data are presented as the mean ± standard deviation (χ̄±s). Differences between measurement data that obey a normal distribution were compared using the t test and paired t test. Comparisons among multiple groups were analyzed using one-way ANOVA. Enumeration data between groups were compared with the chi-square (χ2) test. Logistic regression analysis was used to evaluate the risk of recurrence of patients with UCB. Univariate survival analysis was performed with the Kaplan-Meier method and log-rank test. Multivariate survival analysis was performed using the Cox risk regression model. P<0.05 was considered a significant difference.
Results
Association of CASC2a expression with its clinicopathologic features in patients with UCB
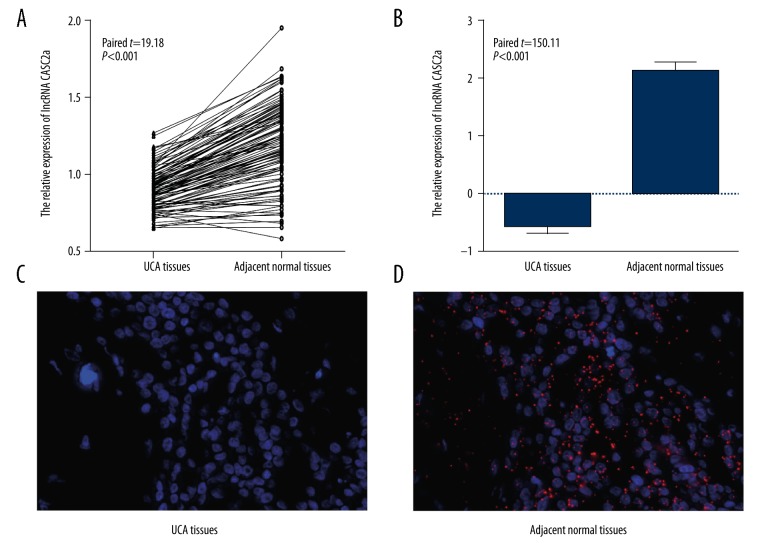
qRT-PCR showed that the expression of CASC2a was lower in UCB tissues than the paired adjacent normal tissues (0.91±0.23 vs. 1.24±0.36, paired-t=19.18, P<0.001, Figure 1A), which was consistent with the result of FISH, that is, a significant 6-fold decrease of CASC2a level in tumor tissue when compared to the paired adjacent tissues (paired-t=150.11, P<0.001, Figure 1B–1D). Additionally, according to the median value of CASC2a expression (median=0.901) in UCB tissues detected by qRT-PCR, 112 patients were divided into the high-expression group (≥0.901, n=56) and low-expression group (<0.901, n=56). As illustrated in Table 3, the results revealed that the expression of CASC2a was strongly linked to the pT classification, pN classification, tumor size and LVI of patients with UCB (all P<0.05), but did not show any association with age, BMI, tumor grade, STSM, or adjuvant chemotherapy (all P>0.05).
Figure 1.
Expression of CASC2a in UCB tissues and adjacent normal tissues by qRT-PCR and FISH. (A) Expression of CASC2a in UCB tissues and adjacent normal tissues by qRT-PCR, (B–D) Expression of CASC2a in UCB tissues and adjacent normal tissues by FISH; CASC2 – cancer susceptibility candidate 2; qRT-PCR – quantitative reverse transcriptase polymerase chain reaction; FISH – fluorescence in situ hybridization.
Table 3.
Relationship between CASC2a expression and clinicopathological features of UCUB.
| CASC2a expression | t/χ2/Z | P | ||
|---|---|---|---|---|
| Low (n=56) | High (n=56) | |||
| Gender | ||||
| Male | 42 | 47 | ||
| Female | 14 | 9 | 1.368 | 0.242 |
| pT classification | ||||
| pT0–1 | 11 | 25 | ||
| pT2 | 15 | 13 | ||
| pT3 | 20 | 15 | ||
| pT4 | 10 | 3 | 3.017 | 0.003 |
| pN classification | ||||
| pN0 | 37 | 48 | ||
| pN+ | 19 | 8 | 5.905 | 0.015 |
| Tumor grade | ||||
| Low grade (grade 1, 2) | 7 | 2 | ||
| High grade (grade 3) | 49 | 54 | 3.020 | 0.082 |
| Tumor size | ||||
| <3 cm | 46 | 54 | ||
| ≥3 cm | 10 | 2 | 5.973 | 0.015 |
| Concomitant CIS | ||||
| Absent | 28 | 33 | ||
| Present | 28 | 23 | 0.900 | 0.343 |
| LVI | ||||
| Absent | 31 | 45 | ||
| Present | 25 | 11 | 8.023 | 0.005 |
| STSM | ||||
| Negative | 52 | 54 | ||
| Positive | 4 | 2 | 0.704 | 0.401 |
| Adjuvant chemotherapy | ||||
| No | 42 | 47 | ||
| Yes | 14 | 9 | 1.368 | 0.242 |
The expression of CASC2a in UCB patients with recurrence after RC
The 5-year recurrence-free rate of 112 patients after RC was 59.82% (67/112). The patients with high CASC2a expression had lower 5-year recurrence-free rate than those with low expression (53.57% vs. 66.07%). Significant differences were found between the UCB patients with recurrence and those without recurrence (Recurrence vs. No-recurrence: 0.86±0.11 vs. 0.98±0.12, t=5.593, P<0.01) (Figure 2).
Figure 2.
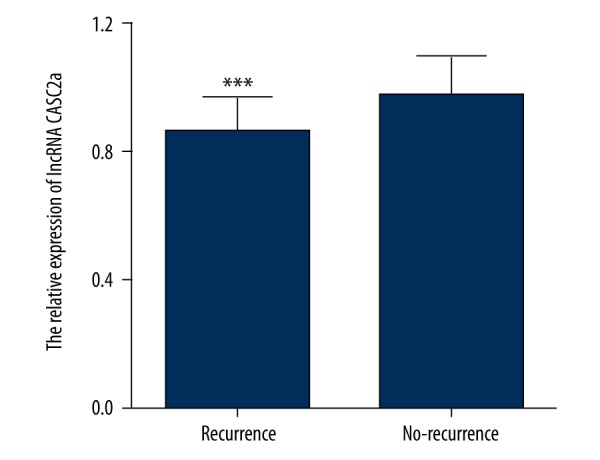
Detection of CASC2 expression in UCB patients with/without recurrence undergoing RC by qRT-PCR. *** P<0.001, compared with patients without recurrence after RC. CASC2 – cancer susceptibility candidate 2; qRT-PCR – quantitative reverse transcriptase polymerase chain reaction; RC – radical cystectomy.
The receiver operating characteristic (ROC) curve analysis
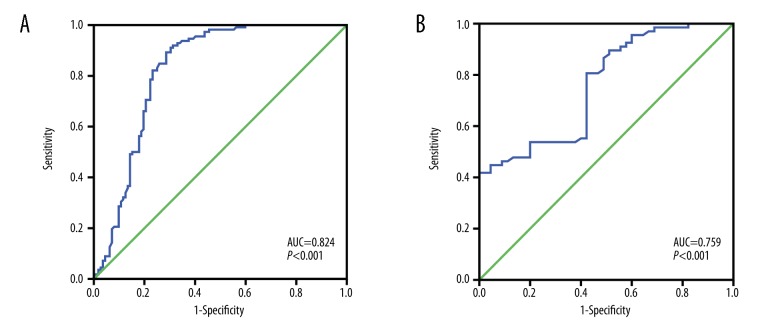
As shown in Figure 3A, the area under the ROC curve (AUC) of the diagnostic potential of CASC2a in UCB patients was 0.824 (95% CI: 0.766~0.883, P<0.001), and the sensitivity and specificity and diagnostic threshold were 89.30%, 71.43%, and 1.076, respectively, indicating that for a subject with the test result value <1.076, there was a 89.30% probability that the subject will be assigned to the UCB group, and for a subject with the test result value ≥1.076, there was a 71.43% probability that the subject will be assigned to the non-UCB group. Additionally, the AUC of the diagnostic value of CASC2a in UCB patients with recurrence undergoing RC was 0.759 (95% CI: 0.671~0.848, P<0.001). The diagnostic threshold was 0.816, and the sensitivity and specificity were 81.55% and 58.21%, respectively (Figure 3B), suggesting that for subjects with a value below 0.759, there was 81.55% probability that they will be assigned to the recurrence group, and for those with a value ≥1.076, there was 58.21% probability that the subject will be assigned to the non-recurrence group.
Figure 3.
ROC curve analysis. (A) Diagnostic of CASC2 expression in UCB by ROC curve analysis; (B) ROC curve for UCB recurrence after RC; ROC – receiver operating characteristics; CASC2 – cancer susceptibility candidate 2; RC – radical cystectomy.
Bivariate logistic regression analysis of recurrence in UCB patients after RC
In the bivariate logistic regression analysis, the dependent variable was UCB patients with or without recurrence after RC, and the independent variables were the clinicopathological features and CASC2a expression of UCB patients. As shown in Table 4, low expression of CASC2a, lymph node metastasis, and not receiving adjuvant chemotherapy increase the risk of recurrence after radical cystectomy in UCB patients.
Table 4.
Bivariate logistic regression analysis of recurrence after radical cystectomy in UCUB patients.
| B | SE | Wals | df | Sig. | Exp (B) | 95% CI | |
|---|---|---|---|---|---|---|---|
| Age | −0.024 | 0.024 | 0.984 | 1 | 0.321 | 0.976 | 0.932~1.024 |
| BMI | −0.032 | 0.073 | 0.190 | 1 | 0.663 | 0.969 | 0.840~1.118 |
| Gender (Male vs. Female) | −0.319 | 0.680 | 0.220 | 1 | 0.639 | 0.727 | 0.192~2.755 |
| pT classification | 3.533 | 3 | 0.316 | ||||
| pT2 vs. pT0–1 | −0.679 | 0.627 | 1.171 | 1 | 0.279 | 0.507 | 0.148~1.734 |
| pT3 vs. pT0–1 | 0.427 | 0.694 | 0.378 | 1 | 0.539 | 1.533 | 0.393~5.978 |
| pT4 vs. pT0–1 | 2.457 | 2.074 | 1.403 | 1 | 0.236 | 11.665 | 0.200~679.838 |
| pN classification (pN+ vs. pN0) | 2.420 | 0.913 | 7.017 | 1 | 0.008 | 11.244 | 1.876~67.373 |
| Tumor grade (low vs. high) | −0.895 | 1.527 | 0.343 | 1 | 0.558 | 0.409 | 0.021~8.149 |
| Tumor size (≥3 cm vs. <3 cm) | 2.348 | 2.274 | 1.066 | 1 | 0.302 | 10.465 | 0.121~902.646 |
| Concomitant CIS (present vs. absent) | 1.601 | 0.614 | 6.809 | 1 | 0.009 | 4.959 | 1.490~16.506 |
| LVI (present vs. absent) | 0.080 | 0.698 | 0.013 | 1 | 0.909 | 1.084 | 0.276~4.257 |
| STSM (positive vs. negative) | −1.438 | 1.833 | 0.616 | 1 | 0.433 | 0.237 | 0.007~8.62 |
| Adjuvant chemotherapy (yes vs. no) | −1.676 | 0.728 | 5.301 | 1 | 0.021 | 0.187 | 0.045~0.779 |
| CASC2a expression | −12.093 | 3.197 | 14.308 | 1 | <0.001 | 0.000 | 0.000~0.003 |
The relationship between CASC2a expression and the recurrence-free survival rate in patients with UCB after RC
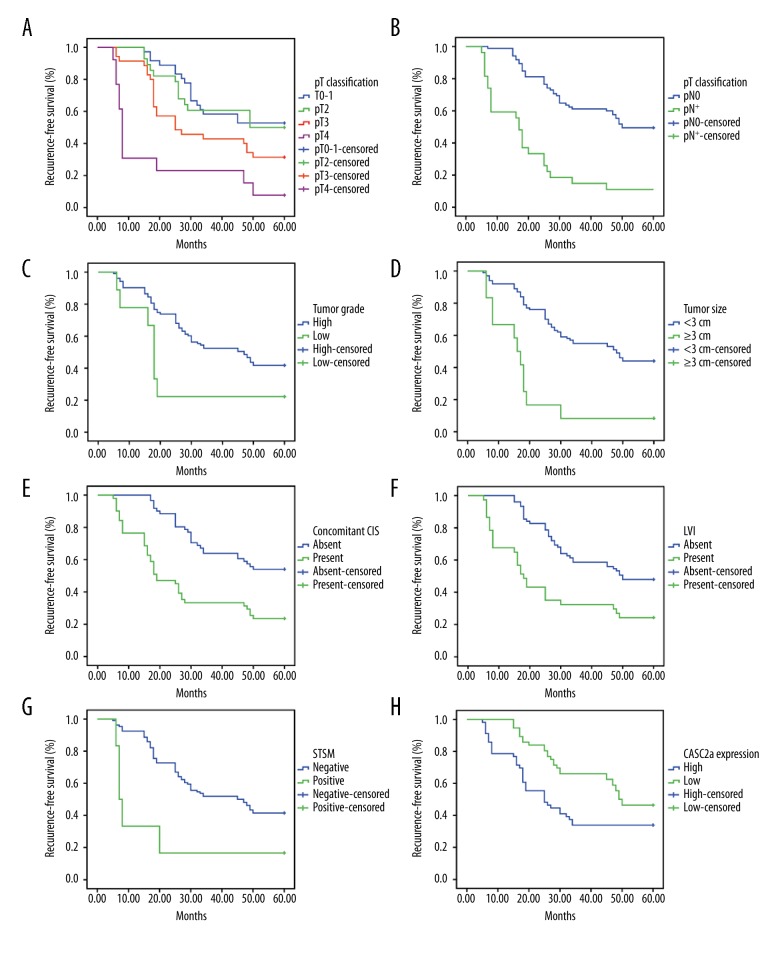
The results of the univariate survival analysis by Kaplan-Meier method are illustrated in Figure 4. Many factors, including pT classification, pN classification, tumor grade, tumor size, CIS, LVI, STSM, and CASC2a expression, were correlated with the recurrence in UCB patients undergoing radical cystectomy (all P<0.05). In addition, after factoring CASC2a expression as well as pathological indexes of statistical significance shown by univariate analysis into the multivariate Cox proportional hazard model, we found that CASC2a expression, pT4, lymph node metastasis, and CIS were independent risk factors for the recurrence of UCB patients undergoing RC (Table 5).
Figure 4.
Kaplan-Meier curves for recurrence-free survival rates according to pT classification, pN classification, tumor grade, tumor size, CIS, LVI, STSM and CASC2 expression in 122 UCB patients. (A) Recurrence-free survival of pT classification, stratified by pT0-1, pT2, pT3 vs. pT4; (B) recurrence-free survival of pN classification, stratified by pN0 vs. pN+; (C) recurrence-free survival of tumor grade, stratified by high grade vs. low grade; (D) recurrence-free survival of tumor size, stratified by ≥3 cm vs. <3 cm; (E) recurrence-free survival of CIS, stratified by present vs. absent; (F) recurrence-free survival of LVI, stratified by present vs. absent; (G) recurrence-free survival of STSM, stratified by positive vs. negative; and (H) recurrence-free survival of CASC2 expression, stratified by low expression vs. high expression; CASC2 – cancer susceptibility candidate 2; LVI – lymphovascular invasion; CIS – concomitant carcinoma in situ; STSM – soft tissue surgical margin.
Table 5.
Multivariate Cox regression analysis.
| B | SE | Wals | df | Sig. | Exp (B) | 95% CI | |
|---|---|---|---|---|---|---|---|
| pT classification | 6.033 | 3 | 0.110 | ||||
| pT2 vs. pT0–1 | −0.106 | 0.404 | 0.068 | 1 | 0.794 | 0.900 | 0.408~1.985 |
| pT3 vs. pT0–1 | 0.266 | 0.348 | 0.584 | 1 | 0.445 | 1.305 | 0.66~2.581 |
| pT4 vs. pT0–1 | 0.983 | 0.460 | 4.579 | 1 | 0.032 | 2.673 | 1.086~6.579 |
| pN classification (pN+ vs. pN0) | 1.207 | 0.309 | 15.248 | 1 | <0.001 | 3.343 | 1.824~6.127 |
| Tumor grade (low vs. high) | 0.206 | 0.424 | 0.236 | 1 | 0.627 | 1.228 | 0.535~2.818 |
| Tumor size (≥3 cm vs. <3 cm) | 0.641 | 0.415 | 2.388 | 1 | 0.122 | 1.898 | 0.842~4.28 |
| Concomitant CIS (present vs. absent) | 0.766 | 0.275 | 7.761 | 1 | 0.005 | 2.152 | 1.255~3.689 |
| LVI (present vs. absent) | 0.430 | 0.308 | 1.958 | 1 | 0.162 | 1.538 | 0.842~2.81 |
| STSM (positive vs. negative) | 0.452 | 0.537 | 0.709 | 1 | 0.400 | 1.572 | 0.549~4.504 |
| CASC2a expression | −0.808 | 0.295 | 2.967 | 1 | 0.045 | 0.602 | 0.338~0.973 |
Discussion
Accumulating studies have shown that abnormal lncRNAs can function as oncogenes or tumor suppressor genes in bladder cancer. For instance, SChLAP1 was elevated in bladder cancer tissues, and SChLAP1 siRNA could inhibit the growth and migration of T24 and 5637 cells while promoting apoptosis [20]. Moreover, LINC00312 up-regulate the expression of MMP-2 and MMP-9 while reducing the expression of TIMP2 via the regulation of miR-197-3p, which can inhibit the migration, invasion, and metastasis of bladder cancer cells [21]. Our study also evaluated the role of lncRNA CASC2a in the early recurrence and diagnostic prediction of patients with UCB undergoing RC.
One of the most important results of our study was that CASC2a was significantly down-regulated in UCB tissues. Of note, CASC2 can be classified into 3 subgroup transcripts: CASC2a, CASC2b, and CASC2c. CASC2a is located in a gene region with allelic loss and can be inactivated via genetic mutations (in a few cases) or epigenetic/regulative changes (in most cases) [22], and the genomic regions affected by such allelic losses often harbor a tumor suppressor gene [19]. Baldinu et al. reported that the mRNA expression levels of CASC2b and CASC2c were similar in normal tissues and neoplastic endometrial tissues, which further demonstrates that CASC2a is a type of transcript that is particularly down-regulated in endometrial carcinoma [15], which was consistent with our results. This suggests that CASC2a as a tumor suppressor gene whose inactivation causes neoplastic transformation could play a role in tumorigenesis of various cancers, including UCB. Additionally, our study also revealed that the diagnostic sensitivity and specificity of CASC2a in UCB patients were 81.55% and 58.21%, respectively, further indicating that CASC2a in UCB has a relatively high diagnostic efficacy, and consequently has a potential diagnostic role in early screening of UCB from healthy individuals. Furthermore, we found that CASC2a was associated with pT, pN, tumor size, and LVI of patients with UCB.
We also found that expression of CASC2a has potential prognostic values in recurrence in UCB patients undergoing RC, which was associated with pT, pN, tumor size, and LVI of patients with UCB, and could be used as an independent prognostic risk factor influencing the recurrence rate of UCB patients, as conformed by Kaplan-Meier method and multivariate Cox regression analysis. Accumulating evidence has confirmed that lncRNAs can affect the prognosis of bladder cancer patients [23–25]. CASC2 has also been demonstrated to be linked with cancer prognosis, and it is closely related to various oncogenes and tumor suppressor genes, such as SFTA1P [26]. Consistent with our study, the reduction of CASC2 was positively correlated with the advanced stage of colorectal cancer [10] and bladder cancer [16], indicating that CASC2 could involve the progression of cancers. Liao et al. used a Cox regression model to analyze survival in 57 patients with glioma, finding that patients with glioma and low CASC2 expression had a shorter survival time than those with high CASC2 expression [27]. Additionally, a study by He et al. found the CASC2 expression is an independent factor that affects prediction of overall survival of non-small cell lung cancer patients [28]. The results of previous studies were in line with our findings and may be explained by the facts that CASC2 inhibits the Wnt/β-catenin signaling pathway [16] and the MAPK signaling pathway [13], or function as a competing endogenous RNA by sponging miR-18a [10], and thus suppressing proliferation, migration, and invasion of cancer cells. Our data are consistent with the results of relevant studies in other cancers. These results support that CASC2 functions as a prognostic predictor for tumors, including UCB. For UCB patients with recurrence after RC, the sensitivity and specificity of CASC2a for cancer diagnosis were 81.55% and 58.21%, respectively, which also supports that CASC2a may be beneficial for bladder cancer prognosis prediction and treatment.
Conclusions
All these findings show that the expression of CASC2a is remarkably down-regulated in patients with UCB, and is correlated with clinical pathological characteristics (pT classification, pN classification, tumor size, and LVI) as well as cancer recurrence after RC, suggesting that CASC2a may function as a target biomarker for treating UCB. In the future, we will perform a study with a larger sample size, and animal experiments are needed to verify the current data, deeply analyzing the specific mechanism of CASC2a in UCB.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
Source of support: Departmental sources
References
- 1.Gershman B, Moreira DM, Tollefson MK, et al. The association of ABO blood type with disease recurrence and mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Urol Oncol. 2016;34:4e1–9. doi: 10.1016/j.urolonc.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 2.Juráček J, Mlčochová H, Staník M, et al. Urinary cell-free microRNAs as potential biomarkers of urothelial carcinoma of the urinary bladder. Cancer Res. 2015;75 Abstract 3979. [Google Scholar]
- 3.Breau RH, Karnes RJ, Farmer SA, et al. Progression to detrusor muscle invasion during urothelial carcinoma surveillance is associated with poor prognosis. Bju International. 2014;113:900–6. doi: 10.1111/bju.12403. [DOI] [PubMed] [Google Scholar]
- 4.Clark PE, Agarwal N, Biagioli MC, et al. Bladder cancer. J Natl Compr Canc Netw. 2013;11:446–75. doi: 10.6004/jnccn.2013.0059. [DOI] [PubMed] [Google Scholar]
- 5.Sun M, Thuret R, Bhojani N, et al. 320 a population-based competing-risks analysis of the survival of patients treated with nephroureterectomy (nu) for upper-tract urothelial carcinoma (UTUC) Eur Urol Suppl. 2011;10:118. [Google Scholar]
- 6.Yarmishyn AA, Kurochkin IV. Long noncoding RNAs: A potential novel class of cancer biomarkers. Front Genet. 2015;6:145. doi: 10.3389/fgene.2015.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo X, Hua Y. CCAT1: An oncogenic long noncoding RNA in human cancers. J Cancer Res Clin Oncol. 2017;143:555–62. doi: 10.1007/s00432-016-2268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou M, Zhong L, Xu W, et al. Discovery of potential prognostic long non-coding RNA biomarkers for predicting the risk of tumor recurrence of breast cancer patients. Sci Rep. 2016;6:31038. doi: 10.1038/srep31038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan W, Du L, Jiang X, et al. Identification of a serum circulating lncRNA panel for the diagnosis and recurrence prediction of bladder cancer. Oncotarget. 2016;7:78850–58. doi: 10.18632/oncotarget.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang G, Wu X, Li S, et al. The long noncoding RNA CASC2 functions as a competing endogenous RNA by sponging miR-18a in colorectal cancer. Sci Rep. 2016;6:26524. doi: 10.1038/srep26524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang GQ, Ke ZP, Hu HB, Gu B. Co-expression network analysis of long noncoding RNAs (IncRNAs) and cancer genes revealsSFTA1P and CASC2abnormalities in lung squamous cell carcinoma. Cancer Biol Ther. 2017;18:115–22. doi: 10.1080/15384047.2017.1281494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Y, Xu R, Xu X, et al. Downregulation of lncRNA CASC2 by microRNA-21 increases the proliferation and migration of renal cell carcinoma cells. Mol Med Rep. 2016;14:1019–25. doi: 10.3892/mmr.2016.5337. [DOI] [PubMed] [Google Scholar]
- 13.Li P, Xue WJ, Feng Y, Mao QS. Long non-coding RNA CASC2 suppresses the proliferation of gastric cancer cells by regulating the MAPK signaling pathway. Am J Transl Res. 2016;8:3522–29. [PMC free article] [PubMed] [Google Scholar]
- 14.Liao Y, Shen L, Zhao H, et al. LncRNA CASC2 interacts with miR-181a to modulate glioma growth and resistance to TMZ through PTEN pathway. J Cell Biochem. 2017;118(7):1889–99. doi: 10.1002/jcb.25910. [DOI] [PubMed] [Google Scholar]
- 15.Baldinu P, Cossu A, Manca A, et al. CASC2a gene is down-regulated in endometrial cancer. Anticancer Res. 2007;27:235–43. [PubMed] [Google Scholar]
- 16.Pei Z, Du X, Song Y, et al. Down-regulation of lncRNA CASC2 promotes cell proliferation and metastasis of bladder cancer by activation of the Wnt/β-catenin signaling pathway. Oncotarget. 2017;8(11):18145–53. doi: 10.18632/oncotarget.15210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greco DB. Revising the declaration of Helsinki: Ethics vs. economics or the fallacy of urgency. Can HIV AIDS Policy Law Rev. 2000;5(4):98–101. [PubMed] [Google Scholar]
- 18.Liu J, Cheng S, Zhang Y, et al. Association between polymorphisms in the integrin gene predicted microRNA binding sites and bladder cancer risk. Int J Clin Exp Med. 2013;7:4398–405. [PMC free article] [PubMed] [Google Scholar]
- 19.Yin J, Tian L. Joint confidence region estimation for area under ROC curve and Youden index. Stat Med. 2014;33:985–1000. doi: 10.1002/sim.5992. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Shi Z, Nan Y, Li M. Inhibiting malignant phenotypes of the bladder cancer cells by silencing long noncoding RNA SChLAP1. Int Urol Nephrol. 2016;48:711–16. doi: 10.1007/s11255-016-1230-2. [DOI] [PubMed] [Google Scholar]
- 21.Wang YY, Wu ZY, Wang GC, et al. LINC00312 inhibits the migration and invasion of bladder cancer cells by targeting miR-197-3p. Tumor Biol. 2016;37:1–11. doi: 10.1007/s13277-016-5303-8. [DOI] [PubMed] [Google Scholar]
- 22.Palmieri G, Paliogiannis P, Sini MC, et al. Long non-coding RNA CASC2 in human cancer. Crit Rev Oncol Hematol. 2017;111:31–38. doi: 10.1016/j.critrevonc.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Berrondo C, Flax J, Kucherov V, et al. Expression of the Long non-coding RNA HOTAIR correlates with disease progression in bladder cancer and is contained in bladder cancer patient urinary exosomes. PLoS One. 2016;11:e0147236. doi: 10.1371/journal.pone.0147236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhan Y, Lin J, Liu Y, et al. Up-regulation of long non-coding RNA PANDAR is associated with poor prognosis and promotes tumorigenesis in bladder cancer. J Exp Clin Cancer Res. 2016;35:83. doi: 10.1186/s13046-016-0354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao XL, Zhao ZH, Xu WC, et al. Increased expression of SPRY4-IT1 predicts poor prognosis and promotes tumor growth and metastasis in bladder cancer. Int J Clin Exp Pathol. 2014;8:1954–60. [PMC free article] [PubMed] [Google Scholar]
- 26.Huang G, Ke ZP, Hu HB, Gu B. Co-expression network analysis of long noncoding RNAs (IncRNAs) and cancer genes revealsSFTA1P and CASC2abnormalities in lung squamous cell carcinoma. Cancer Biol Ther. 2017;18(2):115–22. doi: 10.1080/15384047.2017.1281494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yiwei L, Liangfang S, Haiting Z, et al. LncRNA CASC2 interacts with miR-181a to modulate glioma growth and resistance to TMZ through PTEN pathway. J Cell Biochem. 2017;118(7):1889–99. doi: 10.1002/jcb.25910. [DOI] [PubMed] [Google Scholar]
- 28.He X, Liu Z, Su J, et al. Low expression of long noncoding RNA CASC2 indicates a poor prognosis and regulates cell proliferation in non-small cell lung cancer. Tumor Biol. 2016;37:9503–10. doi: 10.1007/s13277-016-4787-6. [DOI] [PubMed] [Google Scholar]