Abstract
Penile-sparing modalities are gaining widespread adoption for the management of low-stage penile cancer due to an increasing demonstration of sound oncologic, cosmetic, sexual, psychosocial, and quality of life outcomes. This review aims to provide a comprehensive overview of the respective treatment options in the armamentarium of the practicing urologist in dealing with this rare but problematic condition.
Keywords: Mohs surgery, penile cancer, penile-sparing surgery, radiation therapy, topical therapy
INTRODUCTION
Penile cancer can be a debilitating condition with long-lasting effects on life expectancy, sexual function, cosmesis, and quality of life. Risk factors associated with penile cancer are HPV exposure, uncircumcised phallus, phimosis, and smoking.[1] Although it is a relatively rare primary malignancy in the industrialized world, recent estimates indicate there will be over 2000 cases of newly diagnosed penile cancer in 2017 and over 350 estimated deaths.[2] While studies have shown an overall decrease in penile cancer incidence rate in the United States, there does appear to be an increased proportion of tumors diagnosed at a localized stage and Grade 1 or 2 on pathological analysis.[3] The preponderance of low-grade and low-stage penile cancers has provided the impetus for clinicians to find efficacious penile-sparing approaches to optimize oncologic, sexual, and cosmetic outcomes. In this review, we will assess the penile-sparing treatment modalities for low-stage penile cancer. We will begin by providing the oncologic rationale for penile-sparing modalities which herein forms the inherent underpinning for the treatments discussed, namely, topical therapy, laser treatment, radiation, Mohs surgery, and penile-sparing surgery (PSS).
METHODS
A PubMed/MEDLINE search was performed for relevant publications in the English language journals using the following search terms alone or in combination: “penile sparing,” “penile cancer,” “laser,” “radiation,” “Mohs surgery,” “5-fluorouracil,” “imiquimod,” “glansectomy,” and “penile-sparing surgery.” All references from the articles found by our PubMed search were then assessed for applicability to the scope of this article.
ONCOLOGIC RATIONALE FOR PENILE-SPARING MODALITIES
Treatment of penile cancer remains a much-debated topic in the oncologic community. Pathologically, close to 95% of penile carcinoma is of epithelial origin, with squamous cell histology.[4] Carcinoma of the penis remains an aggressive disease with overall poor treatment outcome. Historically, surgical treatment for this disease included radical surgery with resection of the lesion with a 1- or 2-cm margin. However, given the significant psychological and functional concerns associated with a total and partial penectomy,[5] the treatment for low-stage penile cancer (Tis, Ta, T1, and T2 confined to glans) has moved toward less invasive options.
PSS for low-stage penile cancer has slowly gained acceptance over the years. The European guidelines in 2005 changed the paradigm for wide margins by stating that a 5-mm margin would be acceptable,[6] especially for low-stage penile cancer. Minhas et al. published on their cohort of 51 patients with penile cancer and determined that the traditional 2-cm margin was unnecessary for a majority of their patients, with only 4% developing a local tumor recurrence.[7] Furthermore, Philippou et al. determined that a 5-mm margin in their cohort of 179 patients provided adequate oncologic control.[8] They noted that patients with lymphovascular invasion, high tumor stage, and high tumor grade were at highest risk for recurrence and needed closer follow-up care and more aggressive surgical resection.
While the evidence for a reduced tumor-free margin has gained some support, it is clear that PSS may result in an increased risk of positive margin at surgery and of late local recurrence as contents of the preputial cavity remain intact. Given the recent published trends for patients with higher risk low-stage disease,[9] it is imperative that these patients maintain a routine follow-up schedule.
TOPICAL THERAPY
5-fluorouracil (5-FU) is a thymidylate synthase inhibitor that disrupts DNA replication, thereby exerting a cytotoxic effect on cancer cells.[10] About 5% of 5-FU cream is widely regarded as the first-line therapy for carcinoma in situ (CIS) of the penis (Bowen's disease, Erythroplasia of Queyrat, and penile intraepithelial neoplasia) although due to the rarity of this disease, there are no randomized controlled trials to support this. Numerous case reports and small case series have established that 5-FU therapy can be effective for noninvasive penile carcinoma, with a favorable side-effect profile and minimal systemic absorption.[11,12,13] The largest retrospective review to date examined 42 patients treated with 5-FU for CIS of the penis over 10 years. Alnajjar et al. showed a 50% complete response to treatment over a mean follow-up of 34 months.[14] There is no standard application protocol, but typically the cream is applied for 4-6 weeks, for 12 h at a time, every other day.[15] This protocol can be repeated in the case of recurrence. Local side effects appear tolerable and include hypersensitivity, irritation, and pain.[11,14] Systemic absorption of 5-FU cream is theoretically possible but has not been reported in the literature for penile cancer.
Imiquimod is an immune response modifier that acts through toll-like receptor 7 (TLR7) to activate cytokines such as interferon-alpha, interleukin-6, and tumor necrosis factor-alpha. In the skin, it enhances the cutaneous immune response by recruiting and activating Langerhans cells.[16] Imiquimod 5% cream has proven to be an effective treatment for various dermatologic pathologies such as genital warts, superficial basal cell carcinoma, and actinic keratosis. For penile CIS, imiquimod is typically reserved for partial responders or for recurrence after 5-FU therapy. This is supported by several case reports demonstrating success using imiquimod as the primary treatment for penile CIS.[17,18,19,20,21] However, it has never been studied prospectively or compared to 5-FU. The application protocol is similar to that of 5-FU. Adverse events include itching, erythema, hypopigmentation, tenderness, bleeding, and crusting.[17,21] Future studies must examine 5-FU and imiquimod cream in a prospective, randomized controlled manner. Due to their differing mechanisms of action, it will also be important to elucidate their synergistic properties in combination therapy.
LASER TREATMENT
In the well-selected patient, population recurrence rates of laser ablation can approach those of surgical excision. The CO2 laser (depth of penetration 0.1 mm) and the neodymium-doped yttrium aluminum garnet (Nd:YAG) laser (depth of penetration 6 mm) are the two most commonly used lasers although the use of argon and KTP has also been reported.
Overall local recurrence rates for CIS and T1 penile cancer have been reported at 14%–23% with a CO2 laser and 10%–48% with Nd: YAG.[22] As expected, studies including premalignant lesions had lower recurrence rates,[23] whereas other series with larger number of patients with T2 disease had much higher disease recurrence rates.[24] This calls into question the suitability of laser ablation for higher stage disease although it is an appropriate alternative for properly counseled patients who refuse surgery or as adjunct treatment in a multimodal approach. Frimberger et al. reported on 29 patients treated with Nd: YAG laser with recurrence in only two (6.9%).[25] The majority of patients had CIS or T1 disease with overall mean follow-up of 46.7 months. Schlenker et al.[26] reported recurrence in 42% of 54 patients with Tis-T2 tumors over a longer mean follow-up of 87 months. Notably, eight patients had recurrence at >53 months emphasizing the need for long-term follow-up in this treatment group. Despite the high recurrence rate, there was no effect seen on lymph node status or penile cancer-specific death. Both studies used acetic acid mapping before ablation and intraoperative frozen sections to confirm negative margins.
Combination laser therapy has also been reported with CO2 to eradicate macroscopic tumor followed by Nd:YAG for deep coagulation of the tumor bed. Windahl and Hellsten[27] reported 19% overall local recurrence rate and 3% disease-specific mortality in a prospective trial using this two-pronged approach with a mean follow-up of 42 months for patients with CIS-T3 disease, expanding on the previous experience in Sweden.[28] For low-stage tumors, recurrence rates were 14% for CIS and 25% for T1.
The 2014 European Association of Urology (EAU) guidelines on penile cancer give laser therapy a Grade C recommendation for Tis, Ta, or T1a tumors only.[6] The authors suggest follow-up with physical examination for at least 5 years. However, due to not uncommon reports of late recurrence, others have suggested follow-up out to 10 years.[24] As with all organ-preserving therapy, the overarching goal is the better cosmetic outcome and decreased psychological burden without sacrificing oncologic control. Importantly, the majority of men treated in this manner seem to be satisfied with their cosmetic and functional outcome.[22,27,29]
RADIATION THERAPY
Radiation therapy as a primary mode of treatment for penile cancers offers curative potential while maintaining relative preservation of organ form and function. In both the EAU and National Comprehensive Cancer Network (NCCN) guidelines, radiotherapy is included as a penile sparing option for T1a, T1b, and select T2 tumors.[30,31] NCCN guidelines include radiation as an option for T3–T4 tumors as well. Radiation can be delivered in two forms, external-beam radiation therapy (EBRT) or interstitial brachytherapy. Both modalities require circumcision before treatment to ensure adequate exposure and limit necrosis and phimosis. Like most treatment modalities for penile cancer, analysis is limited to smaller retrospective studies with varying inclusion criteria.
EBRT is a widely available treatment modality, delivers a homogenous dose of radiation, and requires less skill than brachytherapy. While original series describe using doses of 50–55 Gy, contemporary doses range from 60 to 75 Gy.[32,33,34] Most available studies on EBRT used significantly smaller doses than contemporary management dictates. In series published from 1993 to 2007, EBRT offered 5-year local control rates of 40%–69.7% with penile preservation rates of 50-65%. Cancer-specific survival in these series ranged from 58% to 86%.[32,33,34,35,36] Factors that predicted poor response to EBRT included dose <60 Gy, treatment course >45-day, daily dose of <2 Gy per day, cT3 tumors, and tumor size >4 cm.[32,35,36] When local control is not adequately achieved with EBRT, subsequent partial or total penectomy may be offered.
Brachytherapy most commonly involves percutaneous placement of interstitial iridium-192 needles into the glans penis which remain in place for 4-5 days, whereas the patient remains supine to deliver doses of 50–65 Gy.[32] Retrospective series from 1984 to 2009 demonstrate 5-year local control ranging from 70% to 87% and 5-year cancer-specific survival of 72%–88%.[32,37,38] Penile preservation at 5 and 10 years was 74%–88% and 67%–70%, respectively. Treatment failure was correlated with tumor size >4 cm and insufficient needle spacing.[1,2,3,32,37,38]
Side effects of radiotherapy include both early and late sequelae. In the early postradiation period, moist desquamation is expected and typically resolves within 4–8 weeks with conservative management. The two most common late sequelae include meatal stenosis and soft-tissue ulceration. Stenosis is reported in 10%–15% of EBRT patients compared to 10%–45% in brachytherapy.[32] Soft-tissue ulceration is reported in 0%–23% of patients and appears to be more common in brachytherapy. Necrosis requiring penectomy has been reported in up to 6.8% of brachytherapy patients.[39] Other side effects reported include fistulae, necrosis, pain, and edema.[4,40] While organ preservation outcomes are well-reported, functional outcomes are relatively poorly reported in current literature.
Radiation therapy for penile cancer in carefully selected cases offers the opportunity for local control and organ preservation. The ideal cancers for radiation appear to be tumors limited to the distal penis, stage ≤T1/T2, and ≤4 cm in size. In retrospective series, brachytherapy appears superior to EBRT in local control and organ preservation, whereas EBRT requires less technical skill and may be more widely available. Extended follow-up is necessary as recurrences have been reported beyond 2 years.[37]
MOHS SURGERY
Mohs micrographic surgery (MMS) entails intraoperative, cross-sectional frozen sections reviewed in real time by the surgeon until a negative plane is encountered. Mohs reported the first series of MMS applied to penile cancer in 1985.[41] Their group experienced excellent results when the cancer was distal (glans or prepuce) with 5-year cure rates of 81% which fell to 57% with shaft lesions. Lesion size and prior treatment also significantly affected outcomes. Importantly, no patient had a functional deficit regarding urinary or sexual outcome. Soon after, Brown et al.[42] reported on 20 patients, 11 of whom had invasive squamous cell carcinoma (SCC) with a 29% recurrence rate over 3 years. In a more contemporary case series, Shindel et al. reported on 33 patients CIS-T3 (majority CIS) who underwent MMS with 32% recurrence rate with 2/22 patients progressing and 1 death from penile CA.[43] Most recurrences were managed successfully with repeat MMS.
Although MMS is generally accepted as appropriate for small, distal lesions, there have been reports of successful outcomes for more aggressive applications.[44] Machan et al. reported on 42 patients with overall recurrence rate of 11.1% including 16 patients with invasive SCC, four tumors with urethral invasion, and two into the corpora. The NCCN guidelines suggest that Mohs surgery may be particularly useful for superficial lesions on the proximal shaft to avoid total penectomy for a low-risk cancer.[45]
While an attractive option in select patients, the use of Moh's surgery for low stage penile cancer has not been widely adopted due to nuanced technical details vital to a successful procedure and availability of skilled practitioners outside of a few specialized centers worldwide. May want to mention something about why it is not used more often, mostly the technical limitations and availability of expertise.
PENILE-SPARING SURGERY
PSS is an attractive option for patients with low-stage penile cancer. Djajadiningrat et al. retrospectively assessed patients who underwent PSS and found that the while the 5-year cumulative incidence of local recurrence was significantly higher at 27% versus 3.8% (P < 0.0001) for radical penile surgery, the overall 5-year cancer-specific survival was no difference between the respective surgical approaches.[46] Lont et al.[47] found that even in cases of local recurrence after PSS, local control could be achieved in 94% of patients (Lont, 2006). Patients undergoing PSS generally have a good cosmetic outcome and adequate erectile function.[48] Li et al. found that out of 29 patients who underwent PSS for low-stage penile cancer, all claimed to be satisfied with urination, and only 1 reported decreased sexual function.[49] In patients who underwent (partial) penectomy as opposed to those who underwent PSS, there were significantly more issues with orgasm (P = 0.031), appearance (P = 0.008), life interference (0.032), and urinary function (P < 0.0001).[50]
The surgical options for patients with low-stage penile cancer can range from a simple circumcision to more involved procedures such as total glansectomy with neoglans formation using a split-thickness skin graft. Since the majority of penile cancer cases involve uncircumcised men, circumcision has been proposed as an attractive penile-sparing modality in patients with lesions involving the prepuce (preferably distal). Circumcision can be associated with a high rate of local recurrence, and hence, motivated patients who will adhere to a strict follow-up regimen should undergo this procedure.[49] Wide-local excision (WLE) has gained increasing adoption due in part to the aforementioned discussion of adequate surgical margins for oncological control. WLE can be used for up to T2 glans lesions and T1G2 tumors of the shaft but should not be performed in tumors with concurrent CIS or involving more than half the glans or with urethral involvement.[51] Glans resurfacing, either total or partial depending on the extent of the lesion, is an attractive option for patients with CIS. In general, the glans epithelium and subepithelium are resected until macroscopically clear margins are encountered, and then split-thickness skin grafting is performed. Shabbir et al. found that 96% of patients had complete take of their graft. About 28% of patients required further surgical intervention for positive surgical margins, but oncological outcomes were not compromised.[52] In patients with small isolated tumors of the glans with adequate skin coverage after surgical extirpation, the surgeon can consider performing a partial glansectomy with primary closure [Figure 1]. In these cases, it is critical to obtain intraoperative frozen sections to ensure negative margins. In uncircumcised patients requiring additional tissue coverage that can be provided with primary closure, a primary glansectomy with outer preputial flap is an attractive option as this area is often uninvolved by tumor [Figure 2]. For more extensive lesions of the glans not amenable to partial glansectomy (Ta, T1, or even T2), a total glansectomy, with or without distal corporectomy depending on the extent of the lesion, with split-thickness skin grafting can be performed [Figure 3]. Frozen sections are necessary in these cases to insure negative surgical margins. Quilting sutures are placed throughout the graft to help immobilize it and optimize graft take. A recent study by Parnham et al. showed that 9.3% of patients had a local recurrence and penile cancer-specific mortality was 10.7%. There was additionally a 9% reoperation rate for complications such as graft loss and meatal stenosis.[53] It is once again important to underscore the need for close long-term follow-up in patients undergoing PSS due to higher rates of local recurrence. Overall, in carefully selected patients, PSS can provide an oncologically sound procedure with better cosmetic and sexual outcomes than its more radical counterparts in low-stage penile cancer.
Figure 1.
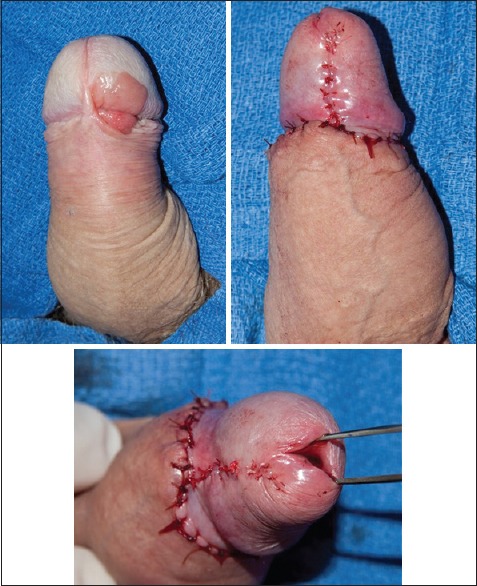
Partial glansectomy with primary closure
Figure 2.
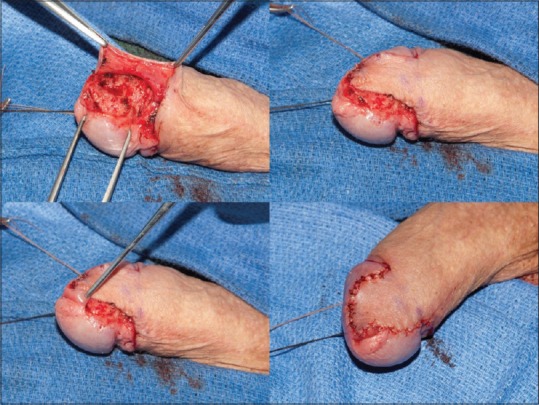
Partial glansectomy with preputial flap
Figure 3.
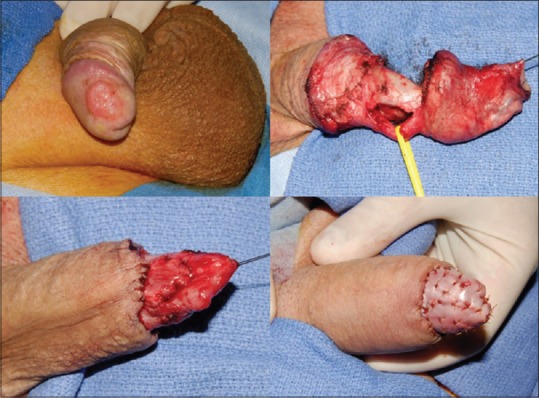
Total glansectomy with distal corporectomy and split-thickness split grafting for neoglans formation
CONCLUSION
Penile-sparing modalities in the treatment of low-stage penile cancer provide patients with excellent oncologic, cosmetic, sexual, psychosocial, and quality of life outcomes. Clinicians should consider the aforementioned options when treating patients with low-stage penile cancer before proceeding to more radical treatment options.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, et al. Penile cancer: Importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer. 2005;116:606–16. doi: 10.1002/ijc.21009. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, Giuliano AR. Incidence trends in primary malignant penile cancer. Urol Oncol. 2007;25:361–7. doi: 10.1016/j.urolonc.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 4.Burt LM, Shrieve DC, Tward JD. Stage presentation, care patterns, and treatment outcomes for squamous cell carcinoma of the penis. Int J Radiat Oncol Biol Phys. 2014;88:94–100. doi: 10.1016/j.ijrobp.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Maddineni SB, Lau MM, Sangar VK. Identifying the needs of penile cancer sufferers: A systematic review of the quality of life, psychosexual and psychosocial literature in penile cancer. BMC Urol. 2009;9:8. doi: 10.1186/1471-2490-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hakenberg OW, Compérat EM, Minhas S, Necchi A, Protzel C, Watkin N, et al. EAU guidelines on penile cancer: 2014 update. Eur Urol. 2015;67:142–50. doi: 10.1016/j.eururo.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Minhas S, Kayes O, Hegarty P, Kumar P, Freeman A, Ralph D, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int. 2005;96:1040–3. doi: 10.1111/j.1464-410X.2005.05769.x. [DOI] [PubMed] [Google Scholar]
- 8.Philippou P, Shabbir M, Malone P, Nigam R, Muneer A, Ralph DJ, et al. Conservative surgery for squamous cell carcinoma of the penis: Resection margins and long-term oncological control. J Urol. 2012;188:803–8. doi: 10.1016/j.juro.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Leijte JA, Kirrander P, Antonini N, Windahl T, Horenblas S. Recurrence patterns of squamous cell carcinoma of the penis: Recommendations for follow-up based on a two-centre analysis of 700 patients. Eur Urol. 2008;54:161–8. doi: 10.1016/j.eururo.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–8. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 11.Goette DK, Carson TE. Erythroplasia of Queyrat: Treatment with topical 5-fluorouracil. Cancer. 1976;38:1498–502. doi: 10.1002/1097-0142(197610)38:4<1498::aid-cncr2820380409>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Lewis RJ, Bendl BJ. Erythroplasia of queyrat: Repor. Of a patient successfully treated with topical 5-fluorouracil. Can Med Assoc J. 1971;104:148–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Bargman H, Hochman J. Topical treatment of Bowen's disease with 5-fluorouracil. J Cutan Med Surg. 2003;7:101–5. doi: 10.1007/s10227-002-0158-6. [DOI] [PubMed] [Google Scholar]
- 14.Alnajjar HM, Lam W, Bolgeri M, Rees RW, Perry MJ, Watkin NA, et al. Treatment of carcinoma in situ of the glans penis with topical chemotherapy agents. Eur Urol. 2012;62:923–8. doi: 10.1016/j.eururo.2012.02.052. [DOI] [PubMed] [Google Scholar]
- 15.Hegarty PK, Shabbir M, Hughes B, Minhas S, Perry M, Watkin N, et al. Penile preserving surgery and surgical strategies to maximize penile form and function in penile cancer: Recommendations from the United Kingdom experience. World J Urol. 2009;27:179–87. doi: 10.1007/s00345-008-0312-x. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H, Wang B, Shivji GM, Toto P, Amerio P, Tomai MA, et al. Imiquimod, a topical immune response modifier, induces migration of Langerhans cells. J Invest Dermatol. 2000;114:135–41. doi: 10.1046/j.1523-1747.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder TL, Sengelmann RD. Squamous cell carcinoma in situ of the penis successfully treated with imiquimod 5% cream. J Am Acad Dermatol. 2002;46:545–8. doi: 10.1067/mjd.2002.120444. [DOI] [PubMed] [Google Scholar]
- 18.Orengo I, Rosen T, Guill CK. Treatment of squamous cell carcinoma in situ of the penis with 5% imiquimod cream: A case report. J Am Acad Dermatol. 2002;47:S225–8. doi: 10.1067/mjd.2002.126580. [DOI] [PubMed] [Google Scholar]
- 19.Taliaferro SJ, Cohen GF. Bowen's disease of the penis treated with topical imiquimod 5% cream. J Drugs Dermatol. 2008;7:483–5. [PubMed] [Google Scholar]
- 20.Danielsen AG, Sand C, Weismann K. Treatment of Bowen's disease of the penis with imiquimod 5% cream. Clin Exp Dermatol. 2003;28 Suppl 1:7–9. doi: 10.1046/j.1365-2230.28.s1.3.x. [DOI] [PubMed] [Google Scholar]
- 21.Micali G, Nasca MR, De Pasquale R. Erythroplasia of Queyrat treated with imiquimod 5% cream. J Am Acad Dermatol. 2006;55:901–3. doi: 10.1016/j.jaad.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 22.Leone A, Inman B, Spiess PE. Need for evidence and consensus on laser treatment for management of select primary penile tumors. Eur Urol. 2017;72:4–6. doi: 10.1016/j.eururo.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 23.Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology. 1998;52:559–65. doi: 10.1016/s0090-4295(98)00308-2. [DOI] [PubMed] [Google Scholar]
- 24.Meijer RP, Boon TA, van Venrooij GE, Wijburg CJ. Long-term follow-up after laser therapy for penile carcinoma. Urology. 2007;69:759–62. doi: 10.1016/j.urology.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 25.Frimberger D, Hungerhuber E, Zaak D, Waidelich R, Hofstetter A, Schneede P, et al. Penile carcinoma. Is Nd: YAG laser therapy radical enough? J Urol. 2002;168:2418–21. doi: 10.1016/S0022-5347(05)64158-4. [DOI] [PubMed] [Google Scholar]
- 26.Schlenker B, Tilki D, Seitz M, Bader MJ, Reich O, Schneede P, et al. Organ-preserving neodymium-yttrium-aluminium-garnet laser therapy for penile carcinoma: A long-term follow-up. BJU Int. 2010;106:786–90. doi: 10.1111/j.1464-410X.2009.09188.x. [DOI] [PubMed] [Google Scholar]
- 27.Windahl T, Andersson SO. Combined laser treatment for penile carcinoma: Results after long-term followup. J Urol. 2003;169:2118–21. doi: 10.1097/01.ju.0000067361.81295.a2. [DOI] [PubMed] [Google Scholar]
- 28.Windahl T, Hellsten S. Laser treatment of localized squamous cell carcinoma of the penis. J Urol. 1995;154:1020–3. [PubMed] [Google Scholar]
- 29.Windahl T, Skeppner E, Andersson SO, Fugl-Meyer KS. Sexual function and satisfaction in men after laser treatment for penile carcinoma. J Urol. 2004;172:648–51. doi: 10.1097/01.ju.0000132891.68094.87. [DOI] [PubMed] [Google Scholar]
- 30.Clark PE, Spiess PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, et al. Penile cancer: Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2013;11:594–615. doi: 10.6004/jnccn.2013.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pizzocaro G, Algaba F, Horenblas S, Solsona E, Tana S, Van Der Poel H, et al. EAU penile cancer guidelines 2009. Eur Urol. 2010;57:1002–12. doi: 10.1016/j.eururo.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 32.Crook J, Ma C, Grimard L. Radiation therapy in the management of the primary penile tumor: An update. World J Urol. 2009;27:189–96. doi: 10.1007/s00345-008-0309-5. [DOI] [PubMed] [Google Scholar]
- 33.McLean M, Akl AM, Warde P, Bissett R, Panzarella T, Gospodarowicz M, et al. The results of primary radiation therapy in the management of squamous cell carcinoma of the penis. Int J Radiat Oncol Biol Phys. 1993;25:623–8. doi: 10.1016/0360-3016(93)90008-j. [DOI] [PubMed] [Google Scholar]
- 34.Neave F, Neal AJ, Hoskin PJ, Hope-Stone HF. Carcinoma of the penis: A retrospective review of treatment with iridium mould and external beam irradiation. Clin Oncol (R Coll Radiol) 1993;5:207–10. doi: 10.1016/s0936-6555(05)80230-4. [DOI] [PubMed] [Google Scholar]
- 35.Sarin R, Norman AR, Steel GG, Horwich A. Treatment results and prognostic factors in 101 men treated for squamous carcinoma of the penis. Int J Radiat Oncol Biol Phys. 1997;38:713–22. doi: 10.1016/s0360-3016(97)00068-0. [DOI] [PubMed] [Google Scholar]
- 36.Gotsadze D, Matveev B, Zak B, Mamaladze V. Is conservative organ-sparing treatment of penile carcinoma justified? Eur Urol. 2000;38:306–12. doi: 10.1159/000020298. [DOI] [PubMed] [Google Scholar]
- 37.Mazeron JJ, Langlois D, Lobo PA, Huart JA, Calitchi E, Lusinchi A, et al. Interstitial radiation therapy for carcinoma of the penis using iridium 192 wires: The Henri Mondor experience (1970-1979) Int J Radiat Oncol Biol Phys. 1984;10:1891–5. doi: 10.1016/0360-3016(84)90268-2. [DOI] [PubMed] [Google Scholar]
- 38.Kiltie AE, Elwell C, Close HJ, Ash DV. Iridium-192 implantation for node-negative carcinoma of the penis: The cookridge hospital experience. Clin Oncol (R Coll Radiol) 2000;12:25–31. doi: 10.1053/clon.2000.9106. [DOI] [PubMed] [Google Scholar]
- 39.Kelley CD, Arthur K, Rogoff E, Grabstald H. Radiation therapy of penile cancer. Urology. 1974;4:571–3. doi: 10.1016/0090-4295(74)90492-0. [DOI] [PubMed] [Google Scholar]
- 40.Cordoba A, Escande A, Lopez S, Mortier L, Mirabel X, Coche-Déqueant B, et al. Low-dose brachytherapy for early stage penile cancer: A 20-year single-institution study (73 patients) Radiat Oncol. 2016;11:96. doi: 10.1186/s13014-016-0676-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohs FE, Snow SN, Messing EM, Kuglitsch ME. Microscopically controlled surgery in the treatment of carcinoma of the penis. J Urol. 1985;133:961–6. doi: 10.1016/s0022-5347(17)49334-7. [DOI] [PubMed] [Google Scholar]
- 42.Brown MD, Zachary CB, Grekin RC, Swanson NA. Penile tumors: Their management by Mohs micrographic surgery. J Dermatol Surg Oncol. 1987;13:1163–7. doi: 10.1111/j.1524-4725.1987.tb02427.x. [DOI] [PubMed] [Google Scholar]
- 43.Shindel AW, Mann MW, Lev RY, Sengelmann R, Petersen J, Hruza GJ, et al. Mohs micrographic surgery for penile cancer: Management and long-term followup. J Urol. 2007;178:1980–5. doi: 10.1016/j.juro.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 44.Machan M, Brodland D, Zitelli J. Penile squamous cell carcinoma: Penis-preserving treatment with Mohs micrographic surgery. Dermatol Surg. 2016;42:936–44. doi: 10.1097/DSS.0000000000000795. [DOI] [PubMed] [Google Scholar]
- 45.Network, N.C.C. Penile Cancer Version 1. 2017. [Last accessed on 2017 Mar 09]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/penile.pdf .
- 46.Djajadiningrat RS, van Werkhoven E, Meinhardt W, van Rhijn BW, Bex A, van der Poel HG, et al. Penile sparing surgery for penile cancer-does it affect survival? J Urol. 2014;192:120–5. doi: 10.1016/j.juro.2013.12.038. [DOI] [PubMed] [Google Scholar]
- 47.Lont AP, Gallee MP, Meinhardt W, van Tinteren H, Horenblas S. Penis conserving treatment for T1 and T2 penile carcinoma: clinical implications of a local recurrence. J Urol. 2006;176:575–80. doi: 10.1016/j.juro.2006.03.063. [DOI] [PubMed] [Google Scholar]
- 48.Veeratterapillay R, Sahadevan K, Aluru P, Asterling S, Rao GS, Greene D, et al. Organ-preserving surgery for penile cancer: Description of techniques and surgical outcomes. BJU Int. 2012;110:1792–5. doi: 10.1111/j.1464-410X.2012.11084.x. [DOI] [PubMed] [Google Scholar]
- 49.Li J, Zhu Y, Zhang SL, Wang CF, Yao XD, Dai B, et al. Organ-sparing surgery for penile cancer: Complications and outcomes. Urology. 2011;78:1121–4. doi: 10.1016/j.urology.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 50.Kieffer JM, Djajadiningrat RS, van Muilekom EA, Graafland NM, Horenblas S, Aaronson NK, et al. Quality of life for patients treated for penile cancer. J Urol. 2014;192:1105–10. doi: 10.1016/j.juro.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 51.Sosnowski R, Kuligowski M, Kuczkiewicz O, Moskal K, Wolski JK, Bjurlin MA, et al. Primary penile cancer organ sparing treatment. Cent European J Urol. 2016;69:377–83. doi: 10.5173/ceju.2016.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shabbir M, Muneer A, Kalsi J, Shukla CJ, Zacharakis E, Garaffa G, et al. Glans resurfacing for the treatment of carcinoma in situ of the penis: Surgical technique and outcomes. Eur Urol. 2011;59:142–7. doi: 10.1016/j.eururo.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 53.Parnham AS, Albersen M, Sahdev V, Christodoulidou M, Nigam R, Malone P, et al. Glansectomy and split-thickness skin graft for penile cancer. Eur Urol. 2016 doi: 10.1016/j.eururo.2016.09.048. pii: S0302-2838(16)30688-1. [DOI] [PubMed] [Google Scholar]


