Abstract
Purpose:
To analyze the prevalence and resistance rates of bacterial agents causing urinary tract infections (UTIs) in Aseer, Saudi Arabia (2013–2016).
Patients and Methods:
This was a 4-year (2013–2016) retrospective study undertaken in Aseer Central Hospital, Saudi Arabia. A total of 49,779 urine and other UT specimens obtained from patients suspected of having a UTI were analyzed. Urine specimens were inoculated onto cystine lactose electrolyte deficient agar following standard procedures. Cultures showing significant bacteriuria were subjected to identification and sensitivity testing using VITEK 2 system. Data of patients and uropathogens were assembled, checked, and analyzed using SPSS software.
Results:
Culture positive samples were 49,779 (59.9% males, 40.1% females; P = 0.000). Year trend showed significant variations (P = 0.000) and the forecast trend line hypothesized a clear rise. Age groups 70–79 years were the most vulnerable group (22.3%). Gram-negative bacilli were 91.8% and the major species were Escherichia coli - 39.7%, Klebsiella pneumoniae - 15.8%; Pseudomonas aeruginosa - 13.8%, Proteus mirabilis - 10.6%, and Acinetobacter baumannii - 5%. Antimicrobials with high sensitivity rate were linezolid (99.1%), daptomycin (89.3%), vancomycin (86.7%), teicoplanin (85.5%), ertapenem (85.1%), fosfomycin (82.1%), and tigecycline (80.2%). High resistant rates to uropathogens were encountered with cephalothin (89.8%), nalidixic acid (86.7%), and ampicillin (81.9%).
Conclusions:
The majority of uropathogens were resistant to antibiotics commonly used in clinical practice. Linezolid, daptomycin, and vancomycin showed the lowest resistance to all uropathogens; this can be revised for empirical treatment of UTIs. Continuous surveillance of uropathogens and their susceptibility is important.
Keywords: Aseer, drug resistance, in vitro assay, Saudi Arabia, urinary tract infections
INTRODUCTION
Urinary tract infections (UTIs) are the most frequent bacterial infections in health settings. UTIs are everyday infections dealt with in outpatient clinics which are ranging from slightly symptomatic cystitis to severe septic shock. Recognition of the predominant uropathogens and their regional resistance patterns is essential to establish the antimicrobial course of action and infection control strategies in hospitals.[1,2] Escherichia coli is the most common commensal bacterium causing infections in humans and animals and serves as a common cause of UTIs and bacteremia in humans.[3] E. coli, Pseudomonas, and Proteus species are the frequently encountered bacterial isolates in UTIs with resistant to commonly used antibiotics in clinical practices and especially those antimicrobials available to patients without prescription. Other studies found E. coli and Klebsiella pneumoniae are the most common,[4] but exact distribution of other pathogen is not fully studies. Strains described as extraintestinal pathogenic E. coli set off a range of infections at extraintestinal sites including the urinary tract (UT), biliary system, and central nervous system. These infections are prevalent both in nosocomial and in community settings.[5] UTIs, although treatable, is now becoming increasingly hard to control because of rampant antimicrobial resistance in the Enterobacteriaceae family, particularly in E. coli.[3,6] These organisms are to blame for substantial social and economic burdens.[7]
The annual prevalence of extended-spectrum beta-lactamase (ESBL) infection ranged from 1.3 to 2.5%. After performing univariate and multivariate regression analysis, the main risk factors for ESBL infections were identified as use of antibiotics the year preceding the admission, duration of catheter use, and bladder washout.[8] Asian countries present the highest rates of drug resistance in general. Imipenem showed an overall resistance rate below 10%, as an example of a drug with - up to now - good rate. Information of drug resistance data at regional and local levels is essential to enhance antimicrobial treatment in urological patients with nosocomial UTIs.[9] Culture and in vitro antimicrobial assays would be necessary before initiating a medication program.[1] Rising resistance rates among uropathogens have obscured management of acute cystitis. Hence, individualized assessment of risk factors for resistance and regimen tolerability is needed to choose the optimum empirical regimen.[7]
The purpose of this study was to analyze the prevalence and resistance rates of bacterial agents causing UTIs in Aseer, Saudi Arabia, in 4 years (2013–2016).
PATIENTS AND METHODS
Design
A retrospective study conducted between January 2013 and June 2016.
Setting
The study was conducted at Aseer Central Hospital (ACH), a large tertiary teaching hospital, southern Saudi Arabia, and the Department of Microbiology, College of Medicine, King Khalid University.
Ethical considerations
The study was conducted in accordance with the Institutional Review Board Declaration of ACH, and the protocol was approved by the Ethics Committee of King Khalid University (REC#2016-07-07).
Specimens
A total of 49,779 urine and other UT specimens obtained from patients suspected of having a UTI were analyzed. More than one sample per patient was possible and repeated according to clinical requests.
Data were collected from cultured positive UT specimens. Valid data entries were 49,779 specimens, urine was the main clinical specimen cultured; resultant pathogens underwent identification to species level and the in vitro antimicrobial assay.
Culture and sensitivity testing
Urine samples were inoculated onto Cystine Lactose Electrolyte Deficient (CLED; BD, Becton Dickinson GmbH) agar by streak plate method following the standard microbiological procedures.[10,11] Isolates obtained were initially identified using selected bench tests and conventional methods.[10,11] We defined a positive culture as a clean-catch midstream urine specimen with a growth of 105 cfu/mL of a single microorganism or mixed flora with a predominant species. Negative urine culture was defined as no growth, insufficient growth, or a mixed microbial flora with no predominant organism.[11]
Identities of isolates were then confirmed using the fully identified VITEK 2 automated bacterial identification system (VITEK 2 Compact; bioMérieux, Paris, France). VITEK 2 utilizes an optimized colorimetric redox indicator to detect active growth of an organism in the presence of the antimicrobial. The organism to be tested is grown on a nonselective medium in appropriate conditions for 16–18 h, before a 0.5 McFarland suspension is prepared. This suspension is inoculated into the appropriate antimicrobial susceptibility testing panel (AST-N291) that contains microwells prelined with increasing concentrations of antimicrobial. The panel is incubated at 35°C on the instrument for up to 16 h and automatically read every 20 min for growth. The minimum inhibitory concentration for each antimicrobial is then determined by the concentration at which the organism fails to grow.
Antimicrobial susceptibility testing
The susceptibility panel containing serial two-fold dilutions of amikacin (concentration range: 8–64 μg/ml), amoxicillin/clavulanic acid (concentration range: 4/2–32/16 μg/ml), and ampicillin (concentration range: 4–32 μg/ml), cefalotin (concentration range: 2–32 μg/ml), cefepime (concentration range: 2–32 μg/ml), cefoxitin (concentration range: 8–32 μg/ml) of ceftazidime (concentration range: 1–32 μg/ml), ceftriaxone (concentration range: 2–32 g/ml), ciprofloxacin (concentration range: 0.5–4 μg/ml), gentamicin (concentration range: 4–32 μg/ml), imipenem (concentration range: 1–12 μg/ml), meropenem (concentration range: 1–12 μg/ml), nitrofurantoin (concentration range: 0.5–12 μg/ml), piperacillin/tazobactam (concentration range: 4/2–48/8 μg/ml), tigecycline (concentration range: 0.75–4 μg/ml), and trimethoprim/sulfamethoxazole (concentration range: 1/19–16/304 μg/ml) were provided by the manufacturer. Stock inoculum suspensions of the bacterial isolates were obtained from 24-h cultures on blood agar plates at 37°C. Inoculum suspensions for the VITEK 2 system were prepared in sterile saline to turbidity equal to a 0.5 McFarland standard.
Statistical analysis
Registered infection data of uropathogens were assembled and checked as a retrospective epidemiological and microbiological survey. Data were collected on patient demographics, comorbid diagnoses and laboratory parameters from the hospital electronic patient record, and culture results from electronic microbiology records. Data were analyzed using SPSS software SPSS Inc. Released 2007. SPSS for Windows, Version 16.0. (Chicago, SPSS Inc.). Descriptive statistics were reported as mean with standard deviation or percentages where appropriate. Continuous variables were analyzed using t-tests for independent samples, and categorical variables were analyzed using Chi-squared test. The results were evaluated with 95% confidence intervals. P < 0.05 was considered statistically significant.
RESULTS
The results of the present survey revealed that out of the 49,779 culture positive samples, 29,820 were males (59.9%) and 19,973 were females (40.1%) (P = 0.0001). Trend according to years showed a significant variations (P = 0.000) with a decline from 2013 to 2014, no change in 2014 to 2015, and rise in 2016 but the forecast trend line hypothesizing a clear rise [Figure 1].
Figure 1.
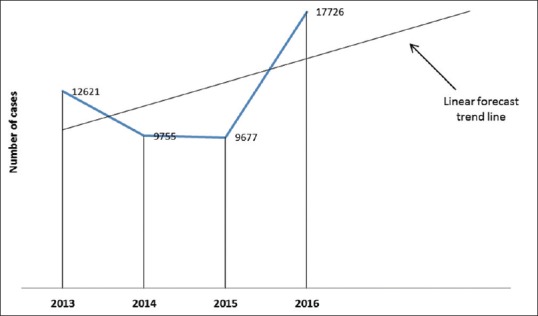
Rate of uropathogens in Aseer region, Saudi Arabia, in 4 years (2013–2016)
The most vulnerable age groups were those between 70 and 79 years old comprising 22.3% followed by 80–89 years old comprising 17.0%; 60–69 years 14% and 20–29 years, 12.1%. Distribution of other age groups is shown in Figure 2.
Figure 2.
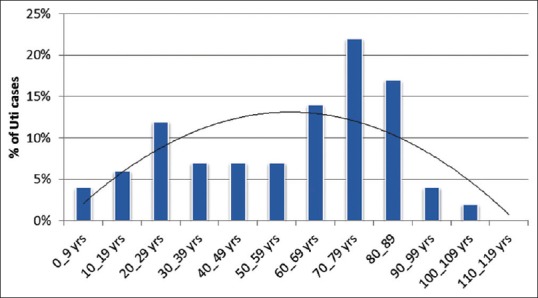
Rate of uropathogens in Aseer region, Saudi Arabia, according to age groups
Gram-negative bacilli were 91.8% including 16,478 cases caused by E. coli (39.7%); 6570 caused by K. pneumoniae (15.8); 5731 caused by P. aeruginosa 13.8%; 4386 caused by P. mirabilis (10.6%); and 2096 caused by A. baumannii (5%) [Figure 3]. Other species with lesser frequencies were Morganella morganii, 4.2%; Providencia stuartii, 4.1%; Enterococcus faecalis, 4.1%; and Enterobacter cloacae, 2.8% [Table 1 and Figure 4].
Figure 3.
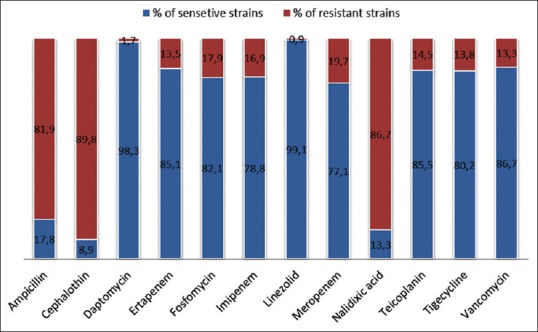
Sensitivity and resistance rates (%) of uropathogens in 4 years (2013–2016) from Aseer, Saudi Arabia, to some antimicrobial agents
Table 1.
Percentages of sensitive and resistant strains of the dominant uropathogens (>1% counts) screened against key antimicrobial agents between 2013 and 2016 in Aseer region, Saudi Arabia
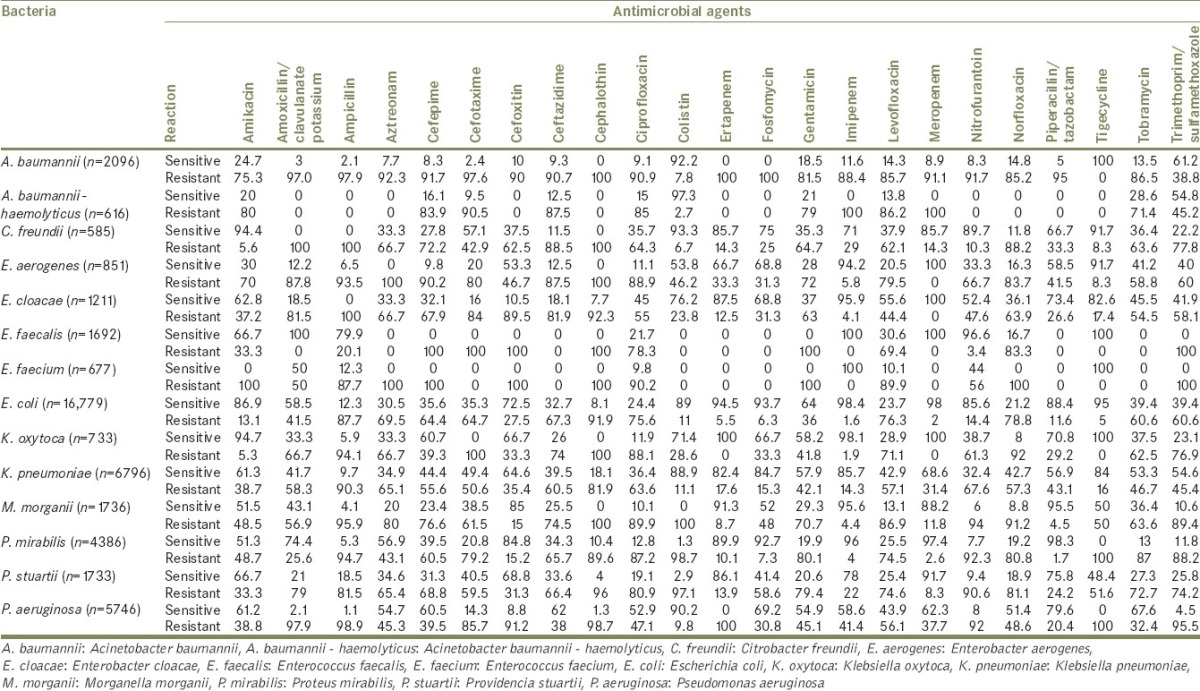
Figure 4.
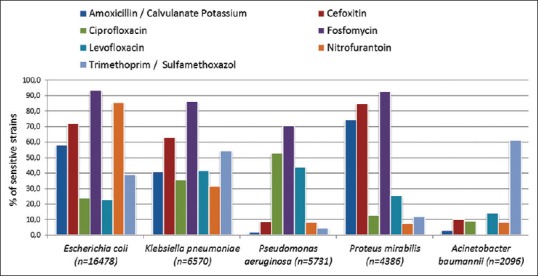
Most common bacterial species causing urinary tract infections in Aseer region and their sensitivity (%) to some empiric antimicrobial agents
Antimicrobials with notably high sensitivity rates to all culture positive uropathogens were linezolid (99.1%) followed by daptomycin (89.3%), vancomycin (86.7%), teicoplanin (85.5%), ertapenem (85.1%), fosfomycin (82.1%), and tigecycline (80.2%). On the other hand, antimicrobials with notably high resistant rates to all culture positive UTIs were cephalothin (89.8%), nalidixic acid (86.7%), and ampicillin (81.9%) [Figure 3].
DISCUSSION
The identification of the most common microorganisms causing infectious diseases and regional resistance patterns is important to determine the treatment policies and infection control guidelines in health-care units.[12] Gram-negative bacteria namely E. coli and K. pneumoniae were the most common uropathogens causing UTIs recorded in this study [Table 1 and Figure 4]. Our results are in agreement with universal findings. According to literature, the most UTIs are caused by Gram-negative bacteria as E. coli, Klebsiella spp., P. mirabilis, P. aeruginosa, Acinetobacter spp., and Serratia spp. and Gram-positive bacteria such as Enterococcus spp. and Staphylococcus spp.[13,14] Similar findings were reported from Aseer region.[15,16]
According to the statistical calculations, there was a significant association between UTIs caused by E. coli and female gender (P < 0.05). Standard approaches to checking susceptibility patterns can be inefficient. It is crucial that practitioners continue requesting for antimicrobial assay and developing novel approaches to identifying patients with risk factors for resistance to question the use of these agents in patients with uncomplicated UTIs.[17] Empirical antimicrobial selection would better establish on information of the local prevalence of specific uropathogens and their antimicrobial sensitivities rather than on universal guidelines because resistance patterns may vary in different regions.[15,18,19,20] In vitro resistance prevalence and the unfavorable ecological consequences of random antimicrobial therapy were contemplated essential in achieving best treatment choices.[21]
The current international and European clinical practice guidelines for treating acute uncomplicated UTIs include nitrofurantoin, trimethoprim-sulfamethoxazole, fosfomycin trometamol, pivmecillinam, fluoroquinolones (ofloxacin, ciprofloxacin, and levofloxacin), and β-lactam agents (amoxicillin-clavulanate, cefdinir, cefaclor, and cefpodoxime). However, the optimal treatment for each specific case is different according to gender, age group, and diagnosis. The findings of this study showed that the resistance rates of most of the uropathogens to most of the guideline-recommended antimicrobial agents are astonishingly high. However, many isolates showed a high sensitivity rate to imipenem (93%), followed by fosfomycin (86%), amikacin (87%), nitrofurantoin (81%), and amoxicillin/clavulanate (74%). The susceptibility of organisms to cotrimoxazole was found 50%. Other studies showed different recommendations. For example, uropathogens were found highly susceptible to nitrofurantoin and gentamicin.[15,22] These authors advised their inclusion in the empirical treatment of UTIs.
Due to a high level of antimicrobial resistance among the pathogens causing UTIs in countries as India,[2] it is cautious to advise or modify therapy, as far as possible, after culture and sensitivity testing has been performed. Regional surveillance programs are warranted for the development of national UTI guidelines. The latest guideline on uncomplicated UTIs features a modern tactic to the use of antibiotics in treating the common type of infection; this was designed to generate a continual improvement.[2,21]
In conclusion, the majority of uropathogens were resistant to antibiotics commonly used in clinical practices. Culture and sensitivity results are necessary before starting antimicrobial routine. Linezolid, daptomycin, and vancomycin were highly effective in vitro as they showed the minimum resistance to uropathogens in this study. These can be reviewed for empirical treatment of UTIs. On the other hand, cephalothin, nalidixic acid, and ampicillin revealed the maximum resistant rates. Continuous surveillance of trends in resistance patterns of uropathogens is critical. Long hospital stay is a significant risk of UTIs given the fact that more than one isolate from the same patient with variable sensitivities was found common in this survey.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Abejew AA, Denboba AA, Mekonnen AG. Prevalence and antibiotic resistance pattern of urinary tract bacterial infections in Dessie area, North-East Ethiopia. BMC Res Notes. 2014;7:687. doi: 10.1186/1756-0500-7-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalal BS, Nagaraj S. Urinary tract infections: A retrospective, descriptive study of causative organisms and antimicrobial pattern of samples received for culture, from a tertiary care setting. Germs. 2016;6:132–8. doi: 10.11599/germs.2016.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: A clinical update. Clin Microbiol Rev. 2005;18:657–86. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behzadi P, Behzadi E, Yazdanbod H, Aghapour R, Akbari Cheshmeh M, Salehian Omran D. A survey on urinary tract infections associated with the three most common uropathogenic bacteria. Maedica (Buchar) 2010;5:111–5. [PMC free article] [PubMed] [Google Scholar]
- 5.Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: An emerging public-health concern. Lancet Infect Dis. 2008;8:159–66. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 6.Russo TA, Johnson JR. Medical and economic impact of extraintestinal infections due to Escherichia coli: Focus on an increasingly important endemic problem. Microbes Infect. 2003;5:449–56. doi: 10.1016/s1286-4579(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 7.Grigoryan L, Trautner BW, Gupta K. Diagnosis and management of urinary tract infections in the outpatient setting: A review. JAMA. 2014;312:1677–84. doi: 10.1001/jama.2014.12842. [DOI] [PubMed] [Google Scholar]
- 8.Bouassida K, Jaidane M, Bouallegue O, Tlili G, Naija H, Mosbah AT. Nosocomial urinary tract infections caused by extended-spectrum beta-lactamase uropathogens: Prevalence, pathogens, risk factors, and strategies for infection control. Can Urol Assoc J. 2016;10:E87–93. doi: 10.5489/cuaj.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tandogdu Z, Cek M, Wagenlehner F, Naber K, Tenke P, van Ostrum E, et al. Resistance patterns of nosocomial urinary tract infections in urology departments: 8-year results of the global prevalence of infections in urology study. World J Urol. 2014;32:791–801. doi: 10.1007/s00345-013-1154-8. [DOI] [PubMed] [Google Scholar]
- 10.Baron EJ, Peterson LR, Finegold SM. 9th. St Louis: Mosby; 1994. Bailey and Scott's Diagnostic Microbiology; pp. 249–57. [Google Scholar]
- 11.Cheesebrough M. 2nd. London: Cambridge University Press; 2006. District Laboratory Practice in Tropical Countries. Part II; pp. 105–14. [Google Scholar]
- 12.Yilmaz Y, Tekkanat Tazegun Z, Aydin E, Dulger M. Bacterial uropathogens causing urinary tract infection and their resistance patterns among children in Turkey. Iran Red Crescent Med J. 2016;18:e26610. doi: 10.5812/ircmj.26610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kashef N, Djavid GE, Shahbazi S. Antimicrobial susceptibility patterns of community-acquired uropathogens in Tehran, Iran. J Infect Dev Ctries. 2010;4:202–6. doi: 10.3855/jidc.540. [DOI] [PubMed] [Google Scholar]
- 14.Beyene G, Tsegaye W. Bacterial uropathogens in urinary tract infection and antibiotic susceptibility pattern in Jimma university specialized hospital, Southwest Ethiopia. Ethiop J Health Sci. 2011;21:141–6. doi: 10.4314/ejhs.v21i2.69055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aseery LH, Alahmari EM, Alshabanah RF, Almohayya TS, Alasmari AM, Al Humayed SM, et al. Antimicrobial sensitivities of pathogens causing community acquired urinary tract infection in adult patients. J Basic Appl Sci Res. 2016;6:31–5. [Google Scholar]
- 16.Hamid ME, Mustafa FY, Alwaily A, Abdelrahman S, Al Azragi T. Prevalence of bacterial pathogens in aseer region, Kingdom of Saudi Arabia: Emphasis on antimicrobial susceptibility of Staphylococcus aureus. Oman Med J. 2011;26:368–70. doi: 10.5001/omj.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulder M, Kiefte-de Jong JC, Goessens WH, de Visser H, Hofman A, Stricker BH, et al. Risk factors for resistance to ciprofloxacin in community-acquired urinary tract infections due to Escherichia coli in an elderly population. J Antimicrob Chemother. 2017;72:281–9. doi: 10.1093/jac/dkw399. [DOI] [PubMed] [Google Scholar]
- 18.Al-Ghamdi MS. Empirical treatment of uncomplicated urinary tract infection by community pharmacist in the Eastern province of Saudi Arabia. Saudi Med J. 2001;22:1105–8. [PubMed] [Google Scholar]
- 19.Lane DR, Takhar SS. Diagnosis and management of urinary tract infection and pyelonephritis. Emerg Med Clin North Am. 2011;29:539–52. doi: 10.1016/j.emc.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Catal F, Bavbek N, Bayrak O, Karabel M, Karabel D, Odemis E, et al. Antimicrobial resistance patterns of urinary tract pathogens and rationale for empirical therapy in Turkish children for the years 2000-2006. Int Urol Nephrol. 2009;41:953–7. doi: 10.1007/s11255-008-9445-5. [DOI] [PubMed] [Google Scholar]
- 21.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103–20. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 22.Haque R, Akter ML, Salam MA. Prevalence and susceptibility of uropathogens: A recent report from a teaching hospital in Bangladesh. BMC Res Notes. 2015;8:416. doi: 10.1186/s13104-015-1408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


