Abstract
Introduction:
The horseshoe kidney (HSK) is the most common type of renal fusion anomaly. The incidence and characteristics of kidney stones in patients with HSK are not well studied. The aim of this meta-analysis was to evaluate the incidence and types of kidney stones in patients with HSK.
Methods:
A systematic literature search was performed using MEDLINE, EMBASE, and Cochrane Database of Systematic Reviews from the databases' inception through November 2016. Studies assessing the incidence and types of kidney stones in patients with HSK were included. We applied a random-effects model to estimate the incidence of kidney stones. The study protocol was registered with PROSPERO (International Prospective Register of Systematic Reviews; no. CRD42016052037).
Results:
A total of 14 observational studies with 943 patients (522 adults and 421 pediatric) with HSK were enrolled. The estimated pooled incidence of kidney stones was 36% (95% confidence interval [CI], 15%–59%) in adults with the HSK. Kidney stones were less common in pediatric patients with HSK with an estimated pooled incidence of 3% (95% CI, 2%–5%). The mean age of adult stone formers with HSK was 44.9 ± 6.2 years, and 75% were males. Within reported studies, 89.2% of kidney stones were calcium-based stones (64.2% calcium oxalate [CaOx], 18.8% calcium phosphate [CaP], and 6.2% mixed CaOx/CaP), followed by struvite stones (4.2%), uric acid stones (3.8%), and others (2.8%).
Conclusions:
Kidney stones are very common in adult patients with HSK with an estimated incidence of 36%. Calcium-based stones are the most prevalent kidney stones in adults with HSKs. These findings may impact the prevention and clinical management of kidney stones in patients with HSK.
Keywords: Horseshoe kidney, incidence, kidney stones, nephrolithiasis
INTRODUCTION
Kidney stone disease, also known as nephrolithiasis, is a common problem worldwide,[1,2,3,4,5,6,7,8] with a prevalence of 7% in the adults, and ≥30% recurrence rate within 10 years.[1,3,9] The incidence of kidney stones is globally increasing with an estimated prevalence ranging up to 15%.[10,11,12] During lifetime, approximately 7% of women and 13% of men will develop a kidney stone.[1,10,11,12]
Horseshoe kidney (HSK) is the most common abnormality renal fusion anomaly,[13] estimated to be 1/400–1600 births with the prevalence of 0.25% among the general population.[14,15,16] Its developmental abnormalities include the fusion of the lower poles, resulting in abnormal position of the ureter in the renal pelvis, and highly placed ureteropelvic junction.[17] Impaired drainage of the collecting system and associating ureteropelvic obstruction may predispose the patient to kidney stones.[14,18,19,20,21,22] Besides, metabolic factors have also been suggested to contribute to stone in patients with HSK.[15,18,23,24,25] Nevertheless, unlike the general population, the incidence and characteristics of kidney stones in patients with HSK are not well examined. The objective of this meta-analysis was to evaluate the incidence and types of kidney stones in patients with HSK.
EVIDENCE ACQUISITION
Search strategy
The protocol for this study is registered with PROSPERO (International Prospective Register of Systematic Reviews; no. CRD42016052037). We also followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.[26] Two investigators (ASP and CT) systematically reviewed the published literature and conference abstracts indexed in MEDLINE, EMBASE, and the Cochrane Databases from database inception through November 2016 without language restrictions, using the terms: “stone” or “nephrolithiasis” combined with “kidney” and “horseshoe”. Other pertinent references were obtained through manual review of these retrieved references.
Inclusion criteria and outcomes
The inclusion criteria were (1) randomized controlled trials (RCTs) or observational studies (case–control, cross-sectional, cohort studies, or case series), (2) both adults and pediatric patient populations with HSK, and (3) data on kidney stones in patients with HSK were provided. Both published studies and conference abstracts were included. Study eligibility was independently determined by the two investigators mentioned earlier. Differing decisions were resolved by joint agreement.
Data extraction
A standardized information collection form was utilized to obtain the resulting data: the first author, study design, year of publication, country where the study was conveyed, number of patients with HSK kidney studied, number of HSK kidney patients with kidney stones, age and gender of patients with HSK, time of diagnosis of kidney stones, type and location of kidney stones, urine supersaturation profiles, treatment of kidney stones, and outcomes of patients with HSK with kidney stones.
Statistical analysis
MetaXL software (EpiGear International Pty. Ltd., Queensland, Australia)[27] was utilized for data analysis. The incidence rates and 95% confidence intervals (CIs) of adverse effects were reported using a DerSimonian–Laird random-effects model.[28] As per our study protocol, a prespecified analysis by adult and pediatric population was performed. A random-effects model was implemented due to the high likelihood of interstudy variances. The Cochran Q-test was completed to appraise statistical heterogeneity. The I2 statistic was added to evaluate the degree of variation across studies related to heterogeneity instead of chance. An I2 of 0%–25% represents insignificant heterogeneity, 26%–50% low heterogeneity, 51%–75% moderate heterogeneity, and >75% high heterogeneity.[29] To assess for publication bias funnel plots were utilized.[30]
RESULTS
Our search strategy yielded 655 articles. Of these, 607 articles were excluded following the review of their title and abstract based on their relevance and the eligibility criteria. The remaining 48 articles underwent full-length review, and an additional 34 were excluded for failing to meet the criteria (23 articles were excluded because they did not report the outcomes of interest and 11 articles were excluded because they were not observational studies or RCTs).
Fourteen observational studies[14,15,18,19,20,21,22,24,31,32,33,34,35,36,37] with 943 patients (522 adults and 421 pediatric) with HSK met all inclusion criteria and were enrolled for this systematic review and meta-analysis of kidney stones in patients with HSK [Tables 1 and 2]. Figure 1 outlines our search methodology and selection process.
Table 1.
Main characteristics of the observational studies included in the meta-analysis of incidence of stone incidence
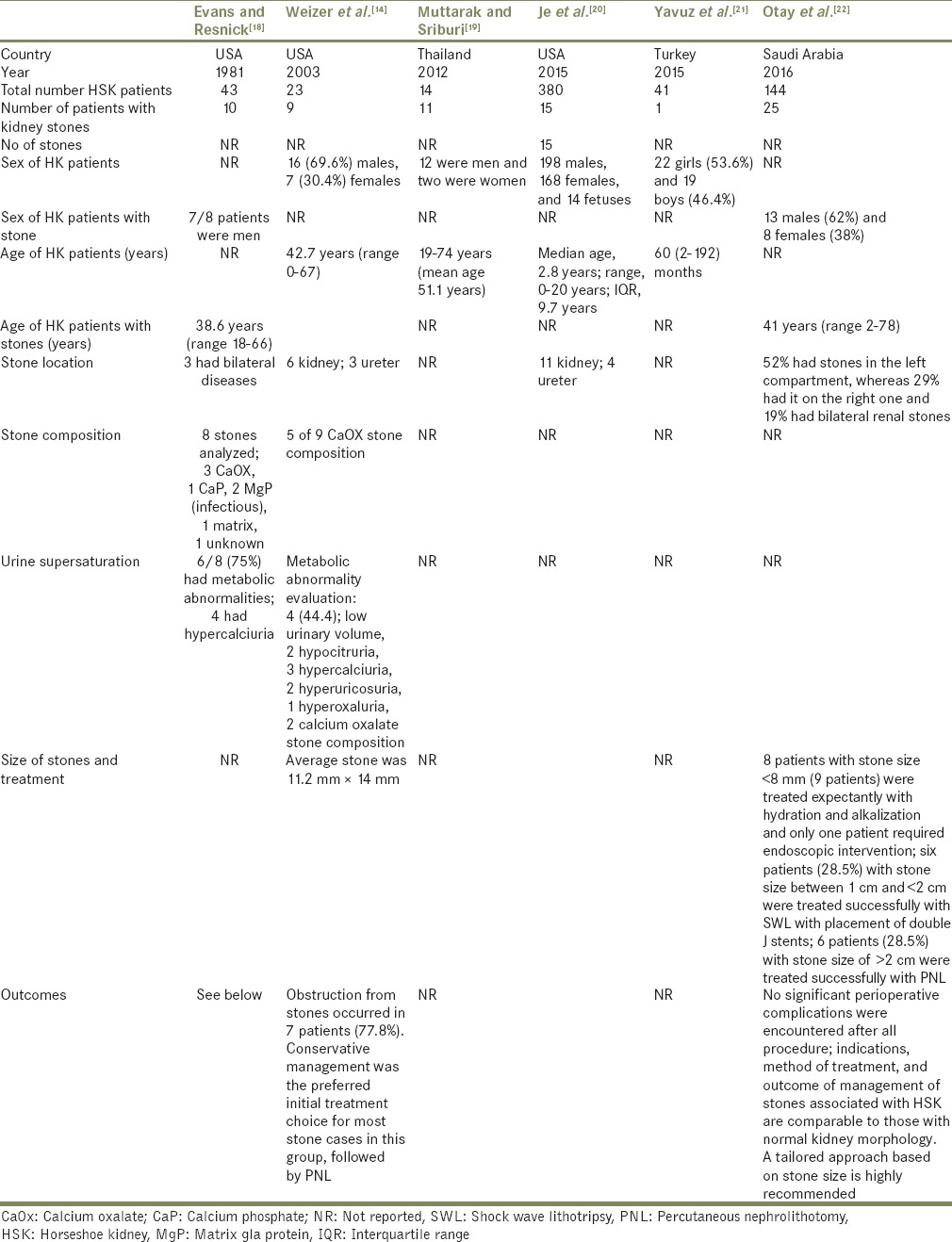
Table 2.
Main characteristics of the observational studies with types of kidney stones in patients with horseshoe kidney
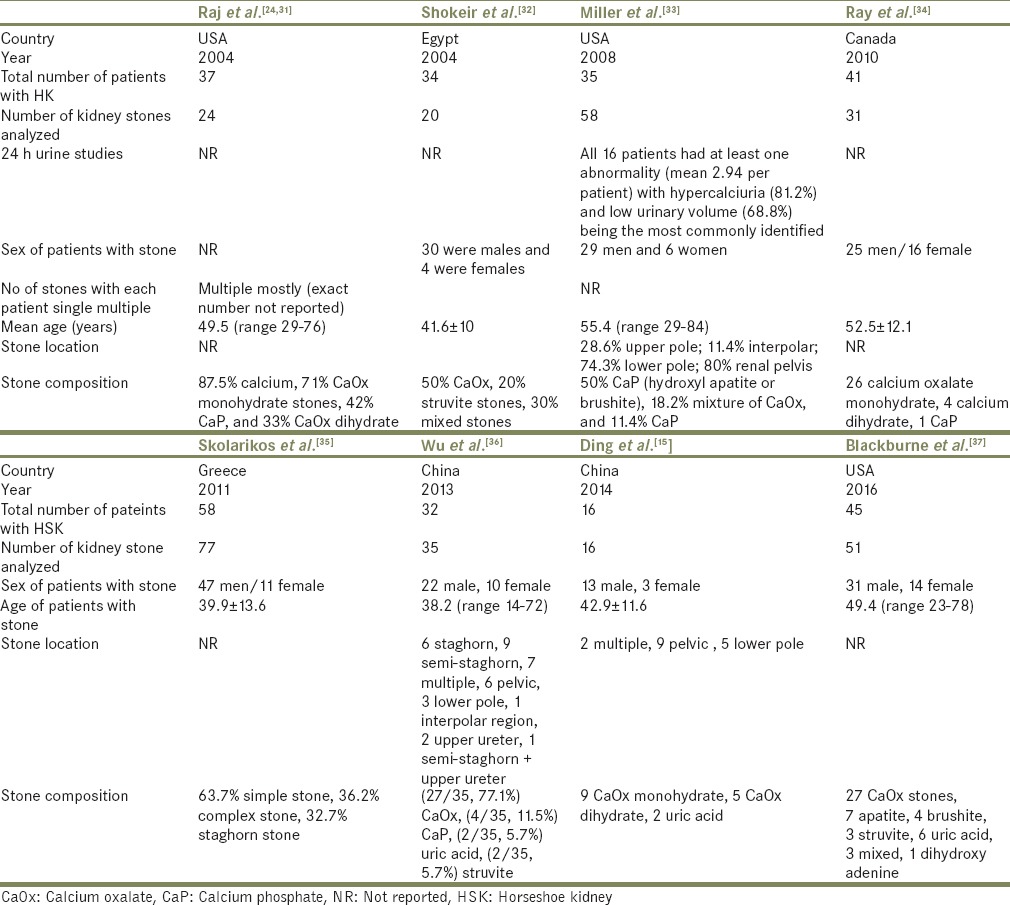
Figure 1.
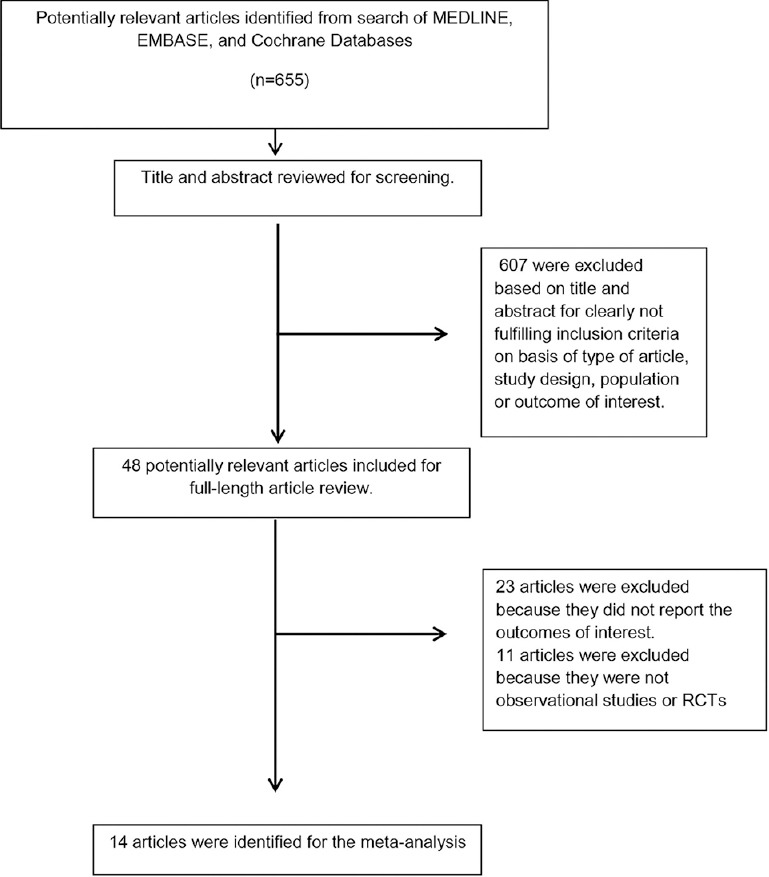
Outline of our search methodology
Incidence of kidney stones in patients with horseshoe kidney
Six studies[14,18,19,20,21,22] evaluated the incidence of kidney stones in patients with HSK as demonstrated in Table 1. The estimated pooled incidence of kidney stones was 36% [95% CI, 15%–59%, I2 = 88%; Figure 2] in adults with HSK. The mean age of adult stone formers with HSK was 44.9 ± 6.2 years, and 75% were males. Kidney stones were less common in pediatric patients with HSK with an estimated pooled incidence of 3% (95% CI, 2%–5%, I2 = 0%).
Figure 2.
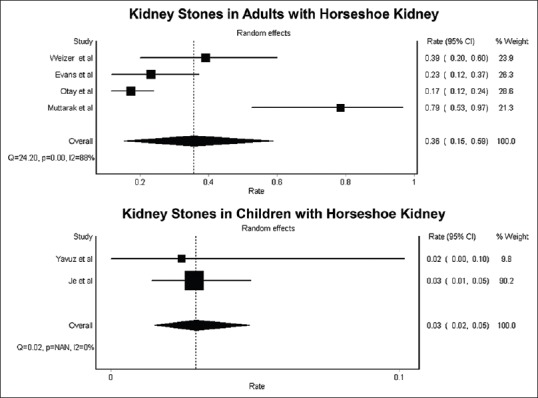
Forest plot of incidence of kidney stones in patients with horseshoe kidney
Geographic information was provided in Table 1; 3 studies from the United States and 2 studies from Western Asia and a study from Southeast Asia. Overall, there were no racial preferences. The estimated incidences of kidney stones in adult patients with HSK was 30% (95% CI, 15%–46%; I2 = 43%) in the United States. Data on the incidence of kidney stones in patients with HSK in other geographical area were limited. The incidence of kidney stones in adult patients with HSK in Asia ranged between 17% and 79%.
Types and locations of kidney stones in patients with horseshoe kidney
Ten studies[14,15,18,24,31,32,33,34,35,36,37] assessed the type of kidney stones in HSK stone formers as shown in Tables 1 and 2. About 89.2% of kidney stones were calcium-based stones (64.2% calcium oxalate [CaOx], 18.8% calcium phosphate [CaP], 6.2% mixed CaOx/CaP), followed by struvite stones (4.2%), uric acid stones (3.8%), and others (2.8%).
Risk factors for kidney stones in patients with horseshoe kidney
This may be owing to ureteropelvic junction obstruction and to the orientation of the calyces, which prevent the passage of the stones.[15,25,38] Metabolic factors have also been suggested to contribute to stone formation including hyperparathyroidism, low urine volumes, hypercalciuria, hyperoxaluria, hyperuricosuria, and hypocitraturia.[15,18,23,24,25]
Treatment and outcomes of kidney stones in patients with horseshoe kidney
An HSK is formed by fusion across the midline of two distinct functioning kidneys, connecting at the lower poles by an isthmus, which may prevent the ascent of kidney stones.[25] Although ureteroscopy (URS) and extracorporeal shock wave lithotripsy (ESWL) are less effective than percutaneous nephrolithotomy (PCNL) for the treatment of kidney stones in patients with HSK, studies have reported successful treatment with URS and/or ESWL, particularly in small (<15–20 mm) calculi.[15,22] PCNL was used for large stones and required one to four stages to achieve an overall stone clearance rate of >80%.[25]
Evaluation for publication bias
A funnel plot was not drawn because of the limited number of studies. As a rule of thumb, tests for funnel plot asymmetry should be used only when there are at least ten study groups. Due to limited number of included studies evaluating the incidence of kidney stones in patients with HSK, the power of the test is too low to distinguish chance from real asymmetry.[39]
DISCUSSION
In this study, we showed that an overall incidence of kidney stones in adults with HSK was 36%. Kidney stones were less common in pediatric patients with HSK with an estimated incidence of 3%. The mean age of adult stone formers with HSK was 45 years, and 75% were males. Calcium-based (CaOx and CaP) stones were the most prevalent types of kidney stones in patients with HSK.
The incidence of kidney stones in patients with MSK from our meta-analysis is much higher than reported in the general adult populations.[10,11,12] There are several plausible explanations. First, HSK-related abnormal position of the ureter in the renal pelvis and highly placed ureteropelvic junction,[17] can result in impaired drainage of the collecting system and predispose HSK patient to kidney stones.[14,18,19,20,21,22] Second, previous studies have demonstrated that metabolic factors including hyperparathyroidism, low urine volumes, hypercalciuria, hyperoxaluria, hyperuricosuria, and hypocitraturia[15,18,23,24,25] are common in patients with HSK, which may contribute to higher stone formation. Third, the previous reports have demonstrated coexistent HSK with medullary sponge kidney,[40,41,42] a known cause of stone disease.[43]
To date, there have been no available clinical trials comparing the treatment efficacy of URS, ESWL, or PCNL on kidney stones in patients with HSK. PCNL is generally recommended due to anatomical abnormalities and is used for large stones with stone clearance rate of >80%.[25] However, studies have also shown that URS and ESWL may be effective in small stones.[15,22]
There are several limitations to our study. First, there were statistical heterogeneities in the analysis of the incidence of kidney stones in adult HSK patients. The potential sources of this heterogeneity included differences in diagnostic methodology of kidney stones. Second, most included studies were conducted in the United States with the majority of the participants being Caucasian. Therefore, the findings from our study may not represent HSK populations from other parts of the world, and the future studies are required.
CONCLUSIONS
Our meta-analysis demonstrates that kidney stones are common in patients with HSK with estimated incidence of 36%. Kidney stones are prevalent in males with HSK. Calcium-based stones were the most common types of kidney stones in patients with HSK.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rule AD, Lieske JC, Li X, Melton LJ rd, Krambeck AE, Bergstralh EJ. The ROKS nomogram for predicting a second symptomatic stone episode. J Am Soc Nephrol. 2014;25:2878–86. doi: 10.1681/ASN.2013091011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheungpasitporn W, Thongprayoon C, Mao MA, O'Corragain OA, Edmonds PJ, Erickson SB. The risk of coronary heart disease in patients with kidney stones: A systematic review and meta-analysis. N Am J Med Sci. 2014;6:580–5. doi: 10.4103/1947-2714.145477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lieske JC, Peña de la Vega LS, Slezak JM, Bergstralh EJ, Leibson CL, Ho KL, et al. Renal stone epidemiology in Rochester, Minnesota: An update. Kidney Int. 2006;69:760–4. doi: 10.1038/sj.ki.5000150. [DOI] [PubMed] [Google Scholar]
- 4.Cheungpasitporn W, Erickson SB, Rule AD, Enders F, Lieske JC. Short-term tolvaptan increases water intake and effectively decreases urinary calcium oxalate, calcium phosphate and uric acid supersaturations. J Urol. 2016;195:1476–81. doi: 10.1016/j.juro.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheungpasitporn W, Thongprayoon C, Mao MA, Kittanamongkolchai W, Jaffer Sathick IJ, Dhondup T, et al. Incidence of kidney stones in kidney transplant recipients: A systematic review and meta-analysis. World J Transplant. 2016;24(6):790–797. doi: 10.5500/wjt.v6.i4.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thongprayoon C, Cheungpasitporn W, Vijayvargiya P, Anthanont P, Erickson SB. The risk of kidney stones following bariatric surgery: A systematic review and meta-analysis. Ren Fail. 2016;38:424–30. doi: 10.3109/0886022X.2015.1137186. [DOI] [PubMed] [Google Scholar]
- 7.Cheungpasitporn W, Rossetti S, Friend K, Erickson SB, Lieske JC. Treatment effect, adherence, and safety of high fluid intake for the prevention of incident and recurrent kidney stones: A systematic review and meta-analysis. J Nephrol. 2016;29:211–9. doi: 10.1007/s40620-015-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheungpasitporn W, Thongprayoon C, O'Corragain OA, Edmonds PJ, Ungprasert P, Kittanamongkolchai W, et al. The risk of kidney cancer in patients with kidney stones: A systematic review and meta-analysis. QJM. 2015;108:205–12. doi: 10.1093/qjmed/hcu195. [DOI] [PubMed] [Google Scholar]
- 9.Shah J, Whitfield HN. Urolithiasis through the ages. BJU Int. 2002;89:801–10. doi: 10.1046/j.1464-410x.2002.02769.x. [DOI] [PubMed] [Google Scholar]
- 10.Long LO, Park S. Update on nephrolithiasis management. Minerva Urol Nefrol. 2007;59:317–25. [PubMed] [Google Scholar]
- 11.López M, Hoppe B. History, epidemiology and regional diversities of urolithiasis. Pediatr Nephrol. 2010;25:49–59. doi: 10.1007/s00467-008-0960-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int. 2003;63:1817–23. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 13.Dhillon J, Mohanty SK, Kim T, Sexton WJ, Powsang J, Spiess PE. Spectrum of renal pathology in adult patients with congenital renal anomalies-a series from a tertiary cancer center. Ann Diagn Pathol. 2014;18:14–7. doi: 10.1016/j.anndiagpath.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Weizer AZ, Silverstein AD, Auge BK, Delvecchio FC, Raj G, Albala DM, et al. Determining the incidence of horseshoe kidney from radiographic data at a single institution. J Urol. 2003;170:1722–6. doi: 10.1097/01.ju.0000092537.96414.4a. [DOI] [PubMed] [Google Scholar]
- 15.Ding J, Huang Y, Gu S, Chen Y, Peng J, Bai Q, et al. Flexible ureteroscopic management of horseshoe kidney renal calculi. Int Braz J Urol. 2015;41:683–9. doi: 10.1590/S1677-5538.IBJU.2014.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obidike S, Woha A, Aftab F. Fused ureters in patient with horseshoe kidney and aortic abdominal aneurysm. J Surg Case Rep. 2014;2014:1–3. doi: 10.1093/jscr/rju113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Natsis K1, Piagkou M, Skotsimara A, Protogerou V, Tsitouridis I, Skandalakis P. Horseshoe kidney: A review of anatomy and pathology. Surg Radiol Anat. 2014;36:517–26. doi: 10.1007/s00276-013-1229-7. [DOI] [PubMed] [Google Scholar]
- 18.Evans WP, Resnick MI. Horseshoe kidney and urolithiasis. J Urol. 1981;125:620–1. doi: 10.1016/s0022-5347(17)55139-3. [DOI] [PubMed] [Google Scholar]
- 19.Muttarak M, Sriburi T. Congenital renal anomalies detected in adulthood. Biomed Imaging Interv J. 2012;8:e7. doi: 10.2349/biij.8.1.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Je BK, Kim HK, Horn PS. Incidence and spectrum of renal complications and extrarenal diseases and syndromes in 380 children and young adults with horseshoe kidney. AJR Am J Roentgenol. 2015;205:1306–14. doi: 10.2214/AJR.15.14625. [DOI] [PubMed] [Google Scholar]
- 21.Yavuz S, Kiyak A, Sander S. Renal outcome of children with horseshoe kidney: A single-center experience. Urology. 2015;85:463–6. doi: 10.1016/j.urology.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Otay AA, Sarhan O, Helaly AA, Albedaiwi K, Ghanbar MA, Nakshabandi Z, et al. Different management options for horseshoe kidney stones: Single center experience. J Urol. 2016;1:e325. [Google Scholar]
- 23.Mottola A, Selli C, Carini M, Natali A. Lithiasis in horseshoe kidney. Acta Urol Belg. 1984;52:355–60. [PubMed] [Google Scholar]
- 24.Raj GV, Auge BK, Assimos D, Preminger GM. Metabolic abnormalities associated with renal calculi in patients with horseshoe kidneys. J Endourol. 2004;18:157–61. doi: 10.1089/089277904322959798. [DOI] [PubMed] [Google Scholar]
- 25.Symons SJ, Ramachandran A, Kurien A, Baiysha R, Desai MR. Urolithiasis in the horseshoe kidney: A single-centre experience. BJU Int. 2008;102:1676–80. doi: 10.1111/j.1464-410X.2008.07987.x. [DOI] [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 27.Barendregt J, Doi S. Wilston, Australia: EpiGear International Pty. Ltd; 2010. MetaXL User Guide. Ver. 1.0. [Google Scholar]
- 28.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: An update. Contemp Clin Trials. 2007;28:105–14. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–72. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 31.Raj GV, Auge BK, Weizer AZ, Denstedt JD, Watterson JD, Beiko DT, et al. Percutaneous management of calculi within horseshoe kidneys. J Urol. 2003;170:48–51. doi: 10.1097/01.ju.0000067620.60404.2d. [DOI] [PubMed] [Google Scholar]
- 32.Shokeir AA, El-Nahas AR, Shoma AM, Eraky I, El-Kenawy M, Mokhtar A, et al. Percutaneous nephrolithotomy in treatment of large stones within horseshoe kidneys. Urology. 2004;64:426–9. doi: 10.1016/j.urology.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Miller NL, Matlaga BR, Handa SE, Munch LC, Lingeman JE. The presence of horseshoe kidney does not affect the outcome of percutaneous nephrolithotomy. J Endourol. 2008;22:1219–25. doi: 10.1089/end.2008.0051. [DOI] [PubMed] [Google Scholar]
- 34.Ray AA, Ghiculete D, D'A Honey RJ, Pace KT. Shockwave lithotripsy in patients with horseshoe kidney: Determinants of success. J Endourol. 2011;25:487–93. doi: 10.1089/end.2010.0213. [DOI] [PubMed] [Google Scholar]
- 35.Skolarikos A, Binbay M, Bisas A, Sari E, Bourdoumis A, Tefekli A, et al. Percutaneous nephrolithotomy in horseshoe kidneys: Factors affecting stone-free rate. J Urol. 2011;186:1894–8. doi: 10.1016/j.juro.2011.06.056. [DOI] [PubMed] [Google Scholar]
- 36.Wu W, Zhao Z, Zhu H, Yang D, Ou L, Liang Y, et al. Safety and efficacy of minimally invasive percutaneous nephrolithotomy in treatment of calculi in horseshoe kidneys. J Endourol. 2014;28:926–9. doi: 10.1089/end.2013.0760. [DOI] [PubMed] [Google Scholar]
- 37.Blackburne AT, Rivera ME, Gettman MT, Patterson DE, Krambeck AE. Endoscopic Management of Urolithiasis in the Horseshoe Kidney. Urology. 2016;90:45–9. doi: 10.1016/j.urology.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 38.Schiappacasse G, Aguirre J, Soffia P, Silva CS, Zilleruelo N. CT findings of the main pathological conditions associated with horseshoe kidneys. Br J Radiol. 2015;88:20140456. doi: 10.1259/bjr.20140456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Irisawa C, Yamaguchi O, Shiraiwa Y, Kikuchi Y, Irisawa S, Irisawa C. A case of medullary sponge disease associated with horseshoe kidney. Nihon Hinyokika Gakkai Zasshi. 1990;81:1255–7. doi: 10.5980/jpnjurol1989.81.1255. [DOI] [PubMed] [Google Scholar]
- 41.Lambrianides AL, John DR. Medullary sponge disease in horseshoe kidney. Urology. 1987;29:426–7. doi: 10.1016/0090-4295(87)90516-4. [DOI] [PubMed] [Google Scholar]
- 42.Cheungpasitporn W, Pawar AS, Erickson SB. Recurrent renal calculi in coexistence of horseshoe kidney and medullary sponge kidney. Urol Ann. 2017;9:214–5. doi: 10.4103/UA.UA_173_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheungpasitporn W, Thongprayoon C, Brabec BA, Kittanamongkolchai W, Erickson SB. Outcomes of living kidney donors with medullary sponge kidney. Clin Kidney J. 2016;9:866–70. doi: 10.1093/ckj/sfv107. [DOI] [PMC free article] [PubMed] [Google Scholar]


