Abstract
Background:
Acute renal failure after cardiac surgery is known to be associated with significant short-term morbidity and mortality. There have as yet been no major reports on long-term quality of life (QOL). This study assessed the impact of acute kidney injury (AKI) and renal replacement therapy (RRT) on long-term survival and QOL after cardiac surgery. The need for long-term RRT is also assessed.
Materials and Methods:
Patients who underwent cardiac surgery between 2005 and 2011 (n = 6087) and developed AKI (RIFLE criteria, n = 570) were included. They were propensity-matched 1:1 to patients without renal impairment (control). Data were prospectively collected, and health-related QOL questionnaire was sent to patients who were alive at least 1-year postoperatively at the time of the study.
Results:
There was no significant difference in the preoperative characteristics between the two groups (age, gender, left ventricular ejection fraction, procedure, urgency, logistic Euroscore), respectively. Median follow-up was 52 months. Survival data were available in all patients. Questionnaires were returned in 64% of eligible patients. Long-term survival was significantly lower, and QOL, in particular the physical aspect, was significantly worse for the AKI group as compared to non-AKI group (38.8 vs. 44.2, P = 0.002), especially so in patients who required RRT. In alive respondents, despite an 18% (66/359) incidence of ongoing renal follow-up, the need for late RRT was only in 1.1% (4/359).
Conclusion:
AKI and especially the need for RRT following cardiac surgery are associated with increased long-term mortality as well as worse quality of life in a propensity-matched control group.
Keywords: Acute kidney injury, postcardiac surgery, quality of life
Introduction
The incidence of acute kidney injury (AKI) after cardiac surgery varies from 2% to 30% depending on the definition.[1] However, nowadays, most clinicians would use the agreed RIFLE criteria.[2] The short-term impact of AKI postcardiac surgery is well documented in the literature[3,4,5] and includes an increased inhospital morbidity and mortality as well as prolonged Intensive Care Unit (ICU) and inhospital length of stay.
Patients without preoperative renal impairment, who subsequently develop AKI including those who require renal replacement therapy (RRT) following cardiac surgery, are reported to have worse outcomes in terms of both early and long-term survival.[6] Hobson et al. studied 1265 patients with no history of chronic kidney disease who developed AKI after cardiac surgery noting worse long-term survival with higher odds ratio for late death even in patients who recover after their AKI. Inhospital mortality in the AKI group was reported at 32%, and survival was 44% in patients with AKI versus 63% in controls at 10 years.[7]
The impact on quality of life (QOL) of patients suffering AKI is mostly reported in the general ICU setting but minimally so following cardiac surgery.[5,8] This study addressed this gap and documents the late QOL and survival outcomes in adult patients developing new AKI following cardiac surgery.
Materials and Methods
Adult patients who underwent elective or urgent (surgery performed after 24 h but within same hospital admission) cardiac surgery between January 2005 and December 2011 were included in this study. A total of 6087 adult patients underwent elective or urgent cardiac surgery during the study period. Patients with preoperative renal failure as per the Society for Cardiothoracic Surgery definition (serum creatinine >200 μmol/L or on dialysis) were excluded. This study was registered as a service evaluation with the hospital's research and development department. As such, the study was performed in accordance with the institutional ethical standards based on the good clinical practice in research. Patients were treated according to the Declaration of Helsinki 1964 (amended Edinburgh 2000). As part of the Declaration of Helsinki, patient's consent was presumed if they returned the QOL questionnaire.
For this study, all patients with a postoperative creatinine rise by at least 50% from their preoperative value[9,10] (RIFLE criteria) and/or those patients who required RRT were grouped as having developed AKI (n = 570). RRT was instituted by way of continuous venovenous hemofiltration according to protocol-based indications (acidosis, hyperkalemia, oliguria, or fluid balance).
Baseline data were prospectively collected on the cardiac surgery database (Dendrite). Mortality data were obtained from the hospital mortality database and validated against data held by the patient's general practitioner. Requirement for late RRT was cross-checked with our hospital renal departmental database and validated by inquiring with patients as part of the questionnaire. Health-related QOL data were obtained by sending out the QOL questionnaire to surviving patients who were at least 1 year following their procedure. The Medical Outcomes Study 12-Item Short-Form Health Survey (SF-12 v2) is a validated self-administered tool for measuring patient-reported functional health status.[11,12] The answers to the 12 questions of the QOL questionnaire were used to generate a physical and a mental score. Patients were allowed 8 weeks to respond.
Patients who developed postoperative new-onset AKI (n = 570, 9.4%) were 1:1 propensity matched to patients without renal impairment (control group). A logistic regression model was used to generate a propensity score for each patient. This assessed the probability of developing AKI and was based on patient and procedure characteristics. The following variables were considered for the propensity score model: age, gender, emphysema, peripheral vascular disease, neurological dysfunction, endocarditis, preoperative serum creatinine, redo cardiac surgery, left ventricular ejection fraction, urgency of operation, need for preoperative inotropes/ventilation/intravenous nitrates/intra-aortic balloon pump, cardiac procedure, recent myocardial infarction and pulmonary hypertension and logistic Euroscore. For each patient in the AKI group, an individual was selected in the non-AKI group by matching on the log of the estimated propensity score, using a nearest neighbor-matching algorithm with calipers of maximum width of 0.2 standard deviations. The distribution of all model factors was compared in the two groups to assess the success of the propensity score model. In line with recommendations, the balance in the covariates across the two groups was considered achieved if the standardized differences were <10%.[13]
The primary aim of this study was to assess the QOL in patients with AKI as compared to their matched non-AKI pair. The secondary aims included survival and long-term need for RRT in the AKI group.
Continuous variables are expressed as mean (standard deviation) or median (range) for Gaussian and skewed distributed data, respectively. The box plots represent the median and the range. Group comparison was carried out using independent t-test or Mann–Whitney U-test. Categorical data are expressed in frequency (%), and difference between the two groups assessed using Pearson Chi-square test. Comparison of survival time in the groups with corresponding significance level was assessed using the Kaplan–Meier method. A two-tailed P < 0.05 was considered as significant. Statistical Package for Social sciences version 16.0 (SPSS-16, SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
Results
Preoperative demographics of the whole group of patients (n = 6087) are listed in Table 1a. After the propensity matching, there were no significant differences in the patient's baseline demographics between the AKI and non-AKI groups [Table 1b]. RRT was required in 54% of AKI patients.
Table 1a.
Preoperative data of acute kidney injury and nonmatched acute kidney injury patients
| Preoperative data | No AKI | AKI | P |
|---|---|---|---|
| n | 5517 | 570 | |
| Age* (years) | 65.3 (10.4) | 69.9 (9.4) | <0.001 |
| Female, n (%) | 1269 (23) | 30 (169) | 0.01 |
| Caucasian ethnicity, n (%) | 4745 (86) | 86 (490) | 0.2 |
| Logistic Euroscore+ | 3.1 (0.9-89.7) | 7.2 (0.9-83.2) | <0.001 |
| Recent MI, n (%) | 1765 (32) | 27 (154) | 0.02 |
| Impaired LVEF, n (%) | 1710 (31) | 45 (259) | <0.001 |
| PVD, n (%) | 938 (17) | 26 (147) | <0.01 |
| Pulmonary disease, n (%) | 662 (12) | 15 (88) | 0.04 |
| Nonelective, n (%) | 1600 (29) | 38 (215) | <0.01 |
| Combined procedures, n (%) | 1490 (27) | 35 (201) | <0.01 |
*Mean (SD), +Median (range). LVEF: Left ventricular ejection fraction, MI: Myocardial infarction, PVD: Peripheral vascular disease, SD: Standard deviation, AKI: Acute kidney injury
Table 1b.
Preoperative data matched nonacute kidney injury and acute kidney injury patients (n=570 for each group)
| Preoperative data | No AKI | AKI | P |
|---|---|---|---|
| Age* (years) | 69.5 (9.9) | 69.9 (9.4) | 0.68 |
| Female, n (%) | 189 (33) | 169 (30) | 0.2 |
| Caucasian ethnicity, n (%) | 467 (82) | 490 (86) | 0.47 |
| Logistic Euroscore+ | 7.2 (0.9-89.7) | 7.2 (0.9-83.2) | 0.97 |
| Recent MI, n (%) | 149 (26) | 154 (27) | 0.98 |
| Impaired LVEF, n (%) | 262 (46) | 259 (45) | 0.12 |
| PVD, n (%) | 149 (26) | 147 (26) | 0.89 |
| Pulmonary disease n (%) | 97 (17) | 88 (15) | 0.47 |
| Nonelective, n (%) | 190 (33) | 215 (38) | 0.09 |
| Combined procedures, n (%) | 179 (31) | 201 (35) | 0.38 |
*Mean (SD), +Median (range). LVEF: Left ventricular ejection fraction, MI: Myocardial infarction, PVD: Peripheral vascular disease, SD: Standard deviation, AKI: Acute kidney injury
Inhospital mortality was significantly higher in the AKI group being 17.5% (100/570) in that group as compared to 2.1% (12/570) when patients did not develop AKI at all [P < 0.01, Figure 1]. In the AKI group, the inhospital mortality was significantly higher when RRT was used being 27.7% (85/307) as compared to 5.7% (15/263) when RRT was not required.
Figure 1.
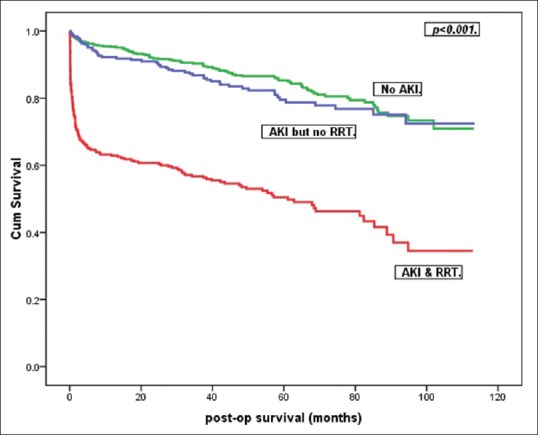
Kaplan–Meier survival curves for the three groups
Survival data were available in all patients. Overall, actuarial survival at 1 year, 5 years, and 8 years was significantly poorer in AKI with RRT group [P < 0.01, Figure 1]. Median follow-up was 52 (0, 113) months.
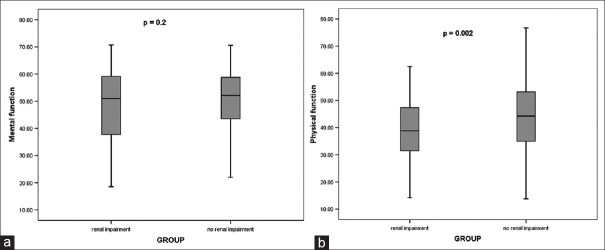
Completed QOL questionnaire was available for 64% (499/777) of patients who were still alive at the time of the study and who were at least 1 year postsurgery. This included a 60% (202/336) response from the AKI group and 67% (297/441) from the non-AKI group. The median follow-up for those patients who returned the QOL questionnaires were 60 (30, 113) and 63 (30, 112) months, respectively (P = 0.09). Interestingly, the median (range) mental scores were not significantly different (51.0 [18.5, 70.8] vs. 52.2 [21.8, 70.6], P = 0.2) [Figure 2a] between the AKI and non-AKI groups but the physical scores were (38.8 [14.2, 62.5] vs. 44.2 [13.8, 76.7], P < 0.01) [Figure 2b]. The differences within the SF-12 physical scores when RRT is required for AKI are illustrated in Figure 3.
Figure 2.
Box plots – median (range) – for the (a) mental and (b) physical performance using Short-Form-12 questionnaire for patients with and without acute kidney injury
Figure 3.
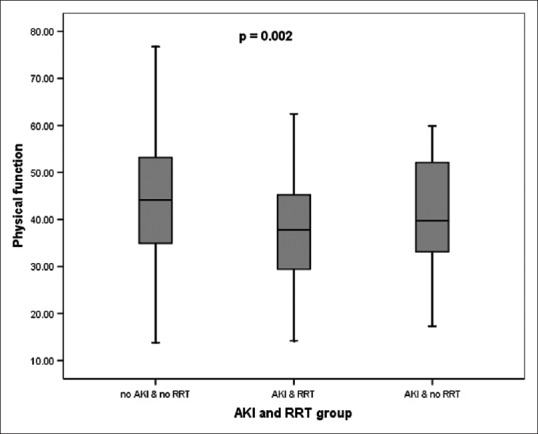
Box plots – median (range) – for the physical performance using Short-Form-12 questionnaire for patients without acute kidney injury, with acute kidney injury and no renal replacement therapy and with acute kidney injury and renal replacement therapy
In the AKI group (n = 570), 66 patients of those still alive (n = 359) had ongoing renal follow-up (18%), but the need for late RRT was only in 1.1% (4/359) patients.
Discussion
AKI following cardiac surgery has a reported incidence of around 30%.[14] Up to 10% may require long-term RRT with reported mortality rates of up to 30% in this subgroup with poor long-term survival.[3,14,15] The late sequelae of new onset AKI (especially those not needing RRT) following cardiac surgery and its effect on QOL are, as yet, not well documented.
In the HANDOUT study published in 2016, the authors investigated the QOL post-RRT in general ICU patients.[16] They reported that their assessment of QOL using the SF-36 did not reveal significant physical limitations in their patient group. This could either be due to the fact that they were able to investigate only a small number of patients (21 patients) as compared to 499 patients in this study and/or they did not have another group for comparison of the QOL. In another study of RRT in general ICU patients, Oeyen et al. reported on the QOL of 56 patients at 4 years.[17] They confirmed similar findings to our study demonstrating that physical QOL was worse than mental health within the RRT group and as compared to non-AKI-RRT group. They were also able to assess the QOL in a longitudinal mode and demonstrated a significant worsening of QOL in the early time period after hospital discharge in the RRT group.
In their report, Hofhuis et al. reviewed the QOL of patients at 6 months of ICU discharge and compared patients who developed AKI to those who did not.[5] They showed that at that time point, there were no significant differences between the two groups in their QOL assessments but these outcomes were much lower when compared to the general population.
The use of RRT in AKI following cardiac surgery has been reported to have profoundly devastating effects. Luckraz et al. studied 92 (2.9%) patients postcardiac surgery requiring RRT and reported a 42% 30-day mortality with actuarial 5-year survival (95% confidence interval) of 52 (42, 62)%.[3] Landoni et al. reported a high inhospital mortality of patients requiring RRT but found a low long-term mortality with reasonable QOL in patients discharged from hospital alive.[18] In a review manuscript published in 2012, the authors concur with the findings that the development of AKI requiring RRT is associated with poor long-term survival with a high mortality within the 1st year postrenal injury.[19]
This study also showed an 18% (66/359) incidence of ongoing renal follow-up among patients in the long term. However, long-term need for RRT was low at 1.1% (4/359) among the survivors in the AKI group. These patients were all from the AKI with RRT group. When considering surviving patients (at least 1 year postoperative) within that group (n = 71), then, the need for long-term RRT is 5.6%. Therefore, in the longer term, much fewer patients were noted to need RRT as compared to published incidence of up to 30%.[3,14,18] One plausible explanation could be that once the cardiac condition was treated by the cardiac surgery, then the kidneys tended to recover over a period of time as the insult to the kidneys (most likely the cardiopulmonary bypass machine) was of an acute and short-lived nature. Another possibility could relate to the fact that patients developing AKI in our unit are managed aggressively with RRT as early as possible, hence possibly limiting the amount of damage to the kidney tubules.
Limitations of the study
This is a single-center, retrospective study of prospectively collected data. Compliance with postal questionnaire is always a practical hindrance although similar effects may be expected within both groups. Baseline preoperative QOL data, for individual patient comparison, were not available. However, these effects apply equally to the AKI and non-AKI groups and could balance out during comparison between the two groups.
Conclusion
In this first large series study assessing the QOL after cardiac surgery in AKI and non-AKI patients, AKI especially when RRT was used had been shown to have a significant impact on the physical aspects of the QOL. A similar finding is described on survival. However, the mental part of the QOL seems to be less affected. Attempts to improve outcome would require prevention of development of AKI in the immediate postoperative phase.
Financial support and sponsorship
This study was financially supported by hospital-based charitable funds.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kramer RS, Herron CR, Groom RC, Brown JR. Acute kidney injury subsequent to cardiac surgery. J Extra Corpor Technol. 2015;47:16–28. [PMC free article] [PubMed] [Google Scholar]
- 2.Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365:417–30. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 3.Luckraz H, Gravenor MB, George R, Taylor S, Williams A, Ashraf S, et al. Long and short-term outcomes in patients requiring continuous renal replacement therapy post cardiopulmonary bypass. Eur J Cardiothorac Surg. 2005;27:906–9. doi: 10.1016/j.ejcts.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 4.Corredor C, Thomson R, Al-Subaie N. Long-term consequences of acute kidney injury after cardiac surgery: A Systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2016;30:69–75. doi: 10.1053/j.jvca.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Hofhuis JG, van Stel HF, Schrijvers AJ, Rommes JH, Spronk PE. The effect of acute kidney injury on long-term health-related quality of life: A prospective follow-up study. Crit Care. 2013;17:R17. doi: 10.1186/cc12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loef BG, Epema AH, Smilde TD, Henning RH, Ebels T, Navis G, et al. Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol. 2005;16:195–200. doi: 10.1681/ASN.2003100875. [DOI] [PubMed] [Google Scholar]
- 7.Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–53. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 8.Abelha FJ, Botelho M, Fernandes V, Barros H. Outcome and quality of life of patients with acute kidney injury after major surgery. Nefrologia. 2009;29:404–14. doi: 10.3265/Nefrologia.2009.29.5.5456.en.full. [DOI] [PubMed] [Google Scholar]
- 9.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup et al. Acute renal failure - Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruz DN, Ricci Z, Ronco C. Clinical review: RIFLE and AKIN – Time for reappraisal. Crit Care. 2009;13:211. doi: 10.1186/cc7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross-validation of item selection and scoring for the SF-12 health survey in nine countries: Results from the IQOLA project. International quality of life assessment. J Clin Epidemiol. 1998;51:1171–8. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 13.Austin PC, Mamdani MM. A comparison of propensity score methods: A case-study estimating the effectiveness of post-AMI statin use. Stat Med. 2006;25:2084–106. doi: 10.1002/sim.2328. [DOI] [PubMed] [Google Scholar]
- 14.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–8. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 15.Conlon PJ, Stafford-Smith M, White WD, Newman MF, King S, Winn MP, et al. Acute renal failure following cardiac surgery. Nephrol Dial Transplant. 1999;14:1158–62. doi: 10.1093/ndt/14.5.1158. [DOI] [PubMed] [Google Scholar]
- 16.Faulhaber-Walter R, Scholz S, Haller H, Kielstein JT, Hafer C. Health status, renal function, and quality of life after multiorgan failure and acute kidney injury requiring renal replacement therapy. Int J Nephrol Renovasc Dis. 2016;9:119–28. doi: 10.2147/IJNRD.S89128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oeyen S, De Corte W, Benoit D, Annemans L, Dhondt A, Vanholder R, et al. Long-term quality of life in critically ill patients with acute kidney injury treated with renal replacement therapy: A matched cohort study. Crit Care. 2015;19:289. doi: 10.1186/s13054-015-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landoni G, Zangrillo A, Franco A, Aletti G, Roberti A, Calabrò MG, et al. Long-term outcome of patients who require renal replacement therapy after cardiac surgery. Eur J Anaesthesiol. 2006;23:17–22. doi: 10.1017/S0265021505001705. [DOI] [PubMed] [Google Scholar]
- 19.Rimes-Stigare C, Awad A, Mårtensson J, Martling CR, Bell M. Long-term outcome after acute renal replacement therapy: A narrative review. Acta Anaesthesiol Scand. 2012;56:138–46. doi: 10.1111/j.1399-6576.2011.02567.x. [DOI] [PubMed] [Google Scholar]