Abstract
Context:
The role of prophylaxis for postoperative nausea and vomiting (PONV) in cardiac surgery is under debate.
Aims:
To study the risk factors for PONV after cardiac surgery and the role of betamethasone with or without droperidol for its prevention.
Setting and Design:
Randomized open-label controlled study comparing standard care with PONV prophylaxis from February to November 2016.
Methods:
Five hundred and two patients with planned nonemergent cardiac surgery were included. Interventions: In the intervention arm, PONV prophylaxis (4 mg betamethasone with/without 0.625 mg droperidol) was administered in high-risk patients (two or more risk factors). Patients in the control arm were treated as per routine hospital practices.
Results:
Female sex, past history of PONV, and migraines were associated with a significantly increased risk of PONV, while motion sickness, smoking status, and volatile anesthetics were not. Pain and treatment with nefopam or ketoprofen were associated with an increased risk of PONV. PONV was less frequent in the active arm compared to controls (45.5% vs. 54.0%, P = 0.063; visual analogic scale 10.9 vs. 15.3 mm, P = 0.043). Among the 180 patients (35.6%) with ≥2 risk factors, prophylaxis was associated with reduced PONV (intention-to-treat: 46.8% vs. 67.8%, P = 0.0061; per-protocol: 39.2% vs. 69%, P = 0.0002). In multivariate analysis, prophylaxis was independently associated with PONV (odds ratio [OR]: 0.324, 95% confidence interval: 0.167–0.629, P = 0.0009), as were female sex, past history of PONV, and migraines (OR: 3.027, 3.031, and 2.160 respectively). No drug-related side effects were reported.
Conclusion:
Betamethasone with/without droperidol was effective in decreasing PONV in high risk cardiac surgical patients without any side effect.
Keywords: Antiemetics, cardiac surgery, postoperative emesis, postoperative nausea and vomiting, prophylaxis, randomized controlled trial
Introduction
Postoperative nausea and vomiting (PONV) is a frequent complication after any surgical intervention, including cardiac surgery where incidence can reach 70%.[1,2] Proposed explanations for these prevalent complications include particularly prolonged surgery with gut hypoperfusion, and endogenous catecholamine surge.[3,4]
Close attention should be paid to PONV after surgery in fragile cardiac patients in light of increased metabolic demands during emesis and the risks of aspiration. Prophylaxis is now a well-accepted approach for decreasing PONV after noncardiac surgery. Drugs typically used for prophylaxis are independently associated with a 26% decrease in PONV, notably droperidol, ondansetron, and dexamethasone. Further decreases are observed with intravenous anesthesia, and/or midazolam.[5,6]
However, the role of prophylaxis for PONV in cardiac surgery is up for debate, following a report from a study showing a considerably lower prevalence of nausea (19.7%) and vomiting (4.3%) after cardiac surgery with fast-track anesthesia when effective rescue treatment was implemented, leading the authors to discourage the use of prophylaxis.[4] A further argument against the use of prophylaxis lies in the fact that droperidol and ondansetron may be associated with increased QT duration and cardiac arrhythmias. In this context of clinical equipoise in the cardiac surgery setting, we aimed to investigate the role of risk factors in the decision to administer PONV prophylaxis after cardiac surgery.
Methods
We designed a randomized open-label study comparing standard care (risk factor assessment not available for clinicians and no consequent intervention) with prophylaxis for postoperative nausea and vomiting after cardiac surgery (PONVACS) in high-risk patients. The trial was carried out in a single French institution and is registered at ClinicalTrials.gov (NCT02744495). Ethical approval for this study (Ethical Committee N° 15049, CPP Ile de France XI) was provided by the regional Ethical Committee, location Saint-Germain en Laye, France (Chairperson Sabine de la Porte) on February 1, 2016 (2015-A01440-49). Patients provided informed consent.
Patients with planned cardiac surgery were screened for inclusion. Inclusion criteria were planned nonemergent cardiac surgery performed using median sternotomy, age ≥18 years, coverage with the French Social Security, and physician approval for study participation. Exclusion criteria included pregnancy, contraindication to antiemetics, chronic antiemetic use, and emergent or complicated surgery.
Data for the presence of specific risk factors for PONV, including Apfel et al. and Koivuranta et al. scores,[7,8] were collected and patients were randomized by permutation blocks of 100 into two arms. As some risk scores incorporate postoperative opioid use as a risk factor, we defined high-risk as having two or more factors among the following: female sex, nonsmoker, migraine sufferer, motion sickness, and a past history of PONV.
Control arm - Data on PONV risk factors were not made available to physicians so prophylaxis was not administered irrespective of the patient's risk score. Patients were treated according to the physician's decision in line with routine hospital practice.
Active arm - PONV risk factors were collected preoperatively and made available to the physicians. Prophylaxis was indicated if a patient had two or more risk factors, and composed of betamethasone (4 mg) immediately after cardiac surgery upon arrival in the Intensive Care Unit (ICU). Patients with three or more risk factors also received droperidol (0.625 mg).
Surgery and anesthetic management
Patients were intubated after induction of anesthesia with midazolam, etomidate, atracurium and sufentanyl. Maintenance was performed with atracurium, sufentanyl, and propofol, +/− volatile anesthetics, but without nitrous oxide. Median sternotomy, normothermic cardiopulmonary bypass, and warm blood cardioplegia were performed whenever needed, without corticosteroids. Full heparinization was antagonized by protamine sulphate. Subxiphoïdal chest tubes (18 or 28 Fr) were used to drain pericardial and mediastinal spaces (with or without additional pleural tubes). Sternal bone was closed with steel wires and stitches, and skin was sutured intracutaneously. At the end of surgery, sedatives were interrupted and patients were transferred to the ICU for fast-track postoperative care. Patients were extubated when they were without risk of reoperation, rewarmed, awake, and able to successfully perform a spontaneous breathing test (median extubation time 4 h). Preoperative cefazolin was continued for 24–48 h, and paracetamol (1 g/6 h) was administered to all patients. No nasogastric tubes were inserted.
Outcomes
All outcomes were assessed at 48 h after surgery.
The primary endpoint was the occurrence of PONV, assessed by nurses over the entire 48 h period. Secondary endpoints were the number of nausea and vomiting events, each assessed according to a visual analog scale (VAS) in terms of severity (where 0 and 100 mm represent no and maximal intensity, respectively), and antiemetics used to treat each episode (rescue therapy was administered following a nurse-driven procedure); postoperative pain (VAS; 0 and 100 mm indicating no and maximal pain, respectively) with number, type, and dose of analgesic drugs used; and postoperative discomfort (VAS). A VAS of 40 mm was considered the threshold of tolerability for all parameters. As a range of analgesic drugs were used, we calculated a “morphine-equivalent“ dosage that approached the equivalent dose of parenteral morphine (mg) received. Morphine-equivalent (mg) = morphine (mg) + ketoprofen (mg)/50 + nefopam (mg)/20 + tramadol (mg)/50.
Safety data collected included side effects, delirium, and QT corrected intervals in the electrocardiograms before and after surgery. We retrospectively defined QTc prolongation as (QTc post- QTc pre-surgery) above the median value (30 ms in our study).
Statistical analyses
Data were expressed in number (%), median (interquartile range), and mean (± standard deviation). Categorical variables were compared using Chi-square test, and continuous variables using Mann–Whitney U-test. Logistic regression, incorporating variables with P < 0.1 in univariate analysis, was then performed as multivariate analysis. A P < 0.05 was considered significant. Analyses were performed with Statview 5.0®, in a nonblinded manner.
Sample size
The study was designed with 80% power to detect a relative difference of 28% between groups (with a significance level of 5%). With the assumption of a 50% risk of PONV in the control group, a total of 400 patients was planned. Inclusion was ultimately prolonged given that few patients included had risk factors, and several protocol violations occurred early in the study. Analyses were performed according to the intention-to-treat, and per-protocol principles.
Results
Postoperative nausea and vomiting and rescue management
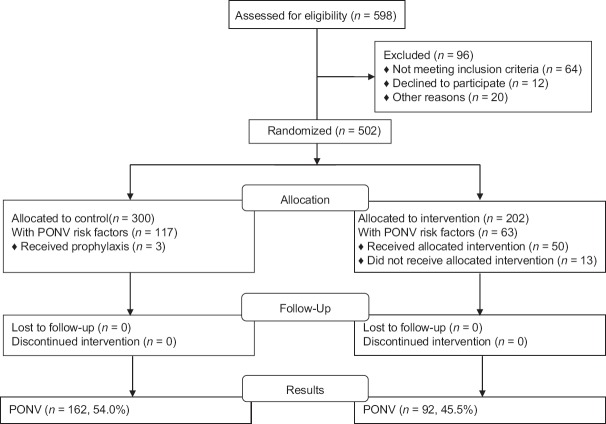
A total of 502 patients were included over a 10-month period from February to November 2016, 300 in the control arm and 202 in the active arm [Figure 1]. Patients had a mean age of 70 years ±7. In terms of cardiac surgery, 321 (64%) underwent a coronary artery bypass graft and 236 (47%) had heart valve surgery (that could be combined). At 1 month postsurgery, none had died.
Figure 1.
Flow chart of patient inclusion and outcome
Analysis of the presence of PONV risk factors showed that patients in the active arm had significantly higher incidence of motion sickness, migraines and a past history of PONV, although the latter factor was borderline significantly different [Table 1]. Apfel and Koivuranta scores did not differ significantly. A high-risk for PONV (≥2 risk factors) was reported in 180 patients (35.9%). In the context of the study, 254 patients (50.6%) experienced PONV, with nausea reported in 176 patients (35.1%; 1.1 ± 1.1, up to six occurrences), and vomiting in 144 patients (28.7%; 1 ± 1.1, up to six occurrences). Patient characteristics in terms of PONV risk factors are presented according to patients who did or did not experience PONV in Table 2 and Figure 2. Incidences of the majority of risk factors were significantly higher in patients who experienced PONV, and significantly higher mean scores were seen for the Apfel and Koivuranta scores as well as for VAS pain.
Table 1.
Clinical characteristics in terms of risk factors for postoperative nausea and vomiting, according to the control arm (n=300) or active arm (n=202)
| Control arm (n=300), n (%) | Active arm (n=202), n (%) | P | |
|---|---|---|---|
| Female sex | 77 (25.7) | 53 (26.2) | 0.89 |
| Nonsmoker | 230 (76.7) | 150 (74.3) | 0.54 |
| Past history of PONV | 37 (12.3) | 14 (6.9) | 0.049 |
| Motion sickness | 46 (15.3) | 14 (6.9) | 0.0044 |
| Migraines | 40 (13.3) | 15 (7.4) | 0.038 |
| Apfel score | 1.4±0.9 | 1.3±0.9 | 0.17 |
| Koivuranta score | 2.3±0.9 | 2.1±0.8 | 0.10 |
| Volatile anesthetics | 260 (86.7) | 167 (82.7) | 0.22 |
PONV: Postoperative nausea and vomiting
Table 2.
Clinical characteristics in terms of risk factors for postoperative nausea and vomiting, according to the presence (n=254) or absence (n=248) of postoperative nausea and vomiting
| PONV (n=254), n (%) | No PONV (n=248), n (%) | P | |
|---|---|---|---|
| Female sex | 87 (34.3) | 43 (17.3) | <0.0001 |
| Nonsmoker | 189 (74.4) | 191 (77) | 0.49 |
| Past history of PONV | 38 (15.0) | 13 (5.2) | 0.0003 |
| Motion sickness | 35 (13.8) | 25 (10.1) | 0.202 |
| Migraines | 38 (15.0) | 17 (6.9) | 0.0036 |
| Volatile anesthetics | 213 (83.9) | 214 (86.3) | 0.45 |
| Apfel score | 1.5±0.9 | 1.2±0.8 | 0.0039 |
| Koivuranta score | 2.4±0.9 | 2.1±0.7 | 0.0029 |
| VAS pain | 41±22 | 37±24 | 0.041 |
| VAS pain >40 | 119 (46.9) | 90 (36.3) | 0.017 |
PONV: Postoperative nausea and vomiting, VAS: Visual analog scale
Figure 2.
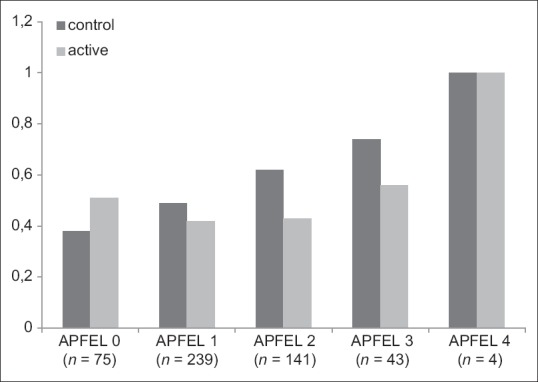
Proportion of postoperative nausea and vomiting according to the number of risk factors for postoperative nausea and vomiting assessed by Apfel scores (x-axis) of 502 patients after cardiac surgery
PONV was considered to be a mild discomfort, as assessed by VAS (11.9 mm ± 19.9), although for 46 patients (9.2%) it was considered the greatest discomfort experienced in terms of their surgical recovery in 45 patients (9.0%) [Table 3]. PONV was considered intolerable (VAS ≥40 mm) in 70 patients (13.9%). Rescue antiemetics were used in 152 patients (30.3%): metoclopramide (10.3 ± 6.8 mg) and ondansetron (1.4 ± 2.2 mg).
Table 3.
Postoperative nausea and vomiting, discomfort induced by postoperative nausea and vomiting assessed by the visual analogic scale, and rescue management, according to the control arm (n=300) or active arm (n=202)
| Control arm (n=300), n (%) | Active arm (n=202), n (%) | P | |
|---|---|---|---|
| PONV | 162 (54.0) | 92 (45.5) | 0.063 |
| Mean nausea±SD | 1.15±1.17 | 1.15±1.17 | 0.97 |
| Mean vomiting events±SD | 1.1±1.2 | 0.8±1 | 0.046 |
| Antiemetics | 96 (32.0) | 58 (28.7) | 0.43 |
| Metoclopramide±SD (mg) | 11.1±6.9 | 9.3±9 | 0.096 |
| Ondansetron±SD (mg) | 2.4±2.9 | 1.1±2.1 | 0.011 |
| Mean VAS PONV score±SD | 15.3±22.6 | 10.9±19.1 | 0.043 |
| VAS PONV ≥40 | 51 (17.0) | 19 (9.4) | 0.016 |
| PONV worst discomfort experienced | 31 (10.3) | 15 (7.4) | 0.27 |
PONV: Postoperative nausea and vomiting, VAS: Visual analog scale, SD: Standard deviation
Prophylaxis
Out of 202 patients included in the active arm, 63 patients (31.2%) had PONV risk factors and 50 (24.8%) received prophylaxis, compared to in the control arm in which 117 patients (39.0%) had risk factors and 3 (1%) received prophylaxis. Protocol violations were reported in 16 patients (3.2%), including administration of corticoids for various reasons in the control arm, and failure to follow the protocol in the active arm.
A summary of PONV, its effect and the use of rescue medication is provided in Table 3. Patients randomized to the active arm experienced borderline less PONV than in the control arm (45.5% vs. 54.0%, P = 0.063; mean VAS PONV score was 10.9 mm vs. 15.3 mm, P = 0.043). When analyzed in the 180 patients with risk factors, prophylaxis was significantly associated with reduced PONV (intention-to-treat: 46.8% vs. 67.8%, P = 0.0061; per-protocol: 39.2% vs. 69%, P = 0.0002). In the multivariate analysis, prophylaxis was independently associated with PONV (odds ratio [OR] 0.324, 95% confidence interval [CI]: 0.167–0.629, P = 0.0009), as were female sex, past history of PONV, and migraines [OR: 3.027, 3.031, and 2.160 respectively, Figure 3].
Figure 3.
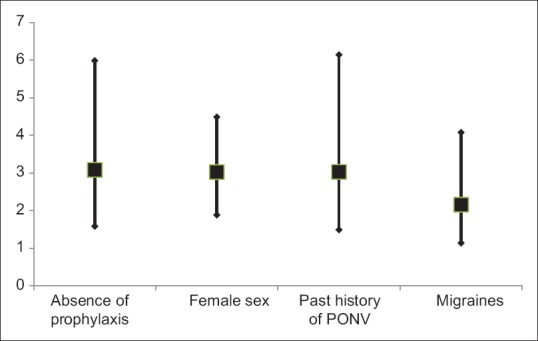
Odds ratio for postoperative nausea and vomiting with 95% confidence intervals of values identified as statistically significant in multivariate analysis. PONV: Postoperative nausea and vomiting
No side effects related to the drugs were reported, with notably no differences in QTc duration [Table 4]. Interestingly, women had significantly longer QTc pre-surgery (439 vs. 421 ms, P < 0.0001), but fewer women experienced QTc prolongation than men (49 women [38%] vs. 204 men [55%], P = 0.0008; mean QTc postsurgery 468 vs. 458 ms, P = 0.002). Ventricular arrhythmias occurred in four patients in the control arm, and in three patients in the active arm (P = 0.8), each of which was related to myocardial infarction.
Table 4.
Safety parameters, according to the control arm (n=300) or active arm (n=202)
| Control arm (n=300), n (%) | Active arm (n=202), n (%) | P | |
|---|---|---|---|
| Drug-related side effects | 0 | 0 | - |
| Delirium | 11 (3.7) | 7 (3.5) | 0.91 |
| Median QTc presurgery | 423 (406-445) | 423 (404-444) | 0.24 |
| Median QTc postsurgery | 459 (440-484) | 456 (439-479) | 0.22 |
| QT prolongation* | 143 (47.7) | 110 (54.5) | 0.14 |
*QTc prolongation: QTc post- QTc presurgery above the median value (30 ms in our study)
Pain and analgesia management
Mean postoperative morphine intake was significantly higher in the active arm compared to the control arm (10.8 vs. 19.8 mg, respectively; P = 0.0011), however morphine-equivalent intake, pain assessment and management did not differ [Table 5]. A significantly higher proportion of patients with PONV reported intolerable pain (VAS >40) than patients without (OR: 1.55; 95% CI: 1.08–2.21; P = 0.17) [Table 2]. Patients treated with nefopam (OR: 1.47; 95% CI: 1.03–2.09; P = 0.034) and ketoprofen (OR: 1.70; 95% CI: 1.08–2.70; P = 0.023) were both significantly more likely to experience PONV. However significance was not seen with tramadol, morphine, or other treatments. Although pain was not independently associated with PONV in the primary multivariate analysis, when risk factors were considered as a binary variable (at risk or not at risk, i.e., <2 or ≥2 risk factors), it reached statistical significance (OR: 1.5; P = 0.03).
Table 5.
Pain and discomfort assessed according to the visual analogic scale, and subsequent analgesic management, according to the control arm (n=300) or active arm (n=202)
| Control arm (n=300), n (%) | Active arm (n=202), n (%) | P | |
|---|---|---|---|
| Morphine | 35 (11.7) | 25 (12.4) | 0.81 |
| Mean morphine (mg) | 10.8±11.3 | 19.8±14 | 0.0011 |
| Mean morphine-equivalent (mg) | 7.1±9.1 | 8±10.3 | 0.60 |
| Nefopam | 168 (56) | 115 (56.9) | 0.84 |
| Mean nefopam (mg) | 52.4±41.3 | 57.2±41.4 | 0.19 |
| Ketoprofen | 57 (19) | 36 (17.8) | 0.74 |
| Ketoprofen (mg) | 232±130 | 203±134 | 0.20 |
| Tramadol | 147 (49) | 86 (42.6) | 0.16 |
| Mean tramadol (mg) | 201±123 | 187±106 | 0.57 |
| Mean VAS pain score | 39.4±23.4 | 38.1±23.3 | 0.58 |
| Mean VAS discomforts score | 38.2±28.1 | 34.2±27.6 | 0.18 |
VAS: Visual analog scale
Discussion
Incidence and prophylaxis of postoperative nausea and vomiting
In our study of 502 patients after cardiac surgery, although 36% were considered high-risk, half (51%) of the study population experienced PONV. While it was generally well-tolerated, PONV was a very significant complication for more than one in ten patients, and required rescue antiemetics in 30%. The incidence of PONV seen in our study is coherent with previous reports, which range from approximately 30% to 45%.[9,10] Our study adds to current knowledge of the prevalence of PONV after cardiac surgery in the fast-track management setting. Several hypotheses as to the cause of PONV are possible including prolonged surgery, a large amount of preoperative opioids, and gut hypoperfusion in patients without prolonged sedation.
Our intervention protocol resulted in a slightly lower incidence PONV. While it failed to reach statistical significance in the overall population, it did result in significantly less vomiting and improved tolerance from the patient perspective, as assessed by VAS. When restricted to patients with at least two risk factors (36% of the overall population), the multivariate analysis showed that prophylaxis resulted in a three-fold decrease in PONV. In contrast in the 322 patients (64% of the population) who did not receive prophylaxis, incidence of PONV was similar in the control and active arms. The statistical design of the study between the active and intervention arms makes it improbable that these results were obtained by chance and likewise the two arms were well-balanced in terms of clinical characteristics. Accordingly, global scores of risk for PONV did not differ between arms, and these results are coherent with published studies consistently showing benefits of prophylaxis, including in cardiac surgery.[9,10,11] The limitation of these published studies is however, that only one treatment at a time was assessed. We performed our study in order to validate a protocol of prophylaxis with multiple drugs (zero, one or two), taking into account individual risk of PONV.[5] Our protocol was relatively well followed, but prophylaxis was not performed intra-operatively. We decided to administer prophylaxis at the end of the prolonged surgery, immediately upon ICU arrival. Given that median extubation time was short (four hours), it is improbable that efficiency would be improved by administering the drugs earlier.
We decided to give first-line betamethasone for several reasons, including cost, effectiveness for PONV prophylaxis, tolerance, and its anti-inflammatory properties that may be valuable for improving shivering, and recovery after ischemia reperfusion, despite no valuable effects for hard outcomes after cardiac surgery.[5,11,12,13,14,15] This approach was also used to allow for administration of rescue antiemetics with first-line ondansetron and metoclopramide.
Midazolam may be preferred over ondansetron if it is used at low doses or in (rare) patients with indications for prolonged mechanical ventilation.[6] Similarly, prophylaxis with metoclopramide is more effective than with ondansetron, and is the preferred option;[10] thus, ondansetron could be administered as rescue therapy if needed. However, we considered that dexamethasone is the preferred choice as an effective treatment for PONV prophylaxis, as it may decrease postoperative inflammation and atrial fibrillation, and improve recovery.[13] Alternatively or in addition, droperidol is effective and safe, although it should not be administered to patients with extrapyramidal disease.[9]
The conclusions we can draw do not extend to the efficiency of different protocols such as adding ondansetron as prophylaxis for very high-risk patients, or prophylaxis with betamethasone or/and droperidol for patients with no, one or two risk factors, respectively. We have now proven that administering 4 mg betamethasone immediately after cardiac surgery for patients with two risk factors (and additional 0.625 mg droperidol if three factors or more are present) is safe and efficient (but is not adequate for preventing PONV in the overall population). Other nonpharmacologic measures, such as gastric emptying by nasogastric tube may be more efficient, although not performed in our patients.[16] However, routine nasogastric tube may promote pulmonary complications. Further studies are necessary to fine-tune the optimal prophylaxis protocol(s) of PONV in cardiac surgery.
Risk factors for postoperative nausea and vomiting
Volatile anesthetics have a particular role in cardiac surgery, including preconditioning. We did not find an association between volatile anesthetics (in 85.1% of our patients) and PONV. Nonetheless, this should not limit their broad use in cardiac surgery.[17] We failed to show a significant association between the presence of motion sickness or smoking status and occurrence of PONV. However based on current knowledge, significant motion sickness should be considered as a risk factor for PONV.[7,8] Clearly, quitting smoking as early as possible in all patients should be a priority, and particularly so in cardiac patients. Other risk factors, such as female sex, past history of PONV, and migraines were associated with two to three-fold increased PONV.[7,8,18] We also believe that our prophylaxis protocol should be intensified after cardiac surgery, as more than half of our patients experienced PONV and risk factors were independent.
Pain and pain management
We hypothesized that prophylaxis of PONV may facilitate analgesic management and subsequently improve postoperative pain and discomfort in high-risk cardiac surgery patients; however we failed to validate this hypothesis. Moreover, there was a converse indication with a higher mean morphine dose administered in the active arm. Nonetheless, we believe that this finding was random because neither morphine-equivalent dosage, nor pain VAS differed between arms. Moreover, PONV were lower in the active arm despite higher morphine dosage. One possible way to manage PONV and hyperalgesia may be to administer clonidine, dexmedetomidine, or ketamine; this hypothesis has yet to be proven.[19] In addition, several analgesic management strategies have been associated with decreased PONV, including paracetamol, non-steroidal anti-inflammatory drugs alone, and combined with dexamethasone (± gabapentine).[20,21,22] Pain management specifically after cardiac surgery is our focus in this study and this area requires dedicated studies to identify the optimal protocol. However, it is of note that we were able to confirm the finding that pain is associated with PONV, independently of its analgesic management.[20] Our findings further support the difficulty of combating pain and PONV after cardiac surgery.
Safety
Although we observed QTc prolongations in some cases before but mainly after cardiac surgery, PONV prophylaxis did not play an apparent role in this. The cardiac disease and intake of other cardiac drugs (not reported in our study) may have been of importance. Female sex was associated with longer pre- and (to a less extent) postsurgery QTc, but less QTc prolongation. In these fragile patients, PONV prophylaxis was considered safe, without any side effects related to the drugs.
Conclusion
betamethasone with/without droperidol was effective in decreasing PONV in high risk cardiac surgical patients without any side effect was effective in decreasing PONV without any side effects. Absence of prophylaxis, female sex, past history of PONV, and migraines were independently associated with about 3-fold increased PONV.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
Assistance with the study: we would like to thank Sarah MacKenzie for English edition, and all the staff that cared for the patients.
References
- 1.Choi DK, Chin JH, Lee EH, Lim OB, Chung CH, Ro YJ, et al. Prophylactic control of post-operative nausea and vomiting using ondansetron and ramosetron after cardiac surgery. Acta Anaesthesiol Scand. 2010;54:962–9. doi: 10.1111/j.1399-6576.2010.02275.x. [DOI] [PubMed] [Google Scholar]
- 2.Koizumi Y, Matayoshi Y, Kondoh K, Nakamura K, Tamura H, Matsuda N, et al. Postoperative nausea and vomiting after open heart surgery. Masui. 2002;51:638–41. [PubMed] [Google Scholar]
- 3.Gan TJ, Mythen MG, Glass PS. Intraoperative gut hypoperfusion may be a risk factor for postoperative nausea and vomiting. Br J Anaesth. 1997;78:476. doi: 10.1093/bja/78.4.476. [DOI] [PubMed] [Google Scholar]
- 4.Kogan A, Eidelman LA, Raanani E, Orlov B, Shenkin O, Vidne BA, et al. Nausea and vomiting after fast-track cardiac anaesthesia. Br J Anaesth. 2003;91:214–7. doi: 10.1093/bja/aeg166. [DOI] [PubMed] [Google Scholar]
- 5.Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004;350:2441–51. doi: 10.1056/NEJMoa032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanjay OP, Tauro DI. Midazolam: An effective antiemetic after cardiac surgery – A clinical trial. Anesth Analg. 2004;99:339–43. doi: 10.1213/01.ANE.0000121772.29181.71. [DOI] [PubMed] [Google Scholar]
- 7.Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: Conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700. doi: 10.1097/00000542-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Koivuranta M, Läärä E, Snåre L, Alahuhta S. A survey of postoperative nausea and vomiting. Anaesthesia. 1997;52:443–9. doi: 10.1111/j.1365-2044.1997.117-az0113.x. [DOI] [PubMed] [Google Scholar]
- 9.Grebenik CR, Allman C. Nausea and vomiting after cardiac surgery. Br J Anaesth. 1996;77:356–9. doi: 10.1093/bja/77.3.356. [DOI] [PubMed] [Google Scholar]
- 10.Woodward DK, Sherry KM, Harrison D. Antiemetic prophylaxis in cardiac surgery: Comparison of metoclopramide and ondansetron. Br J Anaesth. 1999;83:933–5. doi: 10.1093/bja/83.6.933. [DOI] [PubMed] [Google Scholar]
- 11.Halvorsen P, Raeder J, White PF, Almdahl SM, Nordstrand K, Saatvedt K, et al. The effect of dexamethasone on side effects after coronary revascularization procedures. Anesth Analg. 2003;96:1578–83. doi: 10.1213/01.ANE.0000063922.90966.3A. table of contents. [DOI] [PubMed] [Google Scholar]
- 12.Henzi I, Walder B, Tramèr MR. Dexamethasone for the prevention of postoperative nausea and vomiting: A quantitative systematic review. Anesth Analg. 2000;90:186–94. doi: 10.1097/00000539-200001000-00038. [DOI] [PubMed] [Google Scholar]
- 13.Murphy GS, Sherwani SS, Szokol JW, Avram MJ, Greenberg SB, Patel KM, et al. Small-dose dexamethasone improves quality of recovery scores after elective cardiac surgery: A randomized, double-blind, placebo-controlled study. J Cardiothorac Vasc Anesth. 2011;25:950–60. doi: 10.1053/j.jvca.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Whitlock RP, Devereaux PJ, Teoh KH, Lamy A, Vincent J, Pogue J, et al. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): A randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:1243–53. doi: 10.1016/S0140-6736(15)00273-1. [DOI] [PubMed] [Google Scholar]
- 15.Yared JP, Starr NJ, Hoffmann-Hogg L, Bashour CA, Insler SR, O’Connor M, et al. Dexamethasone decreases the incidence of shivering after cardiac surgery: A randomized, double-blind, placebo-controlled study. Anesth Analg. 1998;87:795–9. doi: 10.1097/00000539-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Lavi R, Katznelson R, Cheng D, Minkovich L, Klein A, Carroll J, et al. The effect of nasogastric tube application during cardiac surgery on postoperative nausea and vomiting – A randomized trial. J Cardiothorac Vasc Anesth. 2011;25:105–9. doi: 10.1053/j.jvca.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Uhlig C, Bluth T, Schwarz K, Deckert S, Heinrich L, De Hert S, et al. Effects of volatile anesthetics on mortality and postoperative pulmonary and other complications in patients undergoing surgery: A systematic review and meta-analysis. Anesthesiology. 2016;124:1230–45. doi: 10.1097/ALN.0000000000001120. [DOI] [PubMed] [Google Scholar]
- 18.Rimaitis K, Svitojūte A, Macas A. The influence of dexamethasone and ketolgan on postoperative nausea and vomiting and estimation of risk factors in women undergoing gynecologic laparoscopic surgeries. Medicina (Kaunas) 2010;46:261–7. [PubMed] [Google Scholar]
- 19.Piper SN, Beschmann RB, Mengistu A, Maleck WH, Boldt J, Röhm KD, et al. Postoperative analgosedation with S(+)-ketamine decreases the incidences of postanesthetic shivering and nausea and vomiting after cardiac surgery. Med Sci Monit. 2008;14:PI59–65. [PubMed] [Google Scholar]
- 20.Apfel CC, Turan A, Souza K, Pergolizzi J, Hornuss C. Intravenous acetaminophen reduces postoperative nausea and vomiting: A systematic review and meta-analysis. Pain. 2013;154:677–89. doi: 10.1016/j.pain.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 21.Chandrakantan A, Glass PS. Multimodal therapies for postoperative nausea and vomiting, and pain. Br J Anaesth. 2011;107(Suppl 1):i27–40. doi: 10.1093/bja/aer358. [DOI] [PubMed] [Google Scholar]
- 22.Rafiq S, Steinbrüchel DA, Wanscher MJ, Andersen LW, Navne A, Lilleoer NB, et al. Multimodal analgesia versus traditional opiate based analgesia after cardiac surgery, a randomized controlled trial. J Cardiothorac Surg. 2014;9:52. doi: 10.1186/1749-8090-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]