Abstract
Background:
High cord signals (HCS) on preoperative/postoperative T1, T1 gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA), and T2 magnetic resonance (MR) studies, postoperative failure of HCS to regress and/or cord re-expansion, and a triangular cord configuration are poor prognostic factors for surgical patients with cervical spondylotic myelopathy (CSM).
Methods:
Here, we reviewed the negative prognostic import of high Grades/Types and more extensive locations of preoperative/postoperative HCS on T1, T1 Gd-DTPA, and T2 MR studies in surgical patients with CSM. Additional predictors of poor operative outcomes included postoperative failure of HCS to regress, cord re-expansion at the site of a HCS, and the triangular vs. teardrop or boomerang cord configuration. The Types/Grades of HCS on MR follow:Type/Grade 0 – no/absent signal changes; Type/Grade 1 – mild/light/fuzzy/obscure/low cord signal (LCS) changes; Type/Grade 2 – sharp/intense/well-defined HCS; and Type/Grade 3 – mixed/HCS. The definitions of location/extent of LCS/HCS were: focal (1 level), multifocal (with skip areas), and multisegmental (continuous over >1 segment), while cord configuration was categorized as triangular, teardrop, or boomerang.
Results:
On MR studies, preoperative/postoperative Types/Grades 0–1 changes correlated with better prognoses (e.g., improved Japanese Orthopedic Association (JOA) scores or Nurick Grades), while Types/Grades 2–3 correlated with poorer outcomes. Multiple poor prognostic indicators also included; failure of postoperative HCS on MR to regress (particularly if multisegmental), postoperative cord re-expansion at the site of a prior HCS, and triangular cord configuration.
Conclusions:
Grade/Types 2–3 HCS on T1, T1 Gd-DTPA, and T2-weighted MR images on preoperative/postoperative MR studies, failure of HCS to regress (multisegmental), cord re-expansion at the site of a prior HCS, and a triangular cord configuration (atrophy) all contributed to poorer outcomes for CSM surgery.
Keywords: Cervical surgery, cord configuration, hyperintense/high cord signal (HCS), ossification of the posterior longitudinal ligament, magnetic resonance, prognostic indicators, spondylotic myelopathy
INTRODUCTION
Here, we reviewed the prognostic import of preoperative/postoperative high cord signals (HCS) on magnetic resonance images [MR: T1, T1 gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA), and T1] for patients with cervical spondylotic myelopathy. Outcomes were correlated with patients undergoing anteiror cervical diskectomy/fusion (ACDF), anterior corpectomy/fusion (ACF), laminectomy with/without posterior fusion (LAM), and laminoplasty (LOP) [Tables 1–3; Figures 1–6].[1,2,3,4,5,6,7,8,9,10,11,12,13,14,15] Several Types/Grades helped assess the severity/prognostic import of low cord signals (LCS) and high cord signals (HCS) on preoperative/postoperative MR studies; Type/Grade 0: no/absent low cord signal (LCS), Type/Grade 1: faint/fuzzy/mild/obscure LCS; Grade 2: middle/intense/sharp HCS; and Type/Grade 3: mixed/HCS.[1,5,8,14] Additionally, the location/extent of HCS on T2 sagittal MR studies also impacted outcome and were defined as: focal (single level), multifocal (with skip areas), and/or multisegmental (MS: >1 level; continuous).[1,8] Better outcomes for CSM patients correlated with Type/Grade 0/Type Grade 1 findings on preoperative/postoperative MR studies.[5,9] Significant trends toward improvement also correlated with >50% regression of HCS on postoperative T2 MR, particularly when patients were followed for >18 postoperative months.[8] In summary, worse/poor prognostic indicators for postoperative outcomes in CSM patients, therefore, included; HCS (Types/Grade 2 and 3) on preoperative/postoperative MR studies, failure of HCS to regress (especially if multisegmetal), cord re-expansion at sites of HCS, and triangular cord configuration (indicative of underlying cord atrophy) [Tables 1–3].[1,3,4,6,11,13,14]
Table 1.
Postoperative magnetic resonance imaging documenting adequate spinal cord decompression following cervical laminectomy/fusion (2003–2010)
Table 3.
Postoperative MR imaging documenting adequate spinal cord decompression following cervical laminectomy/fusion (2016-2017)
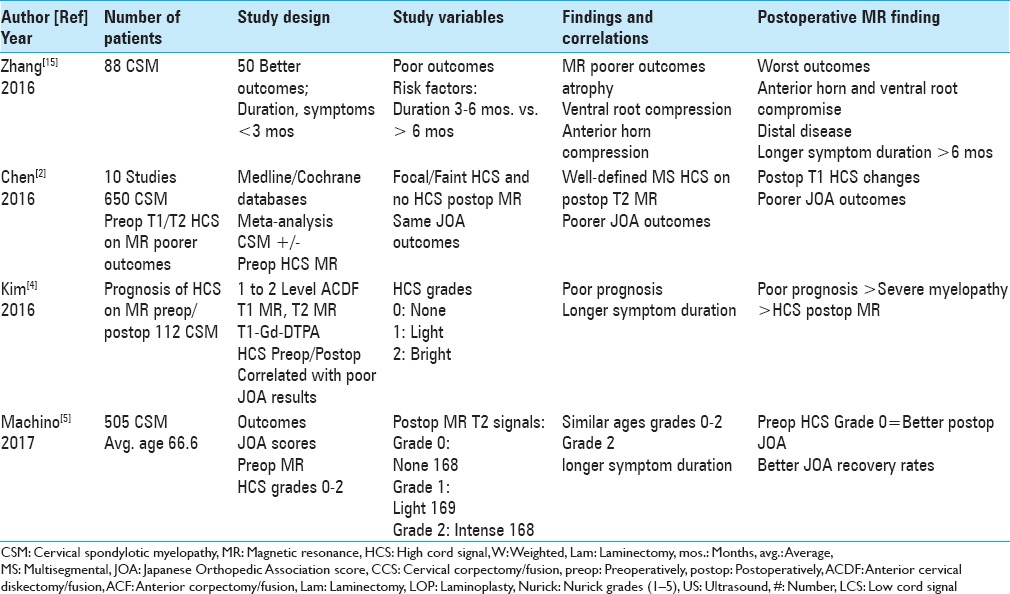
Figure 1.
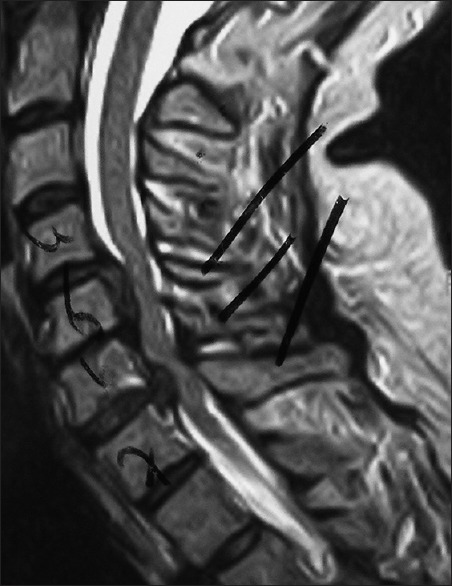
This midline sagittal T2W MR study documented marked cord compression attributed to severe anterior (C4-C6, C6-C7) OPLL, and marked dorsolateral cord compression (C4-C7; ossification of the yellow ligament with laminar shingling). Not the compression was so severe opposite the C5 body that a HCS could not be readily seen on the preoperative T2 MR study
Figure 6.
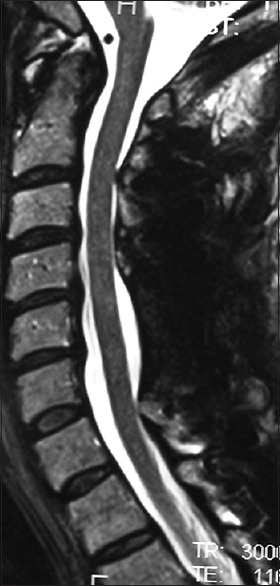
For the same patient in Figure 5, the midline sagittal T2 MR documented excellent postoperative cord decompression. Note the complete perimeter of spinal fluid around the cord at all levels, without any residual evidence of intrinsic LCS or HCS
Figure 4.
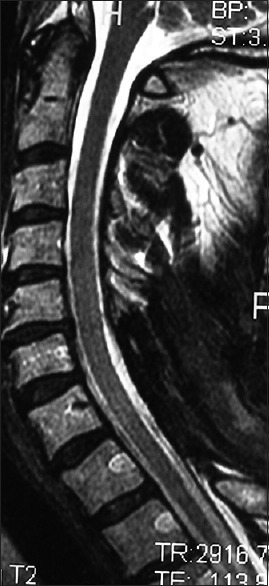
The 6-week postoperative midline sagittal T2 MR in another patient documented adequate cord decompression following a C5-C7 laminectomy, undercutting C4/T1, and posterior C2/T2 fusion. Note, this patient also had no preoperative HCS on the T2 study that correlated with his full postoperative neurological recovery
Figure 5.
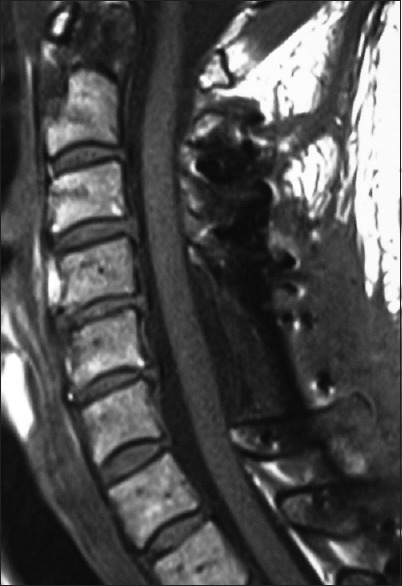
This midline sagittal postoperative 6-week T1 MR documented excellent cord decompression in another patient following a C5-C7 laminectomy, undercutting of C4/T1, with posterior C2/T2 fusion. Note this patient showed neither LCS nor HCS on the preoperative or postoperative T1 or T2 MR studies that nicely correlated with his completely intact neurological status
Types/grades of HCS on preoperative/postoperative MR studies
Several studies proposed different Types/Grades of HCS identified on preoperative/postoperative T1, T1-DTPA, and T2 MR studies [Tables 1–3; Figures 1–6].[1,5,8,14] These included: Type/Grade 0: no/none/absent/LCS; Type/Grade 1: faint/fuzzy/mild/obscure/LCS; Type/Grade 2: middle/intense/sharp HCS; and Type/Grade 3: mixed/HCS. For the 505 CSM patients in Machino et al. series, nearly equal numbers of patients were assigned to one of the three Types/Grades of HCS on T2 MR studies: Type/Grade 0 (none – 168 patients); Type/Grade 1 (light/obscure – 169 patients); and Type/Grade 2 (intense/bright – 168 patients) [Table 3].[5] Notably, the higher the Types/Grades documenting HCS on preoperative/postoperative MR studies, the greater the likelihood of poorer outcomes.[1,5,8,14]
Location/extent of HCS and dorsal cord migration on preoperative/postoperative MR studies in CSM patients
Several series also correlated the location/extent of HCS on T2-weighted (T2W) sagittal MR studies with prognoses for CSM surgery [Tables 1 and 2; Figure 2].[1,7,8] The best results were seen with focal (single level) or multifocal (with skip areas) HCS on T2 MR, while the worst outcomes occurred if HCS were multisegmental (MS: >1 level; continuous). Specifically, when Sarkar et al. (2014) categorized 56 patients’ HCS on T2 MR as focal/single lesions, multifocal/with skip areas, or multisegmental/continuous lesions, the latter yielded the poorest results.[8] Additionally, Naruse et al. (2009) correlated better outcomes for 101 CSM patients undergoing LOP with greater postoperative dorsal cord migration (e.g. with both ultrasound and MR).[7]
Table 2.
Postoperative magnetic resonance imaging documenting adequate spinal cord decompression following cervical laminectomy/fusion (2011-2015)
Figure 2.
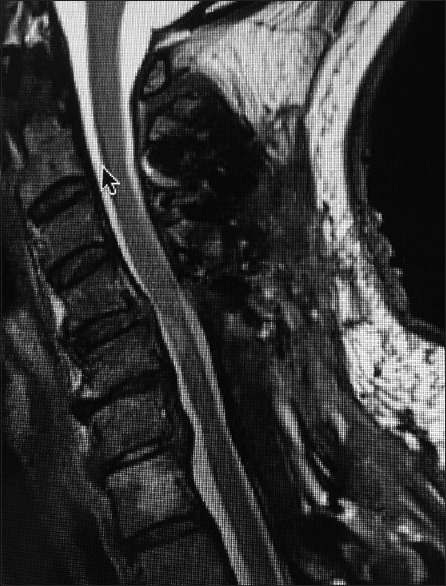
The patient in Figure 1 underwent a laminectomy C5-C7, undercutting of C4 and T1, with posterior fusion C2-T2. Although the postoperative sagittal T2 MR study documented adequate cord decompression at all levels, the HCS opposite the C5 body reflected intrinsic cord damage that correlated with residual/improving myelopathy (preoperative Nurick Grades 4 and 5 to postoperative Nurick Grade 1)
Prognostic import of axial MR cord shape on outcomes for CSM surgery
Axial MR images documenting different cord configurations (e.g. boomerang, teardrop, or triangular) reflected differing degrees/severity of cord atrophy that also impacted outcomes of CSM surgery [Tables 1 and 3].[6,15] Matsuyama et al. (2004) correlated the preoperative MR cord shape with postoperative outcomes in 44 patients with CSM/ossification of the posterior longitudinal ligament (OPLL) undergoing LOP.[6] The best outcomes correlated with the teardrop configuration, intermediate outcomes with the boomerang shape, and the worse outcomes with the triangular cord configuration.[6] Additional poor prognostic factors included: LCS on T1/HCS on T2 preoperative MR studies, and postoperative cord re-expansion at the sites of prior HCS (e.g. reflecting cord atrophy). When Zhang et al. (2016) evaluated poor prognostic risk factors for 88 patients with CSM undergoing cervical surgery, poorer outcomes correlated with MR evidence of more anterior horn compression with/without ventral root compression, distal cord atrophy, and longer symptom duration (e.g. >6 months).[15]
Better outcomes with Types/Grades 0–1 and worse outcomes with Types/Grades 2–3 HCS on T1/T2 preoperative/postoperative MR studies
On preoperative/postoperative T1/T2 MR studies, Types/Grades 0–1 (no/LCS) correlated with better neurological outcomes, while Types/Grades 2–3 (severe/HCS) correlated with poorer outcomes [Tables 1–3; Figures 3–6].[5,8,9,12] Suri et al. (2003) assessed how HCS on preoperative/postoperative T1/T2 MR studies impacted outcomes for 146 CSM patients undergoing ACDF, ACF, LAM, or LOP.[9] Preoperative HCS were seen in 121 of 146 patients (82.9%); 81 had both T1/T2 HCS on MR, while 40 had HCS on only T2 studies. The best outcomes occurred in 14 of 44 patients whose preoperative HCS on T2 MR regressed; the worst outcomes were observed for those with postoperative residual T1/T2 HCS reflecting longstanding, chronic/multiple disc herniations, and/or residual cord compression. When Vedantam and Rajshekhar (2013) looked at preoperative HCS on T2W MR images for 1508 CSM patients (11 studies; PubMed and Cochrane databases), better outcomes correlated with the regression of HCS on postoperative MR scans (e.g. 5 of 10 studies), while poorer results were seen where HCS remained sharp/intense (6 studies) and/or multisegmental (5 studies) [Table 2].[12] In Sarkar et al. (2014), where 54 of 56 patients had HCS on preoperative T2 MR studies, there was a significant trend toward improved postoperative JOA scores/Nurick grades where multisegmental Type 3 (mixed) lesions improved postoperatively to Type 2 lesions [sharp: 71% (>50% regression of HCS on T2 MR; followed >18 months)] [Table 2].[8] In Machino et al. (2017 Spine) series of 505 patients with preoperative HCS on T2 MR, those showing postoperative regression to Grade 0 demonstrated better postoperative outcomes.[5]
Figure 3.
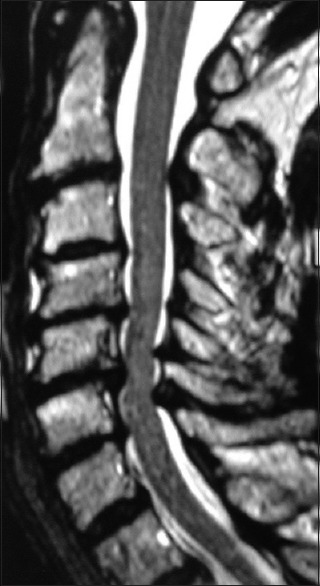
This preoperative midline sagittal T2 MR without a HCS showed moderate anterior osteophytic ridging and maximal dorsolateral cord compression (ossification of the yellow ligament/laminar shingling) from C4-C7 with an excellent cervical lordosis. Here, following a cervical laminectomy of C5-C7, undercutting of C4/T1, and posterior C2-C2 fusion, the patient was fully neurologically intact (Nurick Grade IV to 0)
Poorer prognoses for HCS on MR, triangular cord configuration, and cord re-expansion
In several studies, poorer prognoses correlated with preoperative HCS on MR (T1, T1-Gd-DTPA, and T2), preoperative and persistent postoperative triangular cord configuration (reflecting underlying cord atrophy), and cord re-expansion at the site of a prior HCS [Tables 1 and 2; Figures 1 and 2].[1,2,3,4,6,11,13,14] When Matsuyama et al. (2004) evaluated 44 patients with CSM/OPLL undergoing LOP, the worst postoperative outcomes correlated with preoperative LCS on T1/HCS on T2 MR, postoperative MR-documented cord re-expansion, and triangular cord configuration (e.g. best prognosis-teardrop, intermediate prognosis-boomerang).[6] In Yagi et al. (2010) 50 of 71 CSM/OPLL patients undergoing LOP, the worst JOA outcomes correlated with persistent postoperative HCS on MR; this remained true even for the 16 patients showing postoperative re-expansion [Table 1].[13] Also in 2010, Avadhani et al. found poorer postoperative Nurick grades in 35 CSM patients undergoing LAM, where HCS on preoperative T2 MR failed to resolve or patients developed the late onset of LCS on T1 MR (average 51.3 postoperative months).[1] Additionally, for the 73 CSM patients with HCS on preoperative MR studies, Zhang et al. (2010) correlated poorer surgical outcomes for those with the most severe persistent HCS (Grade 3) on postoperative MR studies.[14] Further, Cho et al. (2011) found that HCS on preoperative T1 Gd-DTPA MR studies constituted the worst prognostic sign for JOA outcomes in 74 CSM patients undergoing 1 to 2 level ACDF (followed on average 39.7 months).[3] In Uchida et al. (2014) study of 148 patients (102 CSM; 46 OPLL), poorer outcomes correlated with LCS on preoperative T1/HCS on T2 MR, while better outcomes were seen with HCS on preoperative T1/no HCS on postoperative T2 MR.[11] When Chen et al. (2016) evaluated 10 studies (Medline/Cochrane databases) involving 650 CSM surgical patients, better JOA outcomes correlated with Types/Grades 0–1 preoperative MR findings, while poorer outcomes were seen with postoperative HCS on T1/T2 studies (especially multisegmental).[2] For 112 patients with CSM undergoing 1 to 2 level ACDF, Kim et al. (2016) also correlated HCS on preoperative/persistent postoperative MR studies (T1, T1 Gd-DTPA, T2) with poorer outcomes.[4]
Early surgery recommended for CSM based on MR findings
Tauchi et al. (2015) recommended that CSM patients with preoperative MR studies already showing LCS on T1 and HCS on T2 MR studies, along with extensive/severe stenosis, cord compression, and kyphosis, should be considered for early surgery (within <4 months; whether ACDF, ACF, LOP, LAM) [Table 2].[10]
CONCLUSION
Here we reviewed multiple MR-based (MR: T1, T2, T1 enahnced studies) prognostic factors for CSM patients undergoing spinal surgery [Tables 1–3].[1,2,3,4,5,6,7,8,9,10,11,12,13,14,15] The best/better outcomes correlated with Grade 0/Grade 1 MR changes on preoperative/postoperative MR studies, or trends toward improvement where postoperative T2 HCS regressed.[5,8,9] Poorer/worse prognoses correlated with HCS seen on all preoperative/postoperative MR scans, their failure to regress, documentation of cord re-expansion at sites of prior HCS, and residual triangular cord configurations.[1,3,4,6,11,13,14]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
REFERENCES
- 1.Avadhani A, Rajasekaran S, Shetty AP. Comparison of prognostic value of different MRI classifications of signal intensity change in cervical spondylotic myelopathy. Spine J. 2010;10:475–85. doi: 10.1016/j.spinee.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Pan J, Nisar M, Zeng HB, Dai LF, Lou C, et al. The value of preoperative magnetic resonance imaging in predicting postoperative recovery in patients with cervical spondylosis myelopathy: A meta-analysis. Clinics (Sao Paulo) 2016;71:179–84. doi: 10.6061/clinics/2016(03)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho YE, Shin JJ, Kim KS, Chin DK, Kuh SU, Lee JH, et al. The relevance of intramedullary high signal intensity and gadolinium (Gd-DTPA) enhancement to the clinical outcome in cervical compressive myelopathy. Eur Spine J. 2011;20:2267–74. doi: 10.1007/s00586-011-1878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim TH, Ha Y, Shin JJ, Cho YE, Lee JH, Cho WH. Signal intensity ratio on magnetic resonance imaging as a prognostic factor in patients with cervical compressive myelopathy. Medicine (Baltimore) 2016;95(39):e4649. doi: 10.1097/MD.0000000000004649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machino M, Imagama S, Ando K, Kobayashi K, Ito K, Tsushima M, et al. The Image Diagnostic Classification of MR T2 Increased Signal Intensity in Cervical Spondylotic Myelopathy: Clinical Evaluation Using Quantitative and Objective Assessment. Spine (Phila Pa 1976) 2017 doi: 10.1097/BRS.0000000000002328. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Matsuyama Y, Kawakami N, Yanase M, Yoshihara H, Ishiguro N, Kameyama T, et al. Cervical myelopathy due to OPLL: Clinical evaluation by MRI and intraoperative spinal sonography. J Spinal Disord Tech. 2004;17:401–4. doi: 10.1097/01.bsd.0000112087.85112.86. [DOI] [PubMed] [Google Scholar]
- 7.Naruse T, Yanase M, Takahashi H, Horie Y, Ito M, Imaizumi T, et al. Prediction of clinical results of laminoplasty for cervical myelopathy focusing on spinal cord motion in intraoperative ultrasonography and postoperative magnetic resonance imaging. Spine (Phila Pa 1976) 2009;34:2634–41. doi: 10.1097/BRS.0b013e3181b46c00. [DOI] [PubMed] [Google Scholar]
- 8.Sarkar S, Turel MK, Jacob KS, Chacko AG. The evolution of T2-weighted intramedullary signal changes following ventral decompressive surgery for cervical spondylotic myelopathy: Clinical article. J Neurosurg Spine. 2014;21:538–46. doi: 10.3171/2014.6.SPINE13727. [DOI] [PubMed] [Google Scholar]
- 9.Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J. 2003;3:33–45. doi: 10.1016/s1529-9430(02)00448-5. [DOI] [PubMed] [Google Scholar]
- 10.Tauchi R, Imagama S, Inoh H, Yukawa Y, Kanemura T, Sato K, et al. Appropriate timing of surgical intervention for the proximal type of cervical spondylotic amyotrophy. Eur J Orthop Surg Traumatol. 2015;25(Suppl 1):S107–13. doi: 10.1007/s00590-014-1504-2. [DOI] [PubMed] [Google Scholar]
- 11.Uchida K, Nakajima H, Takeura N, Yayama T, Guerrero AR, Yoshida A, et al. Prognostic value of changes in spinal cord signal intensity on magnetic resonance imaging in patients with cervical compressive myelopathy. Spine J. 2014;14:1601–10. doi: 10.1016/j.spinee.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 12.Vedantam A, Rajshekhar V. Does the type of T2-weighted hyperintensity influence surgical outcome in patients with cervical spondylotic myelopathy? A review. Eur Spine J. 2013;22:96–106. doi: 10.1007/s00586-012-2483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yagi M, Ninomiya K, Kihara M, Horiuchi Y. Long-term surgical outcome and risk factors in patients with cervical myelopathy and a change in signal intensity of intramedullary spinal cord on Magnetic Resonance imaging. J Neurosurg Spine. 2010;12:59–65. doi: 10.3171/2009.5.SPINE08940. [DOI] [PubMed] [Google Scholar]
- 14.Zhang YZ, Shen Y, Wang LF, Ding WY, Xu JX, He Magnetic resonance T2 image signal intensity ratio and clinical manifestation predict prognosis after surgical intervention for cervical spondylotic myelopathy. J Spine (Phila Pa 1976) 2010;35:E396–9. doi: 10.1097/BRS.0b013e3181c6dbc4. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Cui C, Liu Z, Tong T, Niu R, Shen Y. Predisposing factors for poor outcome of surgery for cervical spondylotic amyotrophy: A multivariate analysis. Sci Rep. 2016;6:39512. doi: 10.1038/srep39512. [DOI] [PMC free article] [PubMed] [Google Scholar]


