Abstract
Human voltage-gated sodium channel NaV1.2 has a single pore-forming α-subunit and two transmembrane β-subunits. Expressed primarily in the brain, NaV1.2 is critical for initiation and propagation of action potentials. Milliseconds after the pore opens, sodium influx is terminated by inactivation processes mediated by regulatory proteins including calmodulin (CaM). Both calcium-free (apo) CaM and calcium-saturated CaM bind tightly to an IQ motif in the C-terminal tail of the α-subunit. Our thermodynamic studies and solution structure (2KXW) of a C-domain fragment of apo 13C,15N-CaM (CaMC) bound to an unlabeled peptide with the sequence of rat NaV1.2 IQ motif showed that apo CaMC (a) was necessary and sufficient for binding, and (b) bound more favorably than calcium-saturated CaMC. However, we could not monitor the NaV1.2 residues directly, and no structure of full-length CaM (including the N-domain of CaM (CaMN)) was determined. To distinguish contributions of CaMN and CaMC, we used solution NMR spectroscopy to assign the backbone resonances of a complex containing a 13C,15N-labeled peptide with the sequence of human NaV1.2 IQ motif (NaV1.2IQp) bound to apo 13C,15N-CaM or apo 13C,15N-CaMC. Comparing the assignments of apo CaM in complex with NaV1.2IQp to those of free apo CaM showed that residues within CaMC were significantly perturbed, while residues within CaMN were essentially unchanged. The chemical shifts of residues in NaV1.2IQp and in the C-domain of CaM were nearly identical regardless of whether CaMN was covalently linked to CaMC. This suggests that CaMN does not influence apo CaM binding to NaV1.2IQp.
Keywords: Voltage-Gated Sodium Channel, Molecular Recognition, Domain Interactions, Allostery, EF-hand protein
Biological Context
The human type 2 voltage-gated sodium channel (NaV1.2) is a large transmembrane protein that is primarily expressed in the central nervous system. In neurons, NaV1.2 is found as a heterotrimer composed of a single pore-forming α-subunit interacting with two auxiliary β-subunits that are also transmembrane proteins (Messner and Catterall 1985). In the central nervous system, the function of NaV1.2 is critical for the initiation and propagation of action potentials. At the molecular level, this depends on conformational responses of the NaV1.2 α-subunit to changes in membrane potential. These cause the pore to open and allow sodium to flow rapidly from the extracellular space into the cytosol (Yang, George et al. 1996, Yu and Catterall 2003, Yarov-Yarovoy, DeCaen et al. 2012). Within milliseconds of opening, the flow of sodium through the channel is halted by a process known as fast inactivation (Gawali and Todt 2016). Mutations within the channel that alter the rapid inactivation of the α-subunit have been linked to multiple debilitating conditions, highlighting the importance of tight control of sodium entry (Ogiwara, Ito et al. 2009, Shi, Yasumoto et al. 2012, Nakamura, Kato et al. 2013).
Fast inactivation is modulated by numerous protein-protein interactions including intramolecular domain interactions between cytosolic elements of the α-subunit itself, and intermolecular association with the β-subunits, and several other auxiliary proteins (O’Malley and Isom 2015, Wildburger, Ali et al. 2015, Ahern, Payandeh et al. 2016). One regulator is calmodulin (CaM), a small, essential, and ubiquitously expressed calcium-sensing protein that binds 4 calcium ions. CaM is composed of two domains (CaMN and CaMC), each of which has a pair of calcium-binding EF-hands.
CaM recognizes two intracellular sites in most NaV α-subunits in a calcium-dependent manner. (Ca2+)4-CaM, but not apo CaM, binds to the highly conserved inactivation gate (also called the DIII-DIV linker) of NaV1.5 with a dissociation constant (KD) of ~0.6 μM mediated by CaMC (Sarhan, Tung et al. 2012). In contrast, the IQ motif [IQxxx[R,K]Gxxx[R,K] found in the C-terminal domain (CTD) of NaV1.2 binds more tightly to apo CaM than (Ca2+)4-CaM (Mori, Konno et al. 2000, Theoharis, Sorensen et al. 2008, Feldkamp, Yu et al. 2011, Wang, Chung et al. 2014). Our recent thermodynamic studies of CaM binding to the IQ motif of rat NaV1.2 determined that the KD for apo CaM binding was ~3 nM, while the KD for (Ca2+)4-CaM was ~85 nM (Hovey, Fowler et al. 2017). The physiological importance of apo CaM binding to the IQ motif was highlighted by the finding that this interaction limits persistent current through NaV1.2, 1.5, and 1.6 (Yan, Wang et al. 2017).
High-resolution structures of apo CaM bound to the IQ motif of NaV1.5 (2L53, 4OVN, 4DCK) and 1.6 (3WFN) showed that CaMC made the majority of contacts with the IQ motif. In contrast, CaMN adopted different positions relative to CaMC and the channel fragment in crystallographic structures (Wang, Chung et al. 2012, Reddy Chichili, Xiao et al. 2013, Gabelli, Boto et al. 2014), and sampled many positions in the only available solution structure (Chagot and Chazin 2011). Currently there is no high-resolution structure available for apo CaM bound to the NaV1.2 IQ motif from any species, although there is one (2KXW) for apo CaMC bound to the rat NaV1.2 IQ motif (Feldkamp, Yu et al. 2011).
To determine whether and how apo CaMN interacts with the NaV1.2 IQ motif in solution, and whether it affects the interface between apo CaMC and the NaV1.2 IQ motif, we have used solution NMR spectroscopy to assign the backbone resonances of 13C,15N- apo CaM and 13C,15N-apo CaMC bound to a 13C,15N-labeled peptide that corresponds to the human NaV1.2 IQ motif. These complexes are referred to as apo CaM+NaV1.2IQp and apo CaMC+NaV1.2IQp throughout this report.
A comparison of the assignments of apo CaM+NaV1.2IQp to assignments of free apo CaM indicated that residues in CaMC underwent large chemical shift perturbations upon binding the peptide, while chemical shifts for residues in CaMN were almost identical to those observed for the same residues in free CaM. Additionally, the chemical shifts of residues in the C-domain of CaM and NaV1.2IQp were nearly identical in apo CaM+NaV1.2IQp and apo CaMC+NaV1.2IQp. These data are consistent with the interpretation that only residues in the C-domain of CaM mediate binding to NaV1.2IQp, while apo CaMN remains free to interact with other regulatory components near the intracellular opening of the NaV1.2 pore.
Materials and Experiments
Protein Expression and Purification
The complexes CaM+NaV1.2IQp and CaMC+NaV1.2IQp, were obtained by bacterial co-expression of CaM or CaMC with a GST-tagged sequence corresponding to the IQ motif of NaV1.2. Full-length human CaM (residues 1-148) or CaMC (residues 76-148) were expressed using a pT7-7 vector (AmpicillinR) (Sorensen and Shea 1998). The peptide NaV1.2IQp was expressed using a modified form of a pBG101 vector (KanamycinR) containing an N-terminal GST-tag and a 3C protease cleavage site (Damo, Feldkamp et al. 2013). It was a generous gift from Walter Chazin, Director of the Center for Structural Biology at Vanderbilt University. The plasmid was modified to code for residues 1901–1927 of human NaV1.2 [KRKQEEVSAIIIQRAYRRYLLKQKVKK]. Because the 3C protease site is between the sequence of GST and the IQ motif, NaV1.2IQp contains a 4-residue N-terminal tag having the sequence G-P-G-S; these residues were labeled with negative integers (−4 to −1) in the BMRB deposits. The final sequence of NaV1.2IQp was GPGSKRKQEEVSAIIIQRAYRRYLLKQKVKK.
Escherichia coli BL21(DE3) cells co-transformed with plasmids for both CaM (or CaMC) and GST-NaV1.2IQp were grown in four baffled flasks containing 1 L cultures of LB at 37°C to an OD600 of 0.7. Cells were pelleted by centrifugation at 4,000 rpm, 4°C for 20 min, and exchanged into 1 L of minimal media containing 1 g/L 15NH4Cl (CIL) and 4 g/L 13C6-glucose (CIL) (Marley, Lu et al. 2001). After a 1 hr recovery period, expression was induced with 1 mL of 0.8 M IPTG. Four hours after addition of IPTG, cells were pelleted by centrifugation at 4,000 rpm, 4°C, for 20 min, and stored at −20°C.
The cell pellet was thawed and resuspended in 30 mL of lysis buffer (50 mM HEPES, 100 mM KCl, 1 mM MgCl2, 5 mM NTA, 50 μM EGTA, 0.01% NaN3 (w/v), pH 7.4), and sonicated. Lysate was centrifuged at 20,000 rpm, 4°C for 50 min; supernatant was passed through a 0.22 μm sterile PVDF filter, added to 10 mL of Glutathione Sepharose resin (Amersham Biosciences), and gently rocked at 4°C. Resin was washed with 50 mL of lysis buffer, 50 mL of a salt wash buffer (lysis buffer with 500 mM KCl), and 50 mL of lysis buffer at 4°C.
To release CaM+NaV1.2IQp or CaMC+NaV1.2IQp, resin was incubated with rhinovirus 3C protease for 4 h at 22 to 25 °C with gentle rocking. The released complex was collected, concentrated and exchanged into a buffer of 10 mM imidazole, 100 mM KCl, 100 μM EDTA, and 0.01% (w/v) NaN3, at pH 6.5. Protein purity (> 95%) was assessed by SDS-PAGE, UV/Vis spectroscopy, and rpHPLC. Protein concentrations were determined with the BCA assay (Pierce Biotechnology; Rockford IL).
NMR Experiments
All NMR spectra were collected at 25°C (298 K) using a 4-channel Unity Inova 600 MHz Oxford AS600 NMR spectrometer (Varian) equipped with a triple resonance gradient probe. The NMR samples consisted of 850 μM apo 13C,15N-labeled CaMC+NaV1.2IQp or 950 μM apo 13C,15N -labeled CaM+NaV1.2IQp in 10 mM imidazole, 100 mM KCl, 100 μM EGTA, 0.01% (w/v) NaN3, at pH 6.5. All spectra were processed using NMRPipe (Delaglio, Grzesiek et al. 1995) and analyzed using CCPN Analysis (Vranken, Boucher et al. 2005). Backbone resonance assignments for both complexes were made using 2D 1H-15N HSQC and 3D HNCO, HNCACB, and CBCA(CO)NH experiments in 90% H2O/10% D2O.
Order parameters were predicted using TALOS+ (Shen, Delaglio et al. 2009). Chemical shift perturbations and differences Δδ (ppm) were calculated with Eq. 1
| [1] |
where ΔδHN and ΔδN are the observed changes in chemical shifts for 1HN and 15N respectively.
Extent of Assignment and Data Deposition
Assigned 1H-15N HSQC spectra are shown for apo CaMC+NaV1.2IQp (Fig. 1A) and apo CaM+NaV1.2IQp (Fig. 1B). Given the largely α-helical secondary structure of both CaM and NaV1.2IQp, the spectra are reasonably well dispersed but the central region is crowded. Panels to the right of each spectrum expand those regions to provide greater clarity in labeling the peaks. For apo CaMC+NaV1.2IQp (Fig. 1A), 95% of the backbone 1HN and 15N resonances were assigned for CaMC and 97% for NaV1.2IQp. For apo CaM+NaV1.2IQp (Fig. 1B), 97% of the backbone 1HN and 15N resonances were assigned for both CaM and NaV1.2IQp.
Figure 1. 1H,15N HSQC Spectra for apo CaMC+NaV1.2IQp (A) and apo CaM+NaV1.2IQp (B).
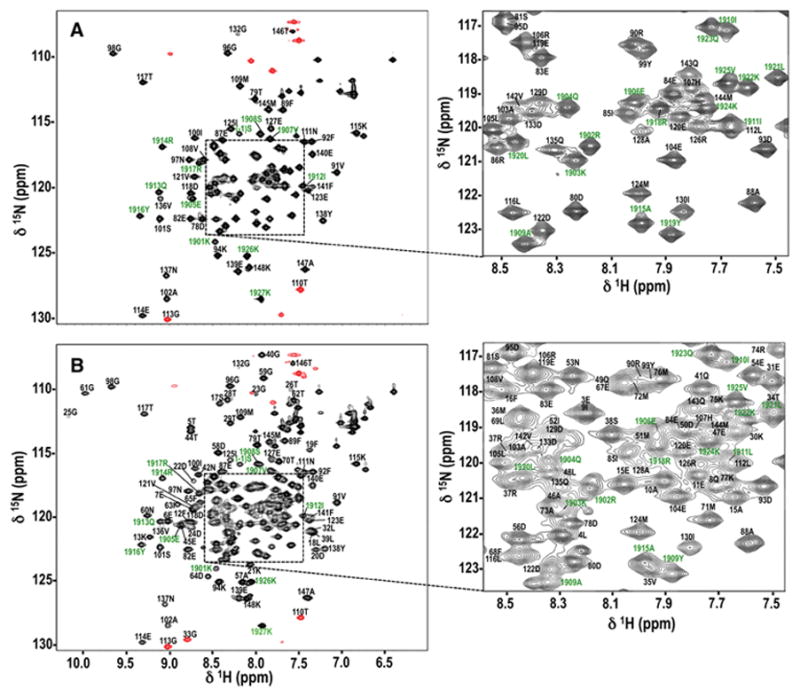
Backbone resonance assignments are labeled in black for residues of CaM or CaMC, and green for NaV1.2IQp. Numbers correspond to the full-length sequence of each protein. Identifiable residues in the N-terminal tag (GPGS) of NaV1.2IQp are labeled with negative numbers. Peaks contoured in red were aliased. Crowded central regions (denoted by a dashed box) were expanded to the right for clarity of labeling.
Crosspeaks were not observed for the first two residues of CaM (M76 and K77 in CaMC, and A1 and D2 in CaM), as well as residues D131 and G134 in calcium-binding site IV in both CaM and CaMC. Additionally, no crosspeak was observed for I27 in calcium-binding site I in the apo CaM+NaV1.2IQp complex. For NaV1.2IQp, all backbone 1HN and 15N resonances of non-proline residues were assigned in both complexes, except for the first residue, G(−4).
For apo CaMC+NaV1.2IQp, 96% of the 13Cα and 13Cβ resonances, and 93% of the 13C′ resonances were assigned for CaMC, and 94% of 13Cα, 93% of 13Cβ, and 90% of 13C′ resonances were assigned for NaV1.2IQp. For the apo CaM+NaV1.2IQp complex, 95% of the 13Cα, 93% of the 13Cβ, and 92% of the 13C′ resonances were assigned for CaM, and 96% of the 13Cα and 13Cβ, and 94% of the13C′ resonances were assigned for NaV1.2IQp.
TALOS+ was used to predict order parameters for both apo CaM and NaV1.2IQp (Fig. 2A and 2B) in apo CaM+NaV1.2IQp using 1HN, 15N, 13Cα, 13Cβ, and 13C′ chemical shifts (Shen, Delaglio et al. 2009). The values predicted for apo CaM agree well with previously determined high-resolution structures of apo CaM such as 1CFD (Kuboniwa, Tjandra et al. 1995). An inset schematic drawing of the typical secondary structure of CaM in Fig. 2A shows that positions predicted to have lower order parameters correspond to the calcium-binding loops (yellow), the loops between the EF-hands within each domain (gray), and the linker connecting CaMN and CaMC (gray). NaV1.2IQp is predicted to have reduced order parameters only at the N-and C-termini (Fig. 2B). This is consistent with NaV1.2IQp forming a single, basic, amphipathic α-helix when interacting with apo CaM, as was observed in high-resolution structures of apo CaM bound to several NaV IQ motifs (Chagot and Chazin 2011, Feldkamp, Yu et al. 2011, Wang, Chung et al. 2012, Reddy Chichili, Xiao et al. 2013, Gabelli, Boto et al. 2014).
Figure 2. Predicted Order Parameters and Analysis of Chemical Shift Perturbations.
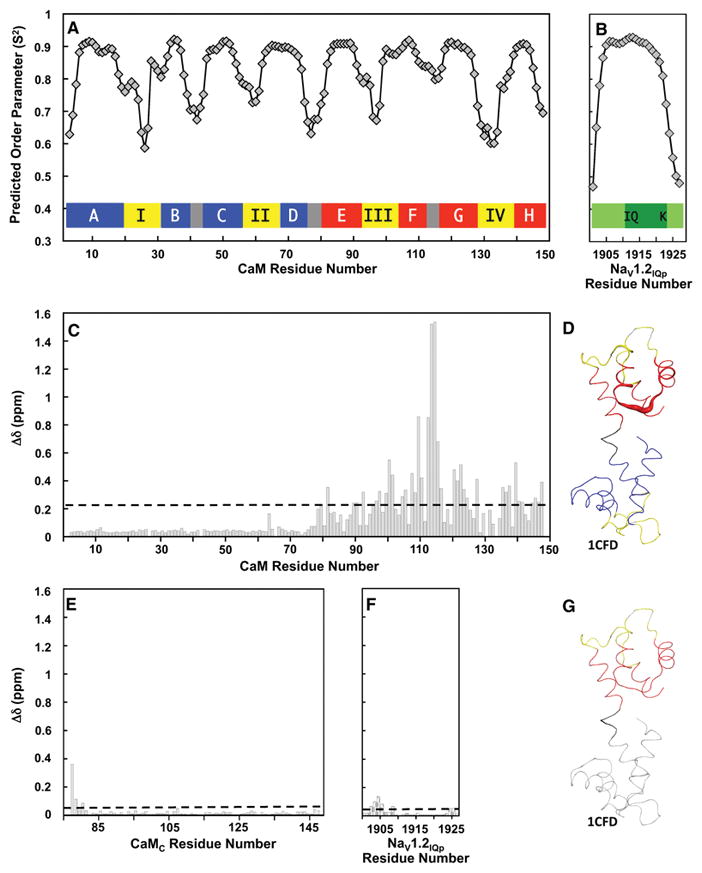
A, B. Order parameters predicted by TALOS+ (Shen, Delaglio et al. 2009) for apo CaM (A) and NaV1.2IQp (B) using the assigned chemical shifts of the apo CaM+NaV1.2IQp complex. The colored bars in (A) depict a schematic of CaM, with the helices of CaMN shown in blue, the helices of CaMC shown in red, the four calcium-binding sites shown in yellow, and the loops and inter-domain linker shown in gray. The helices and calcium-binding sites are labeled A–H and I–IV, respectively. In (B), NaV1.2IQp is shown in green.
C. Histogram of the chemical shift perturbations (Δδ, ppm, Eq. 1) of apo CaM residues upon binding NaV1.2IQp. The dashed line indicates the standard deviation (0.229 ppm) of Δδ values.
D. Chemical shift perturbations attributable to NaV1.2IQp binding are mapped onto the solution structure of free apo CaM in (1CFD.pdb). Residues in calcium-binding sites are yellow; otherwise, residues 1-75 are blue, linker residues 76-80 are black, and residues 81-148 are red. Unassigned residues are gray. Cartoon thickness corresponds to Δδ values in panel 2C. The image was generated using PyMOL (Schrödinger Software).
E. Histogram of the chemical shift differences (Δδ, ppm, Eq. 1) of CaMC residues calculated by comparing apo CaM+NaV1.2IQp to apo CaMC+NaV1.2IQp. The dashed line depicts the standard deviation of the chemical shift change (0.044 ppm).
F. Histogram of the chemical shift differences (Δδ, ppm, Eq. 1) between the peptide residues in apo CaM+NaV1.2IQp and apo CaMC+NaV1.2IQp. The dashed line depicts the standard deviation of the chemical shift change (0.033 ppm). To facilitate comparisons, the same vertical scale was used in panels 2C, 2E and 2F.
G. Chemical shift differences between apo CaM+NaV1.2IQp and apo CaMC+NaV1. 2IQp are illustrated on the solution structure of free apo CaM (1CFD.pdb). Residues 1-75 (not present in CaMC) and unassigned residues in CaMC are gray. CaM residues 76-80 are black, calcium-binding sites III and IV are yellow, and other residues in CaMC are red. Cartoon thickness corresponds to Δδ values in panel 2E. The image was generated using PyMOL (Schrödinger Software).
To determine the effect of binding NaV1.2IQp on apo CaM, we calculated the backbone chemical shift perturbations (CSPs) of CaM residues in apo CaM+NaV1.2IQp relative to free apo CaM (1CFD) (Kuboniwa, Tjandra et al. 1995). The histogram shows that the largest CSPs are found in CaMC, while those in CaMN are smaller than the standard deviation. The dashed line in Fig. 2C corresponds to the standard deviation (0.229 ppm) of the chemical shift changes (average value of 0.163 ppm; median of 0.055 ppm).
Mapping the CSPs onto 1CFD (Fig 2D) indicates that the largest perturbations were in the turn between helices F and G in CaMC, consistent with what was found previously in a solution structure of apo CaMC bound to the NaV1.2 IQ motif (2KXW (Feldkamp, Yu et al. 2011)). The large and numerous CSPs in CaMC, and the very small CSPs in CaMN, suggest that the interaction between apo CaM and NaV1.2IQp is mediated primarily through CaMC.
To determine whether the presence of CaMN alters the interface between CaM and NaV1.2IQp, we calculated chemical shift differences between CaM residues in apo CaM+NaV1.2IQp and apo CaMC+NaV1.2IQp (Fig. 2E) and between NaV1.2IQp residues bound to apo CaM or apo CaMC (Fig. 2F), plotted on the same scale as the comparison of apo CaM+NaV1.2IQp to apo CaM alone (1CFD). The dashed line in Fig. 2E depicts the standard deviation of the chemical shift changes for apo CaMC (0.044 ppm, average value of 0.025 ppm, median value of 0.015 ppm). The dashed line in Fig. 2F corresponds to the standard deviation for NaV1.2IQp (0.033 ppm, average value of 0.029 ppm, median value of 0.018 ppm).
Mapping the chemical shift differences between apo CaMC+NaV1.2IQp (Fig. 1A) and apo CaM+NaV1.2IQ (Fig. 1B) onto 1CFD (apo CaM alone; Fig. 2G) showed that the largest changes were near the linker between CaMN and CaMC. These residues are at the N-terminus of the CaMC construct, but are tethered to CaMN in full-length CaM (Fig. 2E and 2F). Very minor differences were found in comparing NaV1.2IQp in the two complexes (Fig G). The small differences indicate that the interface between apo CaMC and NaV1.2IQp is unaltered by the presence of CaMN. With the chemical shifts of CaMN being independent of peptide binding (Fig. 2C), these data (Fig. 2E, 2F) indicate that the interaction between apo CaM and NaV1.2IQp is independent of CaMN, consistent with the solution NMR structure of apo CaM bound to the IQ motif of NaV1.5.(Chagot and Chazin 2011)
Backbone NMR assignments were deposited with BioMagResBank. Apo CaM+NaV1.2IQp has accession number 27095, and apo CaMC+NaV1.2IQp 27094.
Acknowledgments
We thank C. Andrew Fowler of the University of Iowa Carver College of Medicine (UI CCOM) NMR facility for technical assistance. These studies were supported by NIH T32 NS045549 (RM), UI CCOM FUTURE in Biomedicine Fellowship (AMK), and NIH R01 GM57001 (MAS). The content is solely the responsibility of the authors and does not necessarily represent the official views of any supporting organization or agency.
Footnotes
BMRB Deposit Identification Numbers: 27094 and 27095
References
- Ahern CA, Payandeh J, Bosmans F, Chanda B. The hitchhiker’s guide to the voltage-gated sodium channel galaxy. J Gen Physiol. 2016;147(1):1–24. doi: 10.1085/jgp.201511492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagot B, Chazin WJ. Solution NMR Structure of Apo-Calmodulin in Complex with the IQ Motif of Human Cardiac Sodium Channel NaV1.5. Journal of Molecular Biology. 2011;406(1):106–119. doi: 10.1016/j.jmb.2010.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damo SM, Feldkamp MD, Chagot B, Chazin WJ. NMR studies of the interaction of calmodulin with IQ motif peptides. Methods in Molecular Biology. 2013;963:173–186. doi: 10.1007/978-1-62703-230-8_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. Journal of Biomolecular NMR. 1995;6(3):277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Feldkamp MD, Yu L, Shea MA. Structural and Energetic Determinants of Apo Calmodulin Binding to the IQ Motif of the Nav1.2 Voltage-Dependent Sodium Channel. Structure. 2011;19:733–747. doi: 10.1016/j.str.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabelli SB, Boto A, Kuhns VH, Bianchet MA, Farinelli F, Aripirala S, Yoder J, Jakoncic J, Tomaselli GF, Amzel LM. Regulation of the NaV1.5 cytoplasmic domain by calmodulin. Nature Communications. 2014;5:5126. doi: 10.1038/ncomms6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawali VS, Todt H. Mechanism of Inactivation in Voltage-Gated Na(+) Channels. Curr Top Membr. 2016;78:409–450. doi: 10.1016/bs.ctm.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Hovey L, Fowler CA, Mahling R, Lin Z, Miller MS, Marx DC, Yoder JB, Kim EH, Tefft KM, Waite BC, Feldkamp MD, Yu L, Shea MA. Calcium triggers reversal of calmodulin on nested anti-parallel sites in the IQ motif of the neuronal voltage-dependent sodium channel NaV1.2. Biophysical Chemistry. 2017;224:1–19. doi: 10.1016/j.bpc.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboniwa H, Tjandra N, Grzesiek S, Ren H, Klee CB, Bax A. Solution structure of calcium-free calmodulin. Nature Struct Biol. 1995;2:768–776. doi: 10.1038/nsb0995-768. [DOI] [PubMed] [Google Scholar]
- Marley J, Lu M, Bracken C. A method for efficient isotopic labeling of recombinant proteins. Journal of Biomolecular NMR. 2001;20(1):71–75. doi: 10.1023/a:1011254402785. [DOI] [PubMed] [Google Scholar]
- Messner DJ, Catterall WA. The sodium channel from rat brain. Separation and characterization of subunits. J Biol Chem. 1985;260(19):10597–10604. [PubMed] [Google Scholar]
- Mori M, Konno T, Ozawa T, Murata M, Imoto K, Nagayama K. Novel Interaction of the Voltage-Dependent Sodium Channel (VDSC) with Calmodulin: Does VDSC Acquire Calmodulin-Mediated Ca2+-Sensitivity? Biochemistry. 2000;39(6):1316–1323. doi: 10.1021/bi9912600. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kato M, Osaka H, Yamashita S, Nakagawa E, Haginoya K, Tohyama J, Okuda M, Wada T, Shimakawa S, Imai K, Takeshita S, Ishiwata H, Lev D, Lerman-Sagie T, Cervantes-Barragan DE, Villarroel CE, Ohfu M, Writzl K, Gnidovec Strazisar B, Hirabayashi S, Chitayat D, Myles Reid D, Nishiyama K, Kodera H, Nakashima M, Tsurusaki Y, Miyake N, Hayasaka K, Matsumoto N, Saitsu H. Clinical spectrum of SCN2A mutations expanding to Ohtahara syndrome. Neurology. 2013;81(11):992–998. doi: 10.1212/WNL.0b013e3182a43e57. [DOI] [PubMed] [Google Scholar]
- O’Malley HA, Isom LL. Sodium channel beta subunits: emerging targets in channelopathies. Annu Rev Physiol. 2015;77:481–504. doi: 10.1146/annurev-physiol-021014-071846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiwara I, Ito K, Sawaishi Y, Osaka H, Mazaki E, Inoue I, Montal M, Hashikawa T, Shike T, Fujiwara T, Inoue Y, Kaneda M, Yamakawa K. De novo mutations of voltage-gated sodium channel alphaII gene SCN2A in intractable epilepsies. Neurology. 2009;73(13):1046–1053. doi: 10.1212/WNL.0b013e3181b9cebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy Chichili VP, Xiao Y, Seetharaman J, Cummins TR, Sivaraman J. Structural basis for the modulation of the neuronal voltage-gated sodium channel NaV1.6 by calmodulin. Scientific Reports. 2013;3:2435. doi: 10.1038/srep02435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan MF, Tung CC, Van Petegem F, Ahern CA. Crystallographic basis for calcium regulation of sodium channels. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(9):3558–3563. doi: 10.1073/pnas.1114748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Delaglio F, Cornilescu G, Bax A. TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. Journal of Biomolecular NMR. 2009;44(4):213–223. doi: 10.1007/s10858-009-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Yasumoto S, Kurahashi H, Nakagawa E, Fukasawa T, Uchiya S, Hirose S. Clinical spectrum of SCN2A mutations. Brain Dev. 2012;34(7):541–545. doi: 10.1016/j.braindev.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Sorensen BR, Shea MA. Interactions between domains of apo calmodulin alter calcium binding and stability. Biochemistry. 1998;37:4244–4253. doi: 10.1021/bi9718200. [DOI] [PubMed] [Google Scholar]
- Theoharis NT, Sorensen BR, Theisen-Toupal J, Shea MA. The Neuronal Voltage-Dependent Sodium Channel Type II IQ Motif Lowers the Calcium Affinity of the C-Domain of Calmodulin. Biochemistry. 2008;47(1):112–123. doi: 10.1021/bi7013129. [DOI] [PubMed] [Google Scholar]
- Vranken WF, Boucher W, Stevens TJ, Fogh RH, Pajon A, Llinas M, Ulrich EL, Markley JL, Ionides J, Laue ED. The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins. 2005;59(4):687–696. doi: 10.1002/prot.20449. [DOI] [PubMed] [Google Scholar]
- Wang C, Chung BC, Yan H, Lee SY, Pitt GS. Crystal Structure of the Ternary Complex of a NaV C-Terminal Domain, a Fibroblast Growth Factor Homologous Factor, and Calmodulin. Structure. 2012 doi: 10.1016/j.str.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Chung BC, Yan H, Wang HG, Lee SY, Pitt GS. Structural analyses of Ca2+/CaM interaction with NaV channel C-termini reveal mechanisms of calcium-dependent regulation. Nature Communications. 2014;5:4896. doi: 10.1038/ncomms5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildburger NC, Ali SR, Hsu WC, Shavkunov AS, Nenov MN, Lichti CF, LeDuc RD, Mostovenko E, Panova-Elektronova NI, Emmett MR, Nilsson CL, Laezza F. Quantitative proteomics reveals protein-protein interactions with fibroblast growth factor 12 as a component of the voltage-gated sodium channel 1.2 (Nav1.2) macromolecular complex in Mammalian brain. Molecular and Cellular Proteomics. 2015;14(5):1288–1300. doi: 10.1074/mcp.M114.040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Wang C, Marx SO, Pitt GS. Calmodulin limits pathogenic Na+ channel persistent current. The Journal of General Physiology. 2017 doi: 10.1085/jgp.201611721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, George AL, Jr, Horn R. Molecular basis of charge movement in voltage-gated sodium channels. Neuron. 1996;16(1):113–122. doi: 10.1016/s0896-6273(00)80028-8. [DOI] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, DeCaen PG, Westenbroek RE, Pan CY, Scheuer T, Baker D, Catterall WA. Structural basis for gating charge movement in the voltage sensor of a sodium channel. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(2):E93–102. doi: 10.1073/pnas.1118434109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biology. 2003;4(3):207.201–207.207. doi: 10.1186/gb-2003-4-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]


