Abstract
The prognosis for patients with myasthenia gravis (MG) has improved significantly over the past half century, including substantial reductions in mortality and morbidity. However, approximately 10% of patients fails to respond adequately to current therapies and are considered treatment refractory, or treatment intolerant, and up to 80% have disease that fails to achieve complete stable remission. Although patients with autoantibodies to muscle-specific tyrosine kinase (anti-MuSK positive) are more likely to become treatment refractory than those with autoantibodies to the acetylcholine receptor (anti-AChR positive), each of these serotypes is substantially represented in the refractory MG population. Other risk factors for becoming treatment refractory include history of thymoma or thymectomy and female sex. A modified treatment algorithm for MG is proposed: patients who have disease that fails to respond to the stepwise approach to therapy, are treatment intolerant, or who require chronic rescue measures despite ongoing therapy, should be considered treatment refractory and emerging therapies should be considered. Three emerging monoclonal antibody-based therapies are discussed: the anti-B-cell agent rituximab; the terminal complement activation inhibitor eculizumab; and belimumab, which targets B-cell activating factor. Increased understanding of molecular pathophysiology and accurate antibody subtyping in MG should lead to the use of new therapeutic agents and successful management of treatment-refractory patients.
Keywords: acetylcholine receptor antibodies, monoclonal antibodies, muscle-specific tyrosine kinase antibodies, myasthenia gravis, treatment intolerant, treatment refractory
Introduction
Myasthenia gravis (MG) is an antibody-mediated autoimmune disorder that targets the neuromuscular junction (NMJ), resulting in fluctuating fatigable muscle weakness that typically presents initially with ocular symptoms but frequently generalizes to encompass bulbar, respiratory, and limb girdle functions.1,2 In the past half century, improvements in accurate and prompt diagnosis and treatment have simultaneously increased the estimated prevalence of MG and improved the prognosis for patients, leading to a substantial reduction in mortality attributable to MG.3,4 Among patients with an MG diagnosis, MG-attributable mortality was reduced from 70% in the 1930s to 30% by 1955, and is now well under 10%; in one recent case series of 677 patients with MG, approximately 2% died of MG-associated causes.3,5 During the same period, the estimated prevalence of MG increased over 10 fold, from 1 per 200,000 to 1 per 17,000.3
Improvements in treatment strategies and the prognosis of MG have been relatively broad based, with the treatment goal of establishing complete stable remission (CSR). However, recent work has demonstrated heterogeneity in disease course and treatment response based on patient antibody profile: patients with antibodies to muscle-specific tyrosine kinase (anti-MuSK positive) appear to be less responsive to conventional treatment than those with antibodies to the acetylcholine receptor (anti-AChR positive) or those without anti-MuSK or anti-AChR antibodies.5,6
Of all patients with MG, a fraction (estimated at 10%) have disease that is refractory to treatment with conventional agents such as cholinesterase inhibitors and immunosuppressive agents (including corticosteroids, azathioprine, and cyclosporine).7–9 In this article, we review the natural course of MG, various definitions of the term treatment refractory, clinical factors and underlying mechanisms associated with an increased risk of becoming treatment refractory, a proposed algorithm for treatment of MG, and emerging therapies that may be of use in disease that is considered refractory.
Materials and methods
We conducted a literature search of the US National Library of Medicine PubMed database for articles published from 1 December 2011 to 30 November 2016, using combinations of the search terms ‘refractory’, ‘treatment-refractory’, ‘treatment-resistant’, ‘natural history’, ‘disease course’, ‘myasthenia gravis’, ‘MG’, ‘emerging treatments’, and others pertaining to MG that fails to respond adequately to current therapies. The references located using these search terms were reviewed for relevance and up-to-date information on topics related to refractory MG. In addition, some older references were included for context or background.
Results
Natural history of myasthenia gravis
The natural history of MG has been elucidated in longitudinal studies of nearly 2000 patients with MG conducted by Grob and colleagues between 1940 and 2000.3,4 The initial presenting symptoms in 85% of all patients were ocular (primarily ptosis or diplopia), which reached maximal severity within 1 year in 70% of patients and within 3 years in 85%. Thereafter, ocular symptoms did not worsen in the majority of patients (even over periods of up to 20 years), and up to 20% of patients experienced at least one or more remissions.3 Although the natural history of MG is recognized, there have been some changes in recent years on the pattern of progression of the disease. These changes are likely a consequence of earlier treatment or the trend towards ageing in the general population, where the incidence and prevalence of MG is higher amongst the elderly.10
Of patients presenting initially with only ocular symptoms, 80% went on to develop generalized MG symptoms, most within the first year after onset. In most patients with generalized MG, weakness reached its maximal severity within 6 months of symptom onset, with maximal weakness occurring within 2 years of onset in all but 18%.3 In 382 patients initially diagnosed with generalized MG between 1940 and 1960, 33% died of MG. In a 1981 study of 1036 patients with MG followed over a mean of 12 years, 29% of deaths occurred within the first year of onset, 56% within 3 years, and 73% within 5 years.4
Defining ‘treatment-refractory myasthenia gravis’
Currently, there is no broadly accepted consensus-based definition of ‘treatment-refractory MG’. Operational definitions, often used to establish patient populations for the purposes of analysis or as entry criteria for clinical studies, have typically used the following attributes to define treatment-refractory MG:8,9,11–13
(1) Failure to respond adequately to conventional therapies: in this classic definition, patients have insufficient response (e.g. persistent moderate to severe weakness) to maximal safe doses of steroids and at least one immunosuppressive drug at an adequate dose and duration).
(2) Inability to reduce immunosuppressive therapy without clinical relapse or a need for ongoing rescue therapy such as intravenous immunoglobulin G (IVIg) or plasma exchange (PE): although patients may respond initially to immunosuppressive therapy, the duration of such therapies must be restricted because of the potential for profound side effects associated with their prolonged use (especially the use of corticosteroids).
(3) Severe or intolerable adverse effects from immunosuppressive therapy: this attribute is more accurately described as ‘treatment intolerant’ rather than ‘treatment refractory’. However, because the operational effect (i.e. the inability to effectively treat MG using conventional immunosuppressive therapy) is the same, it has been frequently used as a defining criterion.
(4) Comorbid conditions that restrict the use of conventional therapies: as with the ‘adverse effects’ criterion, this is more accurately described as ‘treatment intolerant’. As described previously, the operational effect is identical to that in ‘genuinely’ treatment-refractory MG.
(5) Frequent myasthenic crises even while on therapy: these life-threatening events, characterized by respiratory or bulbar weakness or paralysis, require urgent hospitalization.
Clinical factors associated with refractory myasthenia gravis
Longitudinal and chart-review studies have been conducted to identify the factors most strongly associated with treatment-refractory MG.5,11 Suh and colleagues used chart reviews to evaluate a set of 128 patients referred to a neuromuscular clinic for treatment of MG between September 2003 and February 2011. Of these, 109 patients were classified as having nonrefractory disease and 19 as having refractory disease, based on the inability to reduce the amount of immunotherapy without clinical relapse, a lack of clinical control despite their immunotherapeutic regimen, or severe side effects of immunosuppressive therapy.11
Suh and colleagues found that patients with refractory MG were significantly younger at onset [median: 36 years; interquartile range (IQR): 28, 51 years] than those with nonrefractory MG (median: 60 years; IQR: 42, 72 years; p < 0.001 by Wilcoxon two-sample test). Patients with refractory MG were also significantly more likely to be female than those with nonrefractory disease (74% versus 47%, respectively; p = 0.03).11 It should be noted that the age of MG onset exhibits a long-recognized bimodal distribution, with a peak around age 30 years driven primarily by an elevated incidence in young women, followed by an increase in incidence after age 50 that reflects a slightly elevated incidence in older men.2,6
Antibody status was available for 115 of 128 patients (90%) studied by Suh and colleagues (19/19 refractory and 96/109 nonrefractory disease). Overall, 71% had anti-AChR antibodies, with 10 of 19 (53%) patients with refractory MG being anti-AChR positive and 72 of the 96 with nonrefractory MG (75%) being anti-AChR positive (p = 0.05); 10% had anti-MuSK antibodies; of those, 9 of the 19 (47%) patients with refractory MG were MuSK positive, while 2 of 96 (2%) anti-MuSK-positive patients had nonrefractory MG (p < 0.001). Of the 19% who were seronegative for both antibodies, none had refractory MG and 22 of 96 (23%) had nonrefractory MG (p = 0.02).11 The analysis showed that patients with anti-MuSK antibodies are much more likely to have refractory disease than those with anti-AChR antibodies; however, the absolute numbers of patients with refractory disease and anti-MuSK and anti-AChR antibodies were approximately equal because of the far higher proportion of the latter among all patients with MG.
Suh and colleagues also found that patients with refractory MG were more likely to have had a thymoma (45% versus 14%; p = 0.02) and to have undergone thymectomy compared with those with nonrefractory disease (68% versus 17%; p < 0.001). Similar proportions of patients with refractory and nonrefractory disease who underwent thymectomy had a thymoma confirmed pathologically (39% versus 50%; p = 0.72), whereas all patients with a computed tomography (CT) or pathologically confirmed thymoma had undergone thymectomy in both groups.11 A summary of the clinical characteristics of patients with refractory and nonrefractory MG from the study by Suh and colleagues is presented in Table 1.11
Table 1.
Comparison of selected attributes of patients with refractory and non-refractory myasthenia gravis.11
| Parameter | Total (n = 128) | Nonrefractory (n = 109) | Refractory (n = 19) | p value* |
|---|---|---|---|---|
| Median age at onset, year (IQR) | 55 (38–69) | 60 (42–72) | 36 (28–51) | <0.001 |
| Female | 51% | 47% | 74% | 0.03 |
| Antibody status available | 90% | 88% | 100% | |
| Anti-AChR positive | 71% | 75% | 53% | 0.05 |
| Anti-MuSK positive | 10% | 2% | 47% | <0.001 |
| Double seronegative | 19% | 23% | 0 | 0.02 |
| Thymectomy | 24% | 17% | 68% | <0.001 |
| Thymoma status available | 60% | 61% | 58% | |
| Thymomatous | 18% | 14% | 45% | 0.02 |
| Non-thymomatous | 82% | 86% | 55% |
Adapted from Suh et al.11 with permission from The Yale School of Biology and Medicine.
For comparison between patients with refractory and nonrefractory myasthenia gravis.
Anti-AChR positive, anti-acetylcholine receptor antibody positive; anti-MuSK positive, anti-muscle-specific tyrosine kinase antibody positive; IQR, interquartile range.
Baggi and colleagues took a different approach to evaluating clinical characteristics associated with refractory MG by comparing antibody-defined groups and determining the likelihood of achieving CSR based on antibody status, age at onset, gender, and clinical stage (i.e. ocular, generalized, bulbar, respiratory) at onset and at maximal worsening. A total of 677 patients were included in the analysis: 517 were anti-AChR positive, 55 were anti-MuSK positive, and 105 were ‘double negative’ (DN; negative for both anti-AChR and anti-MuSK antibodies).5
A preponderance of female patients was seen across all antibody-defined groups; however, the female-to-male ratio was far more pronounced in the anti-MuSK-positive group (5.1:1.0) than in the anti-AChR-positive group (1.9:1.0) or the DN group (2.7:1.0) (p = 0.016 for between-group differences). Patients who were anti-MuSK positive also had more severe disease at onset, with 60.1% exhibiting bulbar dysfunction compared with 35.2% of patients who were anti-AChR positive and 23.8% of those who were DN (p < 0.001). These differences continued to be pronounced at maximal worsening, with 83.6% of patients who were anti-MuSK positive exhibiting bulbar symptoms compared with 58.6% and 43.8% in the anti-AChR-positive and DN groups, respectively. Respiratory crises were more common in anti-MuSK-positive patients (10.9%) than in anti-AChR-positive patients (6.8%) or DN groups (1%). However, it is important to note that because they represent a far greater proportion of patients with MG, the population frequency is higher, and the number of anti-AChR-positive patients with bulbar symptoms was over fivefold higher than the number of anti-MuSK-positive patients with bulbar symptoms at onset (182 versus 33) and at maximal worsening (303 versus 46).5
Baggi and colleagues found a large difference between antibody-defined groups with respect to the likelihood of achieving CSR, associating anti-MuSK-positive status with more difficult to treat MG, as did Suh and colleagues. Comparable proportions of patients in the anti-AChR-positive and DN groups achieved CSR (22.2% and 21.9%, respectively); however, only 2 of 55 (3.6%) patients in the anti-MuSK-positive group did so (p = 0.005 for between-group differences). Kaplan–Meier analysis showed that across the entire study population, achievement of CSR was significantly more likely in patients with onset age younger than 40 years versus 40 years or older (p = 0.0004), in those with ocular-only MG at maximal worsening (p = 0.0054), and in the anti-AChR-positive or DN group versus the anti-MuSK-positive group (p = 0.0382). However, the preponderance of anti-AChR-positive patients in the study population (which is representative of the overall MG population) means that far more of these patients than anti-MuSK-positive patients failed to achieve CSR (402 versus 53, respectively).5
Taken together, the Suh and Baggi studies show that MG associated with anti-MuSK antibodies tends toward a more severe form of MG that is more resistant to treatment with more severe symptomatology (e.g. bulbar/respiratory versus ocular/generalized) than anti-AChR-positive or DN MG.5 Anti-MuSK-positive MG is strongly female predominant, and very few patients are likely to achieve CSR with current therapies. Although the reasons for the severity of anti-MuSK-positive MG remain unclear, evidence exists that anti-MuSK antibodies do not bind complement and may disrupt multiple components of the NMJ, including those that help to anchor and stabilize the AChR molecular scaffold of the postsynaptic membrane. It is critical to re-emphasize that although the proportion of anti-AChR-positive patients with refractory MG is smaller than that of anti-MuSK-positive patients, the number of refractory anti-AChR-positive patients is at least as large as (and possibly much larger than) the number of anti-MuSK-positive patients with refractory disease.5
Another clinical variable that is strongly associated with difficult-to-treat MG is the presence of thymoma. Results from the study by Suh and colleagues showed that patients with thymoma were strongly overrepresented in the population with refractory MG.11 In the case series analyzed by Baggi and colleagues, approximately 70%, 46%, and 50% of patients with anti-AChR-positive, anti-MuSK-positive, and DN MG, respectively, had undergone thymic surgery. Although most patients who underwent thymic surgery demonstrated some degree of thymic abnormality (involuted thymus histology or thymic hyperplasia), progression to frank thymoma was much more common in the anti-AChR-positive group (~30%) than in the anti-MuSK-positive and DN groups (0 and 2%, respectively). The study by Baggi showed that across the full study population, the likelihood of CSR was lower in patients with thymoma than in those with involuted thymus histology or thymic hyperplasia (p = 0.0030 for between-group differences).5
A longitudinal study of patients with MG who have thymoma (N = 197; all anti-AChR positive) provided further support for thymoma as an important prognostic and treatment-response marker. The study by Maggi and colleagues found that 81.2% of patients with MG and thymoma had bulbar or respiratory symptoms at maximal severity compared with 57.2% in an earlier series of patients without thymoma; conversely, ocular-only MG was found at maximal severity in 3.04% of patients with thymoma compared with 11.7% in patients without thymoma.14 Only 9.64% of patients with thymoma achieved CSR compared with rates of over 50% in previous series of patients without thymoma. A multivariate analysis showed that no clinical or pathological (e.g. tumor staging) variables significantly altered the chance of achieving CSR, suggesting that the presence of thymoma overwhelms other potentially prognostic variables.14
Proposed treatment algorithm for myasthenia gravis
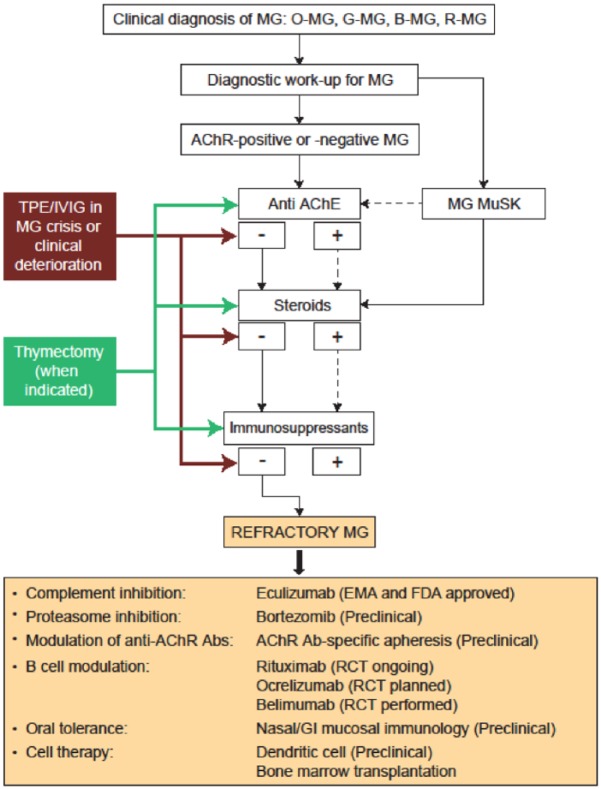
We have previously proposed a framework for making treatment decisions in MG, which we have modified here as Figure 1.15 Similar recommendations have been published elsewhere.16,17 Thymectomy is considered mandatory for patients with a confirmed thymoma. A stepwise approach is recommended for all antibody-defined MG subgroups, beginning with cholinesterase inhibitors (pyridostigmine) to alleviate MG symptoms, followed by immunosuppressive therapy, which is typically initiated using oral corticosteroids (prednisone), with other immunosuppressive agents (e.g. azathioprine, methotrexate, cyclosporine A, cyclophosphamide, and mycophenolate mofetil) substituted or used adjunctively as second-line therapy.15 Plasma exchange or IVIg infusion are usually reserved for acute symptomatic MG exacerbations or for MG crises.15 Therapeutic apheresis may also include immunoadsorption which is particularly suitable for long-term management of treatment-resistant patients due to its semi-selective mechanism of action.18 Another treatment option in refractory MG may include ‘rebooting’ the immune system with high-dose cyclophosphamide. This latter treatment option, however, should only be used as a last resort in selected patients, due to the associated risks and side effects.19
Figure 1.
Proposed algorithm for treatment of myasthenia gravis.15 Presence of antibody or positive response to therapy is indicated by ‘+’; absence of antibody or lack of response to treatment is indicated by ‘−’. Solid blue lines indicate next steps in either diagnosis or treatment. Dashed lines refer to subsequent potential diagnostic or treatment options. Solid red lines refer to treatment options for refractory MG.
Abs, antibodies; AChE, acetylcholinesterase; AChR, acetylcholine receptor; B-MG, bulbar myasthenia gravis; EMA, European Medicines Agency; FDA, US Food and Drug Administration; GI, gastrointestinal; G-MG, generalized myasthenia gravis; IVIg, intravenous immunoglobulin; MG, myasthenia gravis; MuSK, muscle-specific tyrosine kinase; O-MG, ocular myasthenia gravis; RCT, randomized controlled trial; R-MG, respiratory myasthenia gravis; TPE, therapeutic plasma exchange.
Modified from Mantegazza et al.15 Used with permission.
If this stepwise approach to MG therapy fails to produce an acceptable response, if treatment-associated side effects are intolerable or associated with clinical deterioration, or if PE or IVIg continue to be required on a chronic basis despite therapy, the patient should be considered to have treatment-refractory MG and treatment using one of the emerging agents (discussed in the following section) should be considered.
Emerging therapies of potential use in refractory MG
Based on advancements in our understanding of the molecular events involved in MG pathogenesis and progression, a number of potentially useful immunomodulatory treatments have been proposed for use in refractory MG (or possibly as first- or second-line therapies).20 Here, we summarize several monoclonal antibody-based therapies that have been evaluated in case series, for which controlled clinical studies are completed or ongoing. In addition to their efficacy with respect to MG symptoms, these agents may be useful in reducing or eliminating the need for prolonged corticosteroid therapy. It should be noted that prospectively designed, controlled studies have historically not been the norm in MG. All of the conventional therapies discussed previously have been applied on the basis of empirical evidence and clinical experience.
Rituximab
Rituximab is a chimeric murine-human monoclonal antibody of the immunoglobulin G1 (IgG1) κ class that targets CD20, a protein expressed by mature B cells that modulates B-cell activation. Binding of rituximab to CD20 induces complement-dependent cytolysis or antibody-dependent cell-mediated cytotoxicity. Rituximab has been used in various autoimmune disorders (including rheumatoid arthritis and systemic lupus erythematosus) and is standard therapy for non-Hodgkin’s lymphoma.9,13 To date, rituximab has been evaluated in case reports and retrospective case series. Two other therapies on the market that target CD20 on mature B cells are ocrelizumab (for multiple sclerosis) and ofatumumab (for chronic lymphocytic leukemia). These may represent potential therapies for MG.20
In a retrospective, single-center case series, Nowak and colleagues treated 14 patients with refractory generalized MG (six anti-AChR positive and eight anti-MuSK positive) with rituximab.13 Rituximab 375 mg/m2 was infused in treatment cycles of four consecutive weekly infusions, with 6 months between cycles. Of the 13 patients on oral prednisone at the outset of rituximab therapy, all were able to reduce their prednisone dose; five were prednisone free after two rituximab cycles, and eight were prednisone free after three cycles. Mean prednisone dose was reduced by 65.1, 85.7, and 93.8% after cycles 1, 2, and 3, respectively. In addition, among the 12 patients requiring PE treatments, nine no longer needed PE after one rituximab cycle, and none required PE after three cycles. In the six anti-AChR-positive patients, the mean titer of anti-AChR antibodies was reduced by 40.2% after cycle 1 (p = 0.052), 52.1% after cycle 2 (p = 0.0046), and 67% after cycle 3 (data insufficient to generate a p value). Although antibody titers were not available for the eight patients in the anti-MuSK-positive group, the authors note that both antibody-defined groups responded similarly with respect to reductions in prednisone dose and the need for PE.13
In a retrospective, multicenter study, Collongues and colleagues used rituximab to treat seven patients with nonrefractory MG and 13 patients with refractory MG. Patients were designated as refractory based on nonresponse to thymectomy (if performed before the study) and to at least two immunosuppressive drugs.21 Comparing the 2 years prior to and following initiation of treatment, rituximab reduced the annualized relapse rate ± standard deviation (SD) among patients with refractory MG from 2.1 ± 0.3 to 0.3 ± 0.1 (p < 0.001) and among those with nonrefractory MG from 1.9 ± 0.3 to 0.1 ± 0.1 (p < 0.001). Corticosteroid treatment was tapered following the initiation of rituximab, and at 6 months, six patients in the refractory group and two in the nonrefractory group were corticosteroid free; at 1 year, seven in the refractory group and four in the nonrefractory group were corticosteroid free. All patients in the nonrefractory group were completely corticosteroid free after 18 months. After 1 year of rituximab treatment, mean prednisone dosage ± SD was reduced from 38.5 ± 6.6 mg/day to 8.7 ± 3.7 mg/day in patients with refractory MG (p = 0.002) and from 42.8 ± 8.4 mg/day to 6.4 ± 3.5 mg/day in those with nonrefractory disease (p = 0.003). The authors note that the rapid (<1 year) time to clinically significant response is unprecedented when compared with other immunosuppressive agents used in MG therapy, and recommend wider and earlier use of rituximab in MG.21
In light of the strong association between anti-MuSK-positive MG and inadequate treatment response, a particularly interesting study was conducted by Díaz-Manera and colleagues, who compared response to rituximab in six patients with refractory anti-MuSK-positive MG with that in 11 patients with refractory anti-AChR-positive MG.22 Rituximab 375 mg/m2 was administered weekly for 4 weeks, with the cycle repeated monthly for 2 months. Repeat infusions were administered only if MG symptoms re-emerged and interfered with daily activities. After a mean follow up of 31 months, 16 of 17 patients had significantly improved. Notably, all six patients with anti-MuSK-positive MG achieved minimal manifestation status (MMS) that was maintained for a mean follow up of 35 months, with no patients requiring retreatment with rituximab. In contrast, of the 10 patients with anti-AChR-positive MG who improved at 3 months, six required rituximab reinfusion [mean: 17 months (6–34 months) after the first rituximab dose], and none achieved MMS. Mean daily prednisone dosage was reduced from 49 mg/day at baseline to 6.5 mg/day at the last visit in the anti-MuSK-positive group compared with a reduction from 30.5 mg/day to 17.2 mg/day in the anti-AChR-positive group. Rituximab treatment completely depleted peripheral B cells in all 17 patients. Mean anti-MuSK antibody titers in patients with anti-MuSK-positive MG were reduced by 42.7% at 3 months (p = 0.043) and by 87% at last visit (p = 0.002). No significant decrease was seen in anti-AChR antibody titers in patients in the anti-AChR-positive group at 3 months or at last visit. The authors noted the rapidity and durability of response to rituximab treatment and the significantly better response among patients in the anti-MuSK-positive group compared with their anti-AChR-positive counterparts, which the authors attributed to differences in the pathophysiology of the two MG subtypes. Anti-MuSK antibodies are predominantly of the IgG4 subclass, which involves the T-helper 2 cell response; anti-AChR antibodies are predominantly of the IgG1 and IgG3 subclasses, which involve the T-helper 1 cell response.22
A phase II, randomized, multicenter, placebo-controlled, 52-week clinical study of rituximab [ClinicalTrials.gov identifier: NCT02110706] is currently recruiting participants with anti-AChR-positive MG on a stable immunosuppressive regimen (prednisone ± immunosuppressive agents). The primary outcome measure is the percentage of patients who achieve at least a 75% reduction in mean daily prednisone dose at the study endpoint.
Eculizumab
Eculizumab is a fully humanized monoclonal antibody that targets the complement component C5, inhibiting its conversion to C5a and C5b and, in turn, blocking terminal complement activation and formation of the membrane attack complex (MAC).7,23 Complement activation and MAC formation are thought to play a major role in the destruction of the NMJ and loss of AChR function in MG, suggesting that inhibition of these processes may suppress the development and progression of MG.7,24 Eculizumab is currently indicated for the treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome.25
Eculizumab was evaluated in a pilot, phase II, placebo-controlled, multicenter, crossover study in which patients with refractory anti-AChR-positive MG were randomized to receive an intravenous infusion of eculizumab for 16 weeks (600 mg/week for 4 weeks, followed by 900 mg every 2 weeks) or matching placebo, followed by a 5-week washout period; treatment assignments were reversed for a second 16-week treatment period.7 The primary endpoint was the percentage of responders, defined as those with a three-point reduction on the Quantitative Myasthenia Gravis (QMG) total score. Because the QMG scores of patients who received eculizumab in the first treatment period had not returned to baseline after the 5-week washout period, the analysis was based on results from the first treatment period. From baseline to week 16, six of seven (86%) patients treated with eculizumab were responders compared with four of seven (57%) patients who received placebo. In addition, four of seven (57%) patients who received eculizumab achieved at least an eight-point reduction in QMG total score compared with only one of seven (14%) patients in the placebo group. Using results from both treatment periods, a marginally significant between-group difference favoring eculizumab was seen with respect to change in QMG score (p = 0.0577). The authors concluded that eculizumab represents a promising therapy for refractory MG in anti-AChR-positive patients.7
A phase III, randomized, double-blind, placebo-controlled clinical study [ClinicalTrials.gov identifier: NCT00727194] of eculizumab in refractory anti-AChR-positive MG has just been completed. Results from the REGAIN study (N = 125) have been reported.26 The primary efficacy endpoint was change from baseline in Myasthenia Gravis Activities of Daily Living (MG-ADL) total score, which was compared using a worst-rank score analysis.26 The primary endpoint did not differ significantly between patients treated with eculizumab versus placebo; however, eculizumab treatment significantly improved the assessed secondary efficacy outcomes compared with placebo.26 As such, eculizumab was approved for the treatment of MG by the European Medicines Agency, as well as the US Food and Drug Administration. Participants who completed this study have been offered enrollment in a long-term (⩽4 years), open-label extension study [ClinicalTrials.gov identifier: NCT02301624] on the safety and efficacy of eculizumab. As noted previously, anti-MuSK antibodies do not bind complement, and appear to act by way of a different mechanism than anti-AChR antibodies in the pathogenesis of MG. Therefore, those patients with AChR-positive MG are most likely to benefit from treatment with eculizumab, as were the patient population in the phase III study.5
Belimumab
Belimumab is a fully humanized IgG1-λ monoclonal antibody that targets B-cell activating factor (also known as B-lymphocyte stimulator, or BLyS), a key growth factor essential for B-cell activation, maturation, and survival. Belimumab depletes populations of activated and naïve B cells along with plasma cells, but spares memory B cells, helping to maintain the effectiveness of previous vaccinations.27 Belimumab is currently indicated for the treatment of systemic lupus erythematosus as an adjunct to standard therapy.28
Results from a phase II, randomized, double-blind, placebo-controlled, multinational clinical trial of belimumab in patients with anti-AChR-positive or anti-MuSK-positive MG (N = 39) [ClinicalTrials.gov identifier: NCT01480596] have recently been made available. Eligibility for the study was based on the persistence of MG symptoms despite standard-of-care therapy. The study consisted of a 24-week treatment period, during which infusions of belimumab (n = 21) or matching placebo (n = 18) were administered on days 0, 14, and 28, and every 28 days thereafter, with a subsequent 12-week follow-up period. The primary efficacy endpoint was change from baseline to week 24 in the QMG total score. Although the adjusted mean reduction from baseline at 24 weeks in the QMG score (standard error) was greater in the belimumab group [−4.21 (1.14)] than in the placebo group [−2.37 (1.10)], the difference was not statistically significant [95% confidence interval (CI) for between-group difference: −5.08 to 1.40; p = 0.256]. Similar results for between-group comparisons at week 24 were observed for adjusted mean changes from baseline in the MG Composite Scale score (95% CI −2.97 to 3.07) and in the MG-ADL score (95% CI −2.03 to 1.40).
As shown in the therapeutic algorithm (Figure 1), rituximab, eculizumab, and belimumab have been used in randomized controlled trials or in open studies, whereas the others listed are promising therapeutic approaches tested only at the preclinical level.
Discussion
As MG represents the prototype of an antibody-mediated dysfunction at the NMJ, the evolution of MG immunosuppressive treatment has provided many patients with the opportunity to live symptom-free lives, owing to the stepwise application of symptomatic and immunosuppressive treatments and improved management of myasthenic crises. Nonetheless, a significant proportion of patients with MG struggle with refractory disease marked by persistent symptoms that may be severe, with the side effects of prolonged immunomodulatory treatment, or with the need for chronic rescue therapy.
Two important trends are now uniting to improve the clinical picture for patients with refractory MG. The first, which is partly based on improved sensitivity for detecting and classifying autoimmune antibodies, is the capability of defining clinical subtypes of MG and associating them with the likelihood of inadequate treatment response. This capability revealed the heightened frequency of inadequate response in patients with anti-MuSK-positive MG, as well as the substantial population of patients with refractory disease who are anti-AChR positive (possibly larger than the population of patients with refractory disease who are anti-MuSK positive). It also showed that improved treatment paradigms should be based on MG subtypes.
The second trend is the development of targeted therapies (primarily monoclonal antibodies) that address key vulnerabilities (revealed via an improved understanding of the pathophysiology of MG) in the development and progression of MG and that may be amenable to more precise targeting of treatment for antibody-defined subtypes. These therapies include rituximab, which appears more effective in anti-MuSK-positive than in anti-AChR-positive MG, and eculizumab, which specifically targets complement activation involved in anti-AChR-positive MG. These and other emerging therapies may also be useful when applied to patients with MG early in the treatment process (i.e. patients other than those with refractory disease), and their use may be able to reduce the need for more generalized immunosuppressive therapy, especially corticosteroids.
The monoclonal antibody-based therapies discussed herein likely represent only the first wave of precisely targeted therapies that are emerging as a result of these trends. The search for new immunologic pathways or pathogenetic mechanisms as potential targets for new treatments in refractory MG is of particular relevance from the perspective of precision medicine (Figure 1). Interleukin (IL) receptor blockade is one example. Among monoclonal antibodies, tocilizumab, a IL-6 receptor-blocking antibody already approved for rheumatoid arthritis, has been proposed as an alternative to rituximab for patients with neuromyelitis optica (NMO) that is nonresponsive to anti-CD20 therapy.29 Therefore, because NMO and MG share, at least in part, a common IgG-mediated pathogenesis, tocilizumab could also be of interest for MG, and was recently reported as beneficial in two patients with MG showing insufficient responses to rituximab.30 Proteasome inhibition is a promising approach to target plasma cells in autoimmune disorders, as demonstrated by bortezomib in multiple myeloma. Proteasome inhibition causes misfolded proteins to accumulate in the endoplasmic reticulum, leading to apoptosis. Plasma cells are particularly sensitive to proteasome inhibition because of their high rate of protein synthesis; hence antibody-mediated disorders such as MG may be good candidates for further investigation. Indeed, bortezomib has been preliminarily used for systemic lupus erythematosus, with initial positive results to be confirmed with further studies.31 Recently, promising results were reported on the use of bortezomib in combination with other immunosuppressive drugs in patients with severe MuSK-antibody positive MG.32 Induction of tolerance through mucosal administration of antigens has been extensively investigated in several experimental models of autoimmunity. Tolerance induced by orally or nasally administered antigens is thought to be mediated by induction of regulatory cells, particularly T-helper 3 cells that produce transforming growth factor β, a cytokine with regulatory properties.33 Oral or nasal tolerization has been successfully investigated in experimental autoimmune MG in rodents.34,35
The production of recombinant fragments of the AChR to amounts suitable for use in humans could be of great interest for the treatment of MG33,34; in fact, recombinant fragments of the AChR expressed in Escherichia coli have been used in vitro for antigen-specific removal of anti-AChR autoantibodies, and an approach to scale this for clinical purposes has been recently reported.36
In addition, cellular therapies with tolerogenic features may be developed. Tolerogenic human IL-10 modulated, ex vivo derived dendritic cells are able to generate induced regulatory T cells.37 Protocols for the generation of regulatory dendritic cells have already been reported in the literature and used against tumors in clinical trials; further investigation of this novel approach is awaited in the field of autoimmunity.37
The clinical need for improved therapies is clear, not only for refractory MG of all antibody serotypes, but also to reduce the need for and use of more generalized immunomodulatory approaches that still form the backbone of MG therapy. Even among patients who respond to conventional therapies, balancing the inevitable side effects of systemic immunosuppressive agents against their demonstrated efficacy remains an ongoing challenge, and the clinical picture for these patients has changed little over the past two decades. We strongly encourage continued research into, and development of, targeted therapies for MG based partially on serostatus, and hope that despite the challenges inherent in conducting clinical studies in this relatively rare disease, such therapies will be evaluated for use earlier in the treatment process.
Acknowledgments
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE), and are fully responsible for all content. Both authors contributed to all aspects of the creation of this work and have approved the final article.
Footnotes
Funding: This work is supported by Alexion Pharmaceuticals, Inc. (New Haven, CT, USA). Alexion Pharmaceuticals provided funding for the preparation, review, and approval of the manuscript. Editorial and writing assistance has been provided to the authors by Connexion Healthcare (Newtown, PA, USA), and V. Ruvini Jayasinghe PhD of Oxford PharmaGenesis Inc. (Newtown, PA, USA).
Conflict of interest statement: R. Mantegazza has received honoraria from Alexion Pharmaceuticals, Inc. (New Haven, CT, USA). C. Antozzi received no financial support for the research, authorship, and/or publication of this article.
Contributor Information
Renato Mantegazza, Department of Neuroimmunology and Neuromuscular Diseases, Fondazione Istituto Neurologico Carlo Besta, Via Celoria, 11, 20133 Milan, Italy.
Carlo Antozzi, Department of Neuroimmunology and Neuromuscular Diseases, Fondazione Istituto Neurologico Carlo Besta, Milan, Italy.
References
- 1. Spillane J, Higham E, Kullmann DM. Myasthenia gravis. BMJ 2012; 345: e8497. [DOI] [PubMed] [Google Scholar]
- 2. Li Y, Arora Y, Levin K. Myasthenia gravis: newer therapies offer sustained improvement. Cleve Clin J Med 2013; 80: 711–721. [DOI] [PubMed] [Google Scholar]
- 3. Grob D, Brunner N, Namba T, et al. Lifetime course of myasthenia gravis. Muscle Nerve 2008; 37: 141–149. [DOI] [PubMed] [Google Scholar]
- 4. Grob D, Brunner NG, Namba T. The natural course of myasthenia gravis and effect of therapeutic measures. Ann N Y Acad Sci 1981; 377: 652–669. [DOI] [PubMed] [Google Scholar]
- 5. Baggi F, Andreetta F, Maggi L, et al. Complete stable remission and autoantibody specificity in myasthenia gravis. Neurology 2013; 80: 188–195. [DOI] [PubMed] [Google Scholar]
- 6. Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 2015; 14: 1023–1036. [DOI] [PubMed] [Google Scholar]
- 7. Howard JF, Jr, Barohn RJ, Cutter GR, et al. A randomized, double-blind, placebo-controlled phase II study of eculizumab in patients with refractory generalized myasthenia gravis. Muscle Nerve 2013; 48: 76–84. [DOI] [PubMed] [Google Scholar]
- 8. Zebardast N, Patwa HS, Novella SP, et al. Rituximab in the management of refractory myasthenia gravis. Muscle Nerve 2010; 41: 375–378. [DOI] [PubMed] [Google Scholar]
- 9. Silvestri NJ, Wolfe GI. Treatment-refractory myasthenia gravis. J Clin Neuromuscul Dis 2014; 15: 167–178. [DOI] [PubMed] [Google Scholar]
- 10. Binks S, Vincent A, Palace J. Myasthenia gravis: a clinical-immunological update. J Neurol 2016; 263: 826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suh J, Goldstein JM, Nowak RJ. Clinical characteristics of refractory myasthenia gravis patients. Yale J Biol Med 2013; 86: 255–260. [PMC free article] [PubMed] [Google Scholar]
- 12. Drachman DB, Adams RN, Hu R, et al. Rebooting the immune system with high-dose cyclophosphamide for treatment of refractory myasthenia gravis. Ann N Y Acad Sci 2008; 1132: 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nowak RJ, Dicapua DB, Zebardast N, et al. Response of patients with refractory myasthenia gravis to rituximab: a retrospective study. Ther Adv Neurol Disord 2011; 4: 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maggi L, Andreetta F, Antozzi C, et al. Thymoma-associated myasthenia gravis: outcome, clinical and pathological correlations in 197 patients on a 20-year experience. J Neuroimmunol 2008; 201–202: 237–244. [DOI] [PubMed] [Google Scholar]
- 15. Mantegazza R, Bonanno S, Camera G, et al. Current and emerging therapies for the treatment of myasthenia gravis. Neuropsychiatr Dis Treat 2011; 7: 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jani-Acsadi A, Lisak RP. Myasthenic crisis: guidelines for prevention and treatment. J Neurol Sci 2007; 261: 127–133. [DOI] [PubMed] [Google Scholar]
- 17. Skeie GO, Apostolski S, Evoli A, et al. Guidelines for the treatment of autoimmune neuromuscular transmission disorders. Eur J Neurol 2006; 13: 691–699. [DOI] [PubMed] [Google Scholar]
- 18. Antozzi C. Immunoadsorption in patients with autoimmune ion channel disorders of the peripheral nervous system. Atheroscler Suppl 2013; 14: 219–222. [DOI] [PubMed] [Google Scholar]
- 19. Drachman DB, Jones RJ, Brodsky RA. Treatment of refractory myasthenia: ‘rebooting’ with high-dose cyclophosphamide. Ann Neurol 2003; 53: 29–34. [DOI] [PubMed] [Google Scholar]
- 20. Dalakas MC. Future perspectives in target-specific immunotherapies of myasthenia gravis. Ther Adv Neurol Disord 2015; 8: 316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Collongues N, Casez O, Lacour A, et al. Rituximab in refractory and non-refractory myasthenia: a retrospective multicenter study. Muscle Nerve 2012; 46: 687–691. [DOI] [PubMed] [Google Scholar]
- 22. Diaz-Manera J, Martínez-Hernández E, Querol L, et al. Long-lasting treatment effect of rituximab in MuSK myasthenia. Neurology 2012; 78: 189–193. [DOI] [PubMed] [Google Scholar]
- 23. Dubois EA, Cohen AF. Eculizumab. Br J Clin Pharmacol 2009; 68: 318–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Conti-Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: past, present, and future. J Clin Invest 2006; 116: 2843–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alexion Pharmaceuticals, Inc. Soliris (Eculizumab) [prescribing information]. New Haven, CT: Alexion Pharmaceuticals, Inc., 2017. [Google Scholar]
- 26. Howard JF, Jr, Utsugisawa K, Benatar M, et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol 2017; 16: 976–986. [DOI] [PubMed] [Google Scholar]
- 27. Hahn BH. Belimumab for systemic lupus erythematosus. N Engl J Med 2013; 368: 1528–1535. [DOI] [PubMed] [Google Scholar]
- 28. GlaxoSmithKline. Benlysta (Belimumab) [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline, 2017. [Google Scholar]
- 29. Ayzenberg I, Kleiter I, Schröder A, et al. Interleukin 6 receptor blockade in patients with neuromyelitis optica nonresponsive to anti-CD20 therapy. JAMA Neurol 2013; 70: 394–397. [DOI] [PubMed] [Google Scholar]
- 30. Jonsson DI, Pirskanen R, Piehl F. Beneficial effect of tocilizumab in myasthenia gravis refractory to rituximab. Neuromuscul Disord 2017; 27: 565–568. [DOI] [PubMed] [Google Scholar]
- 31. Alexander T, Sarfert R, Klotsche J, et al. The proteasome inhibitor bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann Rheum Dis 2015; 74: 1474–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schneider-Gold C, Reinacher-Schick A, Ellrichmann G, et al. Bortezomib in severe MuSK-antibody positive myasthenia gravis: first clinical experience. Ther Adv Neurol Disord 2017; 10: 339–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Castro-Sánchez P, Martin-Villa JM. Gut immune system and oral tolerance. Br J Nutr 2013; 109(Suppl. 2): S3–S11. [DOI] [PubMed] [Google Scholar]
- 34. Baggi F, Andreetta F, Caspani E, et al. Oral administration of an immunodominant T-cell epitope downregulates Th1/Th2 cytokines and prevents experimental myasthenia gravis. J Clin Invest 1999; 104: 1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barchan D, Souroujon MC, Im SH, et al. Antigen-specific modulation of experimental myasthenia gravis: nasal tolerization with recombinant fragments of the human acetylcholine receptor alpha-subunit. Proc Natl Acad Sci USA 1999; 96: 8086–8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lagoumintzis G, Zisimopoulou P, Trakas N, et al. Scale up and safety parameters of antigen specific immunoadsorption of human anti-acetylcholine receptor antibodies. J Neuroimmunol 2014; 267: 1–6. [DOI] [PubMed] [Google Scholar]
- 37. Kryczanowsky F, Raker V, Graulich E, et al. IL-10-modulated human dendritic cells for clinical use: identification of a stable and migratory subset with improved tolerogenic activity. J Immunol 2016; 197: 3607–3617. [DOI] [PubMed] [Google Scholar]