ABSTRACT
Although outbreaks of Neisseria meningitidis serogroup X occured in a couple of African countries, a limited number of serogroup X meningococcal cases were reported in America and Europe as well as Turkey. Additionally, serogroup X is still not represented in current conjugated meningococcal vaccines. Here, we describe the first pediatric case with meningitis caused by Neisseria meningitidis serogroup X ST-5799 (ST-22 complex) that formed a distinct lineage.
KEYWORDS: menengitis, pediatrics, sequence analyses, phylogenetic network, Turkey
Introduction
Neisseria meningitidis, which has 12 recognized serogroups, is now considered to be the leading cause of bacterial meningitis in Turkey as well as many parts of the world.1 The vast majority of invasive disease is related to six meningococcal serogroups: (Men) A, B, C, W, X and Y.2 Although Men X outbreaks have emerged in sub-Saharan Africa in recent years, developed countries have reported only sporadic cases.3-7 On the other hand; Men X is not included in conjugate meningococcal vaccines that have been available for years. Herein, we describe first pediatric case with meningococcal meningitis caused by serogroup X in Turkey.
Case report
A previously healthy and well developed 16-month-old boy presented to the emergency department of our hospital with two days of fever accompained by new onset lethargy. He was born at 31 weeks’ gestation and his medical history was unremarkable otherwise. The physical examination revealed fever (39,7°C at tympanic temparature measurement), tachypnea (56/min), tachycardia (128/min), nuchal rigidity, positive Kernig and Brudiznski signs. The blood pressure of the patient was 90/60 mmHg and the rest of the physical examination findings were unremarkable. There was no skin rash. Laboratory investigations were as follows; Hb 9.7 g/dL, peripheral blood white blood cell count, 7980/mm3 (neutrophils 76%, lymphocytes 14%, monocytes 4%, eosinophil 4%); platelet count, 402000/mm3, erythrocyte sedimantation rate (ESR), 35 mm/hour; C-reactive protein (CRP), 197 mg/L. The cerebrospinal fluid (CSF) analysis revealed 3000 leukocytes mm3 (90% polymorphonuclear leukocyte), high protein level (302 mg/dL), low glucose level (5 mg/dL, simultaneous blood glucose level 143 mg/dl) and high lactate level (120 mg/dl). Gram negative diplococcus were seen on the Gram-stained preparation. Acute bacterial meningitis was diagnosed, and ceftriaxone (100 mg/kg/d, intravenously), and dexamethasone (0.8 mg/kg/d, intravenously for 2 days) were empirically initiated. His 50% Haemolytic Complement activity of serum (CH50) was normal. Neisseria meningitidis grew in agar plate cultures of CSF and blood. The isolate was serologically confirmed as MenX. Multiplex polymerase chain reaction (PCR) was used to identify the meningococci from CSF after DNA isolation and the identification of serogroups was based upon the oligonucleotides such as sia D gene, which is for Men W, B, C, and Y, orf-2 and ctrA-x genes, which are for Men A and Men X, respectively.8,9
Genome sequence and phylogenetic network analyses were performed as previously described.10-12 The isolate from our patient belonged to ST-5799 (ST-22 complex) of serogroup X. A core genome comparison (1546 loci) of all relatively complete (≤500 contigs) ST-22 complex genomes on https://pubmlst.org/neisseria/ (n = 244, accessed 21/06/17) indicated that the ST-22 complex is diverse and geographically widespread (Fig. 1a). No other serogroup X isolates were identified among the genomes which were mainly from serogroup W isolates, although a serogroup Y-associated clade and several well distributed isolates belonging to other serogroups were also identifed (Fig. 1b). Our isolate formed a distinct lineage with four UK carrier isolates (Fig. 1c) that also possessed serogroup X-related genes. The four carrier isolates were collected at intervals from a single individual during 2011 and 2012 (personal communication Prof. Robert C. Read, University of Southampton, UK). Other available carriage-associated genomes were widely distributed elsewhere in the population structure.13
Figure 1.
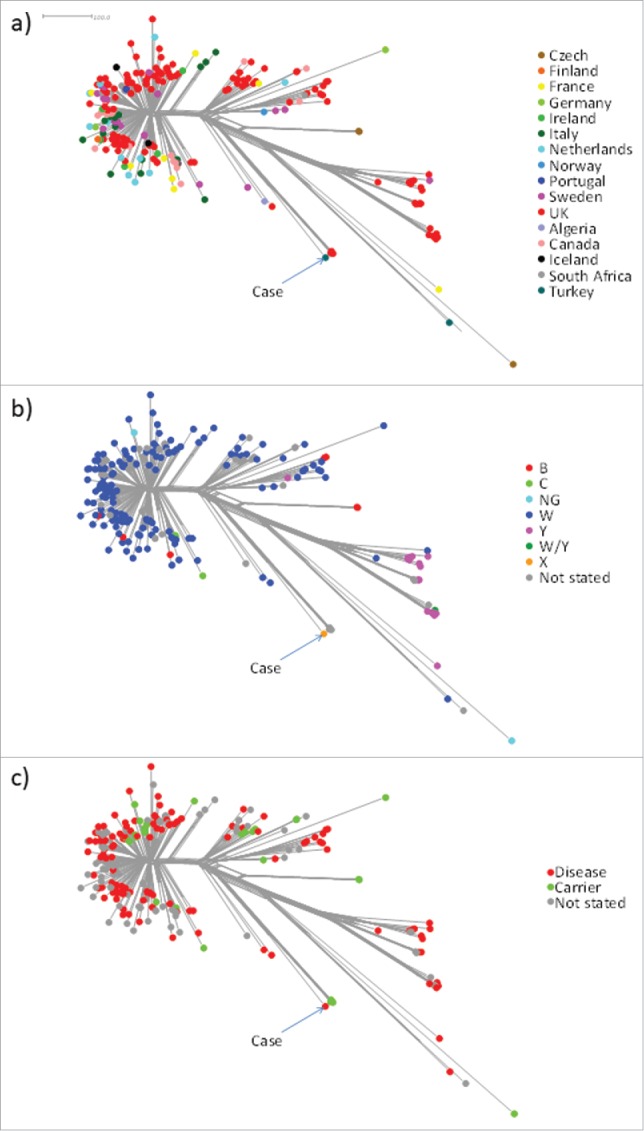
Phylogenetic network analysis of all ST-22 complex genomes on the PubMLST Neisseria database (n = 244; accessed 21/06/17). The network is based on a distance matrix whereby each isolate is compared to every other isolate in terms of 1546 core genes. The total number of non-identical genes between any two isolates represents the ‘distance’ as depicted in the scale bar. Clusters and sub-clusters represent clonal expansion from a common ancester a) illustrates the widespread geographic origins of the corresponding isolates. b) depicts the serogroup distribution among the corresponding isolates which were mainly serogroup W with a distinct, predominantly serogroup Y clade, and several well-distributed minor serogroups putatively arising though capsule switch events. c) depicts the distribution of case (disease) and carrier isolates which were generally well interspersed.
On clinical follow-up; his fever, tachypnea, tachycardia and meningeal irritation findings disappeared in 2 days. Four days later, up to 38.8°C fever reemerged with no clinical deteoriation and no persistence of meningeal irritation findings. At this time laboratory investigations revealed; Hb 9.9 g/dL,peripheral blood white blood cell count, 7980/ mm3 (neutrophils 70%, lymphocytes 30%); platelet count, 560.000/ mm3, ESR, 38 mm/hour; CRP, 5.9 mg/L. For the suspicion of intracranial complications of acute bacterial meningitis, cranial CT scan was performed. Cranial CT findings were found as normal. In the light of these findings, the reason of the recurrence of fever was considered as dexamethasone rebound fever. The patients fever was normalized in 3 days without therapeutic modification. It was noticed that at the 7th day of hospitalization; the patient could not sit without support although he had been able to walk before the illness. At the 14 days of hospitalization, the patient was able to sit without support and he was discharged from the hospital in a well condition.
Discussion
N.meningitidis is the most common cause of bacterial meningitis in Turkey and serogroups W, B, A, and Y have caused bacterial meningitis in a decreasing frequency.1 Although MenX strains were firstly described in the 1960s, MenX has been isolated from a soldier with a diagnosis of meningococcal meningitis in Turkey in 2010.14 This is the first pediatric case caused by MenX since meningitis surveillance was started in our laboratory in 2005, where 445 invasive meningococcal isolates were characterized up to 2016. The sequence type (ST) of the isolate recovered from the Turkish soldier belonged to ST-767 (ST-167 complex) whereas our isolate belonged to the ST-22 complex. ST-22 complex meningococci have been found previously among MenW15 and MenY strains.7 In Niger, 94% of the patients with MenX were under 20 years of age with median 8 years and were caused by the same clone, which was ST-181.3 In another study from Niger, it was reported that patients with NmX meningitis were significantly younger than that of the cases with NmA (mean age, 7.8 years). The most isolated clone was also ST-181 in this study.4 Therefore, continued surveillance is important to understand the nature of potentially new epidemic clones of N. meningitidis.
We showed that the most frequent serogroup of meningococci in Turkey is W according to data of our surveillance16 and we also illustrated a possible explanation for the presence of W that the acquisition of MenW is based on pilgrims attending the Hajj. After the detection of MenX, we firstly thought that the possible source might be Africa. In Africa, serogroup X outbreaks have been described in Niger3,4 and in Kenya5 as well as other countries of Africa that are included in the African meningitis belt. Moreover, Turkey announced 2005 as “the year of Africa” and it was declared that Africa has a crucial value for Turkey within the scope of Turkey's new foreign policy in 2008 with an increasing trade capacity (http://turkishpolicy.com/Files/ArticlePDF/turkeys-rising-role-in-africa-winter-2010-en.pdf). The epidemiology of serogroup X in Africa has been partially described.17 Furthermore, analyses showed that the infecting strains belonged to a new sequence type, ST-5403 (clonal complex unnasigned), in Kenya, and ST-181 (ST-181 complex), a sequence type yet found in Chad, Mali,6 and Niger. The sequence type and clonal complex of our isolate (ST-5799; ST-22 complex) were unrelated to previously described African serogroup X isolates. A further Turkish ST-22 complex case (serogroup W) was unrelated and belonged to a distinct clade. Aside from the genomes, there were two other ST-5799 isolates listed on the database, both of which were serogroup X. These included a isolate listed as ‘other’ from Germany (2004, ID 7944) and a carrier isolate from Turkey (2006; ID 8086). Thus, the origin of this MenX clone is unclear. Further genomic analysis would be required to determine if the isolates are related to the clade identified in this study.
Although capsular switches are commonly reported between meningococcal serogroups including B, C, and W, the data about capsular switching from serogroup X to another serogroup are limited. Nonetheless, some recombination events are reported between serogroups X and A recently.18 The serogroup X-associated sublineage identified in the present study is also a product of capsule switching, probably from serogroup W, though confirmation of this would require further analysis. This case also highlightes the importance of continued surveillance because of the import of new or epidemic clones of N. meningitidis, including MenX, which is not included in the available meningococcal vaccines.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This publication made use of the Neisseria Multi Locus Sequence Typing website (https://pubmlst.org/neisseria/) developed by Keith Jolley and sited at the University of Oxford (Jolley & Maiden 2010, BMC Bioinformatics, 11:595). The development of this site has been funded by the Wellcome Trust and European Union. This publication made use of the Meningitis Research Foundation Meningococcus Genome Library (http://www.meningitis.org/research/genome) developed by Public Health England, the Wellcome Trust Sanger Institute and the University of Oxford as a collaboration and funded by Meningitis Research Foundation.
References
- [1].Ceyhan M, Gurler N, Ozsurekci Y, Keser M, Aycan AE, Gurbuz V, Salman N, Camcioglu Y, Dinleyici EC, Ozkan S, et al.. Meningitis caused by neisseria meningitidis, hemophilus influenzae Type B and streptococcus pneumoniae during 2005–2012 in Turkey. Hum Vaccin Immunother. 2014;10:2706-12. https://doi.org/ 10.4161/hv.29678. PMID:25483487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. New Eng J Med. 2001;344:1378-88. https://doi.org/ 10.1056/NEJM200105033441807. PMID:11333996 [DOI] [PubMed] [Google Scholar]
- [3].Djibo S, Nicolas P, Alonso JM, Djibo A, Cournet D, Riou J, Chippaux JP. Outbreaks of serogroup X meningococcal meningitis in Niger 1995–2000. Trop Med Int Health. 2003;8:1118-23. https://doi.org/ 10.1046/j.1360-2276.2003.01126.x. PMID:14641847 [DOI] [PubMed] [Google Scholar]
- [4].Boisier P, Nicolas P, Djibo S, Taha M-K, Jeanne I, Mainassara HB, Tenebray B, Kairo KK, Giorgini D, Chanteau S, et al.. Meningococcal meningitis: unprecedented incidence of serogroup X-related cases in 2006 in Niger. Clin Infect Dis. 2007;44:657-63. https://doi.org/ 10.1086/511646. PMID:17278055 [DOI] [PubMed] [Google Scholar]
- [5].Materu S, Cox HS, Isaakidis P, Baruani B, Ogaro T, Caugant DA. Serogroup X in meningococcal disease, Western Kenya. Emerg Infect Dis. 2007;13:944-45. https://doi.org/ 10.3201/eid1306.070042. PMID:17582900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gagneux S, Wirth T, Hodgson A, Ehrhard I, Morelli G, Kriz P, Genton B, Smith T, Binka F, Pluschke G, et al.. Clonal groupings in serogroup X Neisserria meningitidis. Emerg Infect Dis. 2002;8:462-66. https://doi.org/ 10.3201/eid0805.010227. PMID:11996679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yazdankhah SP, Kriz P, Tzanakaki G, Kremastinou J, Kalmusova J, Musilek M, Alvestad T, Jolley KA, Wilson DJ, McCarthy ND, et al.. Distribution of serogroups and genotypes among disease-assciated and carried isolates of Neisseria meningitidis from Czech Republic: Greece, and Norway. J Clin Microbiol. 2004;42:5146-3. https://doi.org/ 10.1128/JCM.42.11.5146-5153.2004. PMID:15528708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Taha MK. Simultaneous approach for nonculture PCR-based identification and serogroup prediction of Neisseria meningitidis. J Clin Microbiol. 2000;38:855-57. PMID:10655397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tsolia MN, Theodoridou M, Tzanakaki G, Kalabalikis P, Urani E, Mostrou G, Pangalis A, Zafiropoulou A, Kassiou C, Kafetzis DA, et al.. The evolving epidemiology of invasive meningococcal disease: a two-year prospective, population-based study in children in the area of Athens. FEMS Immunol Med Microbiol. 2003;36:87-94. https://doi.org/ 10.1016/S0928-8244(03)00083-X. PMID:12727371 [DOI] [PubMed] [Google Scholar]
- [10].Diallo K, Gamougam K, Daugla DM, Harrison OB, Bray JE, Caugant DA, Lucidarme J, Trotter CL, Hassan-King M, Stuart JM, et al.. Hierarchical genomic analysis of carried and invasive serogroup A Neisseria meningitidis during the 2011 epidemic in Chad. BMC Genomics. 2017;18:398. https://doi.org/ 10.1186/s12864-017-3789-0. PMID:28532434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ladhani SN, Beebeejaun K, Lucidarme J, Campbell H, Gray S, Kaczmarski E, Ramsay ME, Borrow R. Increase in endemic Neisseria meningitidis capsular group W sequence type 11 complex associated with severe invasive disease in England and Wales. Clin Infect Dis. 2015;60:578-85. https://doi.org/ 10.1093/cid/ciu881. PMID:25389259 [DOI] [PubMed] [Google Scholar]
- [12].Jolley KA, Maiden MC. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics. 2010;11:595. https://doi.org/ 10.1186/1471-2105-11-595. PMID:21143983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Deasy AM, Guccione E, Dale AP, Andrews N, Evans CM, Bennett JS, Bratcher HB, Maiden MC, Gorringe AR, Read RC. Nasal Inoculation of the Commensal Neisseria lactamica Inhibits Carriage of Neisseria meningitidis by Young Adults: A Controlled Human Infection Study. Clin Infect Dis. 2015;60:1512-20. https://doi.org/ 10.1093/cid/civ098. PMID:25814628 [DOI] [PubMed] [Google Scholar]
- [14].Kiliç A, Bedir O, Basustaoglu AC, Bratcher HB, Jolley KA, Mert G. Neisseria Meningitidis serogroup X sequence type 767 in Turkey. J Clin Microbiol. 2010;48:4340-41. https://doi.org/ 10.1128/JCM.01417-10. PMID:20739496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bond KA, Stevens K, Bulach D, Carville K, Ong KS, Howden BP. Rising incidence of invasive meningococcal disease caused by Neisseria meningitidis serogroup W in Victoria. Med J Aust. 2016;204:265-66. https://doi.org/ 10.5694/mja15.01222. PMID:27078598 [DOI] [PubMed] [Google Scholar]
- [16].Ceyhan M, Celik M, Demir ET, Gurbuz V, Aycan AE, Unal S. Acquisition of meningococcal serogroup W-135 carriage in Turkish Hajj pilgrims who had received the quadrivalent meningococcal polysaccharide vaccine. Clin Vaccine Immunol. 2013;20:66-68. https://doi.org/ 10.1128/CVI.00314-12. PMID:23136117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Agnememel A, Hong E, Giorgini D, Nuñez-Samudio V, Deghmane AE, Taha MK. Neisseria meningitidis Serogroup X in Sub-Saharan Africa. Emerg Infect Dis. 2016;22:698-702. https://doi.org/ 10.3201/eid2204.150653. PMID:26982628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhu B, Yao P, Zhang L, Gao Y, Xu L, Xie N, Shao Z. Genetic Analysis of Neisseria meningitidis Sequence Type 7 Serogroup X Originating from Serogroup A. Infect Immun. 2017;85(6) pii: e01019-16. https://doi.org/ 10.1128/IAI.01019-16 [DOI] [PMC free article] [PubMed] [Google Scholar]


