ABSTRACT
Diarrhea is one of the world's leading killers of children, and globally, rotavirus is the most common cause of severe diarrhea among under 5 children. In Bangladesh, rotavirus kills nearly 6,000 under 5 children in each year. To reduce the burden of childhood rotavirus diseases, universal rotavirus vaccination is recommended by World Health Organization. The objective of this study is to assess the cost-effectiveness of introducing universal childhood rotavirus vaccination with the newly developed ROTAVAC vaccine in national Expanded Programme of Immunization in Bangladesh. We developed a decision model to examine the potential impact of vaccination in Bangladesh and to examine the effect if the vaccination is applied in the nationwide immunization program schedule. Introduction of childhood universal rotavirus vaccination in Bangladesh scenario appears as highly cost-effective and would offer substantial future benefits for the young population if vaccinated today. The cost per DALY averted of introducing the rotavirus vaccine compared with status quo is approximately US$ 740.27 and US$ 728.67 per DALY averted from the health system and societal perspective respectively which is “very cost-effective” using GDP threshold level according to World Health Organization definition. The results of this analysis seek to contribute to an evidence-based recommendation about the introduction of universal rotavirus vaccination in national Expanded Programme of Immunization (EPI) in Bangladesh.
KEYWORDS: Bangladesh, childhood, cost-effective, rotavirus, vaccination
Introduction
Diarrhea is one of the world's leading killers of children, and globally, rotavirus is the most common cause of severe diarrhea among under 5 children and approximately 215, 000 children aged <5 y die each year due to rotavirus infections.1,2 It is a leading cause of infantile gastroenteritis accounting for 20% of diarrhea-associated deaths.3 The mortality burden of rotavirus diseases is greatest in Asia and Africa.4 To tackle this public health problem, many life-saving interventions already exist, such as oral rehydration therapy and micro-nutrient supplementation that have already proven effective for preventing of diarrheal related episodes.5 Besides, improving water quality and sanitation, food quality, and hygiene are also preventive measures for the diarrheal related diseases, although those are generally long-term solutions and are linked with socioeconomic and development of communities.6 However, these strategies have not had a great impact in reducing the incidence of rotavirus diarrhea globally.1 Also these interventions remain time consuming and often require a substantial amount of money. This is crucial for a resource poor country like Bangladesh where a huge number of the population lives in urban slums and are at high risk for diarrheal diseases. It was estimated that in every year, rotavirus kills nearly 6,000 under 5 children in Bangladesh.7 The symptoms of rotavirus are vomiting and fever with associated diarrhea; the average duration of illness is 6 days; and the incubation period is at least 2 to 3 d.8 To reduce the burden of childhood rotavirus diseases, rotavirus vaccination is highly recommended, especially for those countries where diarrheal diseases cause more than 10% of deaths.9
Recently, the WHO updated its previous position papers on rotavirus vaccines and reaffirmed its 2009 recommendation that rotavirus vaccines should be included in all National Immunization Programs (NIPs) globally.10 However, the decision to introduce new vaccines is a quite complex issue as the new vaccines are always costly to the health system. Therefore, it is important for the policy maker to understand whether the expenditure is justifiable on epidemiological and fiscal grounds as investment in vaccine programs necessarily denies funds from competing health priorities. In addition, rotavirus vaccines are relatively expensive compared with the other childhood vaccines.11 Therefore, policy makers need to understand the expected health and economics benefit of a future rotavirus vaccination program. In 2016, approximately 81 countries have offered childhood universal rotavirus vaccination and the Global Alliance for Vaccines and Immunization (GAVI) provides financial support to certain countries for introducing rotavirus vaccination program in their immunization programmes.12 However, GAVI-supported countries are also required to make co-payments for vaccines, and understanding whether these vaccines are cost-effective is important for budgetary planning and negotiating procurement costs.13 And these countries will eventually graduate from these programs and the support will stop. The cost-effectiveness of rotavirus vaccines has been evaluated more extensively over the past decade.14 Value for money has become an increasingly necessary criterion among others in new vaccine introduction strategy and cost-effectiveness analysis can help to guide decision about introduction of vaccine versus other health intervention.15 There are several cost-effectiveness studies of rotavirus vaccination among many lower-and middle income settings, however, there has been no such study in the Bangladeshi context. The objective of this study is to assess the cost-effectiveness of universal childhood rotavirus vaccination with the newly developed low cost ROTAVAC vaccine in national Expanded Programme of Immunization (EPI) in Bangladesh.16 The analysis was based on the health system and societal points of view. In health system perspective the costs of rotavirus related medical care and the cost of vaccination program were included; whereas in the societal perspective both direct medical (e.g. medicine, diagnostic), direct non-medical cost (e.g., transportation, lodging) indirect cost (e.g., income loss) and cost of vaccination program were included.
Results
Input parameters for estimating the childhood rotavirus disease burden, service utilization and costs, vaccine coverage, efficacy duration of protection years and vaccine delivery related costs are summarized in methods section. The model's results for costs and outcome for static cohorts from 2016 to 2017 for the universal childhood rotavirus vaccination are presented below.
Health impact of vaccination
The outcomes presented in Table 1 reflect the projected health outcomes when indirect effects are ignored. We estimate that introducing rotavirus vaccination will avert approximately 1.8 million rotavirus cases over a 2-year period. Once the vaccination program is fully implemented, vaccination is estimated to avert over 50 thousand outpatient admissions, 40 thousand inpatient admissions and 49.46 thousand DALYs averted and more than 2.5 thousand deaths per year.
Table 1.
Key vaccination program outcomes over 2 y period.
| Parameters | Health System Perspective | Societal Perspective |
|---|---|---|
| Population cohort (′000) | 15,175.00 | 15,175.00 |
| Average costs per vaccine, US$ | 1 | 1 |
| Average delivery costs per fully vaccinated child, US$ | 0.84 | 0.84 |
| Total inpatients cost averted, US$ (′000) | 5,673.94 | 6,675.23 |
| Total outpatient cost averted, US$ (′000) | 176.59 | 392.42 |
| Total costs averted, US$ (′000) | 5,850.53 | 7,067.65 |
| Net costs of the vaccination, US$ (′000) | 73,228.24 | 72,080.99 |
| Total number of case averted, (′000) | 180.61 | 180.61 |
| Total number of death averted (′000) | 5.01 | 5.01 |
| Total DALYs averted (′000) | 98.92 | 98.92 |
| Incremental cost-effectiveness ratio (ICER) per | ||
| - DALY averted, US$ | 740.27 | 728.67 |
| - Case averted, US$ | 405.46 | 399.1 |
| - Life saved, US$ | 14,596.44 | 14,367.76 |
| GDP Thresholds (for references) | ||
| Cost-effective (3* GDP/capita) | ||
| Very cost-effective (GDP/capita) | Yes | Yes |
Costs and DALYs are discounted at 3% per year
Cost and healthcare utilization
Our results reveal that within the modeling time horizon, introducing universal vaccination for young population, approximately US$ 5.8 million costs could be saved from health system perspective whereas at least US$ 5.63 million costs were saved by preventing inpatient visits. The costs averted due to vaccination are higher from societal perspective (US$ 7.06 million) due to inclusion of household out-of-pocket cost and including the time costs of their caregivers.
Cost effectiveness estimates
A universal rotavirus vaccination in Bangladesh would cost the public approximately US$ 73.22 million for the under-five population cohort although several inpatient admissions and outpatient visits could be averted. The cost per DALY averted of introducing the rotavirus vaccine compared with status quo is US$ 740.27 and US$ 728.27 per DALY averted from the health system and societal perspective respectively. Both incremental cost-effectiveness ratios (ICERs) fall below the 2015–2016 fiscal year gross domestic product (GDP) per capita in Bangladesh (US $ 1,466) which is used as a threshold for determining the cost-effectiveness of an intervention. Therefore, these results demonstrate the likely cost-effectiveness of universal childhood rotavirus program in Bangladesh according to WHO criteria (Table 2).
Table 2.
Input baseline parameter and sensitivity analysis.
| Parameters | Baseline value | Sensitivity analysis |
|---|---|---|
| Population cohort | 15,175,00054 | |
| Age of the population | Under five children | |
| Life table | Country specific85 | |
| Duration of illness (days) | 64,6 | 4.786 to 1087 |
| Price of vaccine (per vial) | US $ 175 | US $ 0.5 to US $ 10 (Author assumption) |
| Cost of vaccine delivery process | US $ 0.83576 | US $ 0.5 to US $ 2 (Author assumption) |
| Vaccination coverage (%) | 65%80 | 40% to 96%16 |
| Incidence of Rotavirus (per 1000) | 10.863 | 8 to 19.663 |
| Vaccine Effectiveness | 55.1%16 | 40 to 85%.67,68 |
| Treatment cost for IPD | $ 8483 | US $ 51.99 to US$ 96.5418 |
| Treatment cost for OPD | 3.8849 | 1 to 5 (Author assumption) |
| Case fatality (per 1000) | 0.366 | 0.3 to 4.1466 |
| Vaccine protection (years) | 216 | 1 to 388 |
| Disability weight | 0.1258 | 0.1258 to 0.28189 |
Cost-effectiveness threshold
1 × GDP per capita (2016)—WHO threshold for ‘highly cost-effective’ (US $) 1,46690
3 × GDP per capita (2016)—WHO threshold for ‘cost-effective’ (US $) 4, 398
Scenario analysis
Fig. 1 and Fig. 2 represents the results of varying select model input values on the cost-effectiveness of rotavirus vaccination from the health system perspective. The results of the deterministic sensitivity analysis showed that vaccine price, vaccine delivery cost, disease incidence, case fatality rate, vaccine coverage and vaccine efficacy were among the most important parameters that can change the ICER. These results are very conservative because we consider a simple static model and ignore the herd immunity of rotavirus vaccination although other study reported that in long-term a significant proportion of the population might be protected against infections.17 In Fig. 1, on the basis of WHO threshold, from the health system perspective, the rotavirus vaccine was highly cost effective in some of the least favorable scenarios for vaccine introduction, namely low incidence rate, low vaccine coverage, low efficacy, low healthcare utilization costs, low mortality rate and moderate price of vaccine. However, the price of the vaccine is the most influential parameter and our analysis showed that at current scenario, if the price of vaccine was at its highest (e.g., US$ 10 and above per vial i.e. market price range of currently available rotavirus vaccine was US$16 to US$ 25 in Bangladesh), then it appeared as a cost-ineffective option, although up to US$ 9.40, the universal rotavirus vaccination is cost-effective in Bangladesh. Similar results were also observed for societal perspective (the figure is not presented here). This suggests that the model results are robust to changes in the value of all major variables, however, there is a need to ensure that the pricing of the vaccine is appropriate in the Bangladesh context. In Fig. 2, vaccine price, vaccine delivery related cost, incidence of disease, case fatality rate and vaccine efficacy and duration of vaccine protection are the most influential factors.
Figure 1.
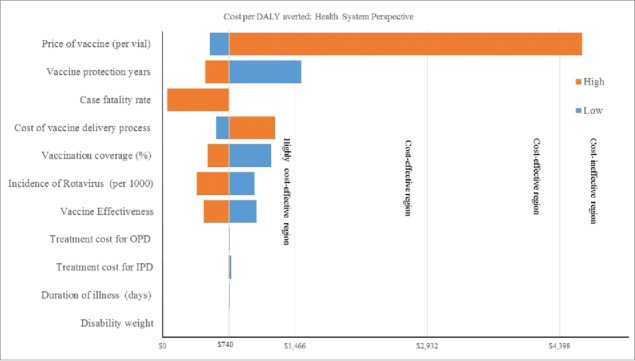
Costs per DALY averted: Health system perspective.
Figure 2.
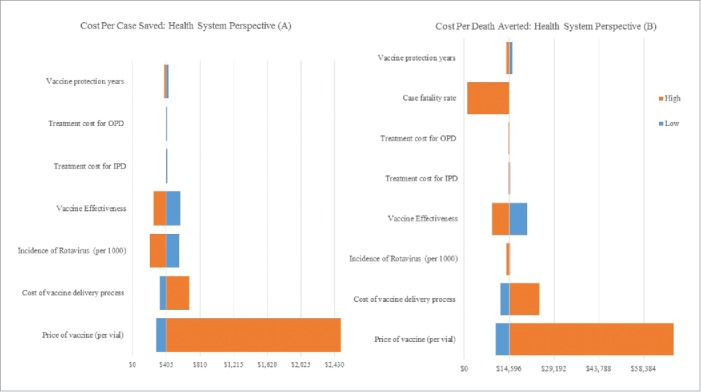
Changes in input parameter on ICER per case averted (A) and death averted (B): health system perspective.
Discussion
Several observations emerge from our analysis. The results of this evaluation suggest that universal childhood rotavirus vaccination would be highly cost-effective and would substantially reduce childhood illness and death due to rotavirus vaccination in Bangladesh. The analysis was performed using country level data where possible, and relied on regional estimates of Lower and Middle Income Countries (LMIC) when national data were not available. Due to limited country representative data on the burden of rotavirus in Bangladesh, very limited studies are available and this is the first cost-effectiveness analysis of rotavirus vaccination in Bangladesh. Our analysis showed that introduction of universal rotavirus vaccination would be a highly cost-effective investment from the health system (US $ 740.27 per DALY averted) and societal perspective (US $ 728.67 per DALY averted). Even in the lowest scenario the future rotavirus vaccination program will be a cost-effective option for health system and societal perspective although we ignore the indirect effects of the vaccination. These cost-effectiveness ratios could provide a useful initial point for comparing the value for money of investments for rotavirus vaccination against the other rotavirus prevention programs such as water and sanitation interventions.
Results generated from this study are consistent with other results on the cost-effectiveness of universal rotavirus vaccination from previous studies conducted in many LMI countries in worldwide.6,18 A study using simulated agent-based model observed that introducing rotavirus vaccination in Indian population is a cost-effective option and which will significantly alleviate disease and financial burdens in Indian households.18 A country-led analysis from Senegal found that cost-effectiveness of rotavirus vaccination was US$ 92 and US$ 73 per DALY averted from the health system and societal perspective which is approximately 10 times lower than country context gross domestic product.6 In Kenya, the cost effectiveness ratio per DALYs averted ranged between US$ 142 to US$ 288 from the societal perspective which is 15 times lower than its GDP per capita.19 Our results showed a cost per DALY averted relatively higher than the above studies and approximately 2 times lower than GDP per capita in Bangladesh. The short time horizon (2 years) we used compared with the above studies is likely a reason for higher cost-effectiveness ratio per DALYs averted. Another reason is that we used the lower case fatality and incidence rate as those data comes from the estimation of rotavirus related hospitalization. Our estimation indicated that by introducing rotavirus vaccination we can prevent approximately 2500 deaths although this underestimates the real burden of rotavirus disease, as the community occurring rotavirus was not captured due to limited data. In a review study, it was observed that in LMI countries in Asia the cost per DALY averted lay between US$ 22 to US$ 2,007 due to rotavirus vaccination, however, the price of the vial ranged between US$ 1 to US$ 30.20 This study finds that that if the price of the ROTAVAC vaccine is 2.6 and 9.4 times higher than the current price of a vial (US$ 1), the vaccination program still a ‘very cost-effective’ and ‘cost-effective’ option. The conclusion from these findings is that the universal childhood rotavirus vaccination program remains cost-effective even at the higher price of vaccine, which is encouraging for those countries which are no longer GAVI subsidized. In Vietnam, a universal rotavirus vaccination study found that if the price per vaccine is US$ 7.26 or less then the immunization program was the cost-effective from a societal perspective.15 In Uzbekistan, the universal rotavirus vaccination would be cost effective with the price ranges up to US$ 2, however case fatality and vaccine efficacy are the most influential parameters which made the intervention cost-effective as our study also demonstrated.21
Our uncertainty analysis showed (Fig. 1) shows that from the health system perspective the future vaccination will be cost-effective even at the higher price of vaccine (up to US$ 9.4). The societal cost per DALYs averted was in line with these results (figure was not presented here). We did not consider the indirect effects from averted transmission, and considered the young population which has no own indirect costs like income loss and opportunity costs which might affect the cost-effectiveness ratio. Our results showed that the cost per case and death averted were US$ 405 and US $ 14,596 from the health system perspective whereas US$ 399 and US$ 14,368 from the societal perspective. Like our study, a national rotavirus study conducted in Brazil from the health system perspective found that the cost per DALY and per life saved were US$ 643 and US$ 21,643 respectively.22 In Thailand, from a health system perspective the cost per life saved was US$ 11,800 which is lower than Bangladesh scenario. These studies used relatively longer time horizon (5 years) compared with our study, and generally for a longer time horizon the intervention becomes increasingly cost effective at reducing the incidence of the particular disease.17 Our uncertainty analysis (Fig. 2) indicated that the cost per case and death averted ranged between US$ 210 vs US$ 2,544 and US$ 1,058 vs US$ 91,578 for the higher and lower estimation respectively.
In the last few decades, vaccines with a wide price range have been developed targeting rotavirus disease. Hence, cost-effectiveness analysis (CEA) study is important to inform decisions about which vaccines to introduce into EPI schedules.23 A systematic literature review concludes that CEA is an important decision-making tool for vaccine introduction although country-specific reliable and high quality of data are crucial for such analysis.24 However, due to lack of technical capacities and empirical data, sometimes, LMIC decisions-makers depend on the previous cost-effective evaluation studies conducted in different countries.25 Further, national decision-makers are always interested to investigate whether the new vaccine delivery is affordable and sustainable in the long-term for their country. Most of the vaccination studies preferred to estimate the cost-effectiveness results using DALYs although the limitation of such analysis are well known.26-28 In the last decade, the Pan American Health Organization (PAHO) created the ProVac Initiative in 2004 to support government especially in Latin America and the Caribbean to understand the evaluation of vaccines for evidence-based decision-making process. For their initiatives they developed a spreadsheet software program named TRIVAC which was used to estimates the incremental cost-effectiveness ratio of 3 childhood vaccines (Haemophilus influenzae type b vaccine, pneumococcal conjugate vaccine and rotavirus vaccines in Latin America.29,30 TRIVAC is a static cohort model calculates a variety of indicators including cost per DALYs averted, case averted, admission and outpatient visit averted and percentage of under-five mortality averted with aim to the decision making process. However, this model has been criticized as being based on average cost-effectiveness ratio instead of incremental cost-effectiveness ratios.31 The pros and cons of the model reported elsewhere.30
Our study, like most others, uses the GDP thresholds level proposed by WHO. The GDP threshold might be a useful screening tool but should not be the only consideration for vaccination investment as there are other issues such as feasibility, affordability, alternative interventions and other local consideration which are not accounted for in the threshold level decision rule. The success of vaccination programs depends on many other factors such as human resources and cold chain and logistics management (as maintaining vaccine at optimum temperature have become more complex due to nature of the vial). Further, effective and efficient vaccine delivery is also required to improve the equity of service. However, in resource-poor countries, decision-making processes need to take both of the technical and political consideration.32 Many studies highlighted the importance of political factors in introducing the new vaccine in a country33-35 and ‘political rationality’ was even more important for decision-making process than ‘technical rationality.36 Again, analysis using this GDP threshold may not reflect the true opportunity costs of other available interventions and thus it does not represent true value and affordability for a specific country.37,38 Further, there is weak theoretical justification for the cut-off levels for cost-effectiveness analysis and the non-health benefit of policy are ignored.39 Thus local threshold levels might be suitable as a cost-effectiveness reference level which will be based on social values, budget availability and willingness to pay within the target country.31 Again, many studies argued that cost-effectiveness analysis alone cannot capture the broader impact of vaccination.20, 40-42 However, we take the view that as Jauregui et al. concluded, economic tools such as cost-benefit analysis and cost-effectiveness analysis (although imperfect) have the potential to strengthen the use of evidence concerning new vaccine introduction of a country.43 And policymakers often need to understand the allocation of public expenditure for vaccination programs compared with other sectors such as water and sanitation investment, and the use of cost-effectiveness thresholds provides a useful perspective on this issue.44
To inform policy makers in resource-poor settings like Bangladesh, our study demonstrated the introduction of universal rotavirus vaccines in routine childhood immunization would be a good investment with respect to health care and costs. A number of similar studies conducted in other LMICs demonstrated the childhood rotavirus vaccination is highly cost-effective and appeared ‘good value of money’ from the public perspective. However, those studies used various assumptions, a wide range of epidemiological, clinical, and economic parameters and also used different model and so the findings may not be directly comparable6,15,19,21,45-51 An optimum decision should be made by comparing universal rotavirus vaccination with alternative public health and healthcare interventions in Bangladesh (such as diarrheal case management strategies, other diarrheal prevention program, sanitation and hygiene related interventions and even introducing with the other vaccines). Again, cost-effectiveness results are highly dependent on input parameter, especially mortality, vaccine price and efficacy-related information. Hence, reliable estimates on childhood mortality, diseases outcome and cost estimation are important so that a standardized comparisons of cost-effectiveness across a range of health interventions could be made.
Although our analysis concludes that the future rotavirus vaccination would be highly cost-effective, there are some limitations in this study as we made several assumptions which could affect the cost-effectiveness ratio. For example, uncertainty with the respect of rotavirus incidence rate, mortality, price and efficacy of vaccine. Furthermore, this analysis did not consider dynamics or the herd protection effects, although rotavirus vaccination protects a substantial part of the young and adult population. Further, in certain endemic regions, it was observed that incorporating the herd protection made the vaccination program cost-effective which was not previously.52 Again, we did not explore other rotavirus vaccines available in market which might be add depth to our study. However, sensitivity analysis showed that, despite these uncertainties, the universal childhood rotavirus vaccination will be cost-effective from the health system and societal perspective. The study based on a simple static model using the direct effect of childhood universal rotavirus vaccination has helped to contribute the knowledge generation of future rotavirus vaccination program in Bangladesh as the government of Bangladesh is committed to introducing rotavirus vaccination program in national EPI schedule.53 In addition, it has helped to highlight important gaps of country representative data, especially the lack of good quality information on rotavirus incidence and mortality and the economic burden of rotavirus illness.
Conclusion
Introducing of childhood rotavirus vaccination in Bangladesh scenario is estimated to be highly cost-effective and would offer substantial future benefits for young population vaccinated today. Other technical, programmatic, political priority, and social issues need to be considered in the process of making the decisions on the introduction of rotavirus vaccine in Bangladesh. The results of this analysis seek to contribute to an evidence-based recommendation about the implementation of rotavirus vaccination.
Methods
Model
We developed a decision model using Microsoft ® Excel spreadsheet to examine the potential impact of low-cost universal oral rotavirus vaccination in Bangladesh and to examine the effect if the vaccination is applied in the nationwide immunization program schedule. We estimate the economic and health burden due to rotavirus disease and the cost-effectiveness of rotavirus vaccination in Bangladesh from the health system and societal perspective. Principal model parameters are described in Table 2. The primary measurements of cost-effectiveness in the current study are the incremental cost per DALY averted, incremental cost per case averted and incremental cost per death averted. Incremental cost-effectiveness ratios (ICERs) are calculated by dividing the difference in cost with and without the universal childhood rotavirus vaccination program by the difference in health outcomes with and without the intervention. For these perspective we collected several parameters from various published papers and regional data sources. The model estimates the various health associated outcomes, health care costs averted and the reduction in disease burden after childhood rotavirus vaccine introduction in national settings. For comparing pre and post universal rotavirus vaccination scenario, we estimated the events and possible costs to capture the baseline rotavirus disease burden and then assessed the number of rotavirus disease-associated events and possible costs that would occur after the introduction of low-cost rotavirus vaccine into the national immunization program. The model was applied to the 2016 annual static birth cohort (N = 15,175,000)54 and analysis was based on 2 y period with additional sensitivity analysis up to 1 to 3 y time horizon. The cost-effectiveness analysis was reported based on the health system and for societal perspective according to the Panel on Cost-Effectiveness in Health in Medicine.55 For the health system perspective we included the costs of medical care related to rotavirus illness and the cost of vaccination program; whereas in the societal perspective both direct medical (e.g., medicine, diagnostic), direct non-medical cost (e.g., transportation, lodging) and indirect, cost (e.g., income loss) were included, however intangible costs, like pain and discomfort were excluded from the estimation. In the current analysis, the costs averted by vaccination were subtracted from the costs invested in vaccination, and they then were divided by the number of Disability-Adjusted Life Year (DALYs) or the number of deaths and cases averted due to introducing childhood rotavirus vaccination. All future costs and benefits were discounted at a rate of 3% annually.56 For reporting the cost-effectiveness scenario we used the common cost-effectiveness threshold level proposed by the World Health Organization: an intervention is considered cost-effective if cost per DALYs averted is less than 3 times of the national annual per capita GDP, whereas the costs less than the GDP per capita is considered highly cost-effective.57 It was observed that, Disability-adjusted life year (DALYs) is the preferred choice for measuring outcomes in lower and middle-income countries rather than other methods.11 The concept of DALYs was used to quantify the disease burden incorporating life lost due to premature death and the time spent in unhealthy states.58 The DALY is a time based measure which combines years of life lost (YLL) due to premature mortality and the years of healthy life lost to living in a state of less than perfect health (years lost to disability or YLD) in country specific context.59 Therefore, DALY is the summation of YLL+YLD and 1 DALY can be considered as equivalent to one lost year of healthy life. Using DALYs, it is possible to measure the gap between current health status and an ideal situation, where everyone lives according to their life expectancy without disease and disability,18 however, the detailed philosophical and methodological aspect of the DALY calculation have been described elsewhere.58,60 Like earlier studies,52,61 to estimate the DALY avoided due to rotavirus vaccination we applied the 4 equations (1–4) as described below:
| (1) |
| (2) |
| (3) |
| (4) |
In the above equations, Eff t is the effectiveness of the rotavirus vaccine in year t, Cover is the percentage of under 5 children that would be vaccinated if the vaccine were provided for free, CFR, I and N are the case fatality rate, incidence of rotavirus illness and number of under 5 children, Length is average duration of illness (i.e., number of days sick with rotavirus), DW is the disability weight, LE is the life expectancy and Durr is the duration of the vaccine effectiveness.
Incidence and case fatality rate
The epidemiological data comes from different studies conducted in this setting. The high burden of all diarrheal diseases, including rotavirus, was observed in the impoverished part of the population like urban slums and poor regions of the country.62 The population-based incidence of hospitalization for rotavirus diarrhea by age and year varied from 10.8 to 19.6/1000 children less than 5 y old in Bangladesh63 and the case fatality rate of rotavirus diseases is still unknown. An earlier hospital-based study conducted in urban Bangladesh and estimated that the childhood mortality attributable to rotavirus was 2 to 3%, however, the analysis was based on the 2% sample of the diarrheal cases of the admitted person in that hospital, which might underestimate the true burden.64 Further, the above hospital is prepared to handle the rotavirus and other diarrheal cases, hence case fatality rate will be lower than the national. However, recently, a nationally representative study conclude that approximately 23% under 5 children did not seek any of the treatment during childhood diarrhea.65 In the similar context, a nationally representative survey conducted in India found that overall under 5 mortality rate from rotavirus-associated gastroenteritis ranged from 0.03 up to 4.14 per 1000 live births depending on the socio-demographic region of the country.66
DALY weights
In this analysis, we used the DALY weights of 0.12 for rotavirus related illness as there is no published DALY weight specifics to rotavirus disease and this value was used for previous rotavirus study.58 This weight will be used to measure the pain, suffering and discomfort associated with rotavirus diseases, but the short duration means that the morbidity associated with the rotavirus had relatively a little effects on cost-effectiveness results.52 The sensitivity ranges goes from 0.12 to 0.281 like earlier study to observe the possible effects of the universal rotavirus vaccination.
Rotavirus vaccine, cost and efficacy
Currently, there are 2 rotavirus vaccine RotaTeq® (RV5- 3 dose) and Rotarix® (RV1 dose) that have been licensed in many countries.1 These vaccines have an efficacy of 85–98%, which is already shown to reduced all-cause hospitalizations by 42 to 59%.67,68 In the international private market, the price for RotaTeq® (3 dose) and Rotarix® (2 dose) per course is approximately $226 and $213 respectively which is relatively higher that the $192 and $184 paid for the same vaccines by the Vaccines for Children Program.69,70 However, in GAVI-eligible countries the subsidized price ranges between US $0.30–$0.60, which is a major concern for policy-makers for those countries that “graduate” for GAVI-eligibility and have to purchase vaccine through some other mechanism. It was observed that both of the RV1 and RV5 have similar efficacy against severe rotavirus gastroenteritis in countries where a high diversity of strains co-circulate, suggesting an important role for heterotypic protective immunity.1 However, the protective effectiveness of these vaccines is not the same in all regions of the world as the effectiveness is high in developed countries but decreases substantially in low and middle income countries.71-74
The Expanded Program on Immunization (EPI) in Bangladesh is one of the successful programs in health sector which started with 6 conventional vaccines against 6 vaccine-preventable diseases and introduced latter measles and rubella (MR) vaccine, Measles Second Dose (MSD), Pneumococal Conjugated Vaccine (PCV) and IPV vaccine. Under the Comprehensive Multi-Year Plan of the National Immunisation Programme of Bangladesh 2011–2016, EPI had aimed for introduction of rotavirus vaccine by the end of 2014 but had not yet included rotavirus vaccination in the routine immunization program. The RV1 and RV5 were available in private market and people can purchase the vaccines from pharmacies as well as from private healthcare facilities. Recently, a new rotavirus vaccine named ROTAVAC has been developed in India based on the 116E rotavirus strain and manufactured by Bharat Biotech International Limited of India. A vaccination trial in India found that ROTAVAC vaccine (3 doses) had efficacy in the first year of life was 56.3% and in second year of life was 48.9% of the severe rotavirus gastroenteritis which is encouraging for resource poor setting like Bangladesh.16 ROTAVAC is currently licensed only in India and planned to make the vaccine available in the public market at a price of US$ 1 per dose.75 However, to introduce ROTAVAC in an immunization program, additionally a vaccine delivery related cost will be incurred from the implementer's perspective. Vaccine delivery costs are associated with vaccination campaign, cold chain and waste management, training and staffing as well as social mobilization for the particular vaccine. Vaccines and other logistics (including vaccine carrier and stationaries) need to transport from center's cold store to field site during the vaccination campaign which are key component of delivery costs. As per the earlier study, we assume that the cost of vaccine delivery process will be US$ 0.835 per dose per individual.76 In this analysis we estimated the cost-effectiveness using ROTAVAC vaccine with a protection up to 2 y. For simplicity of the model we ignored the indirect effect of rotavirus vaccination, although it was observed that pediatric rotavirus vaccination protects young and adults from rotavirus disease will, providing added value to the immunization program.77 In this sense our results understate the degree of benefit and the cost effectiveness of the vaccine. However, modeling studies of Pitzer and colleagues predicted that the rotavirus vaccine would not provide long-term indirect protection in the population as a whole, and also observed that displacement of mortality to older individuals is not significant.78
Coverage of the vaccine
The Expanded Program on Immunization (EPI) is the highest priority in Bangladesh and it is recommended that children complete the schedule of immunizations during their first year of life. The current coverage rate of all vaccines is quite impressive; currently the coverage of BCG, pentavalent vaccine (3-doses) and polio vaccine (3-doses) is above 91% according to the latest survey.79 To assess the efficacy of ROTAVAC vaccine a randomized placebo controlled trial study was conducted in India. The trial found that at least 96% of subjects received all 3 doses of the vaccine although it would be lower in real life universal vaccination program.16 However, the same vaccination coverage rate also observed in a pentavalent rotavirus vaccination trial in rural Bangladesh.74 Recently a large oral cholera vaccine trial was conducted in urban Bangladesh where the migration rate is higher than the other parts of the country and found that the 2 dose vaccine coverage was at least 65% of that area.80 In this analysis we assumed that the rotavirus vaccination will be similar to moderated coverage like the cholera vaccination coverage. However, higher migration implies greater transmission and thus possibly there might be a larger role played by herd immunity. In this model like earlier studies we used the vaccine coverage from 40% to 96% for uncertainty analysis (Table 2).
Costs of illness due to childhood rotavirus
Rotavirus infection is a significant cause of childhood hospitalization and approximately 65% hospitalized children were exposed to rotavirus.81 Recently, a study showed that, approximately 44% of the diarrheal patients received inpatients care and remaining patients used the out-patient services.82 In addition to substantial morbidity, there is growing evidence of the economic burden for households and for providers created by rotavirus. In Bangladesh, a recent study showed that the average cost of rotavirus illness was approximately US$ 84, including both direct and indirect costs.83 In public facilities the treatment costs were shared between public and households level while in private facilities, households bear all the treatment costs. A study conducted in a similar region showed the average inpatients and outpatients treatment cost of rotavirus illness was US$ 74.26 and US$ 3.88 respectively.18,49 However, it was also found that approximately 85% and 45% of the total cost of illness was incurred by the hospital for treating the rotavirus inpatients and outpatients respectively.84
Sensitivity analysis
We conducted one-way sensitivity analyses where we varied the value of each input according to other published and unpublished values to ascertain the impact of uncertainty in input values on the cost-effectiveness ratio. In scenario analyses, the cost-effectiveness ratios were estimated using the low or the high values of selected parameters, and compared with the base-case scenario i.e., no-vaccination strategy.
Disclosure of potential conflicts of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgments
ICDDR,B acknowledges with gratitude the commitment of University of Strathclyde PhD Scholarship to its research efforts. ICDDR,B is also grateful to the Governments of Bangladesh, Canada, Sweden and the UK for providing core/unrestricted support. The authors would also like to thank Itamar Megiddo, University of Strathclyde for his comments on an earlier draft of the manuscript.
References
- [1].World Health Organization Rotavirus vaccines WHO position paper - January 2013. Wkly Epidemiol Rec. 2013;88:49-64. Available from: http://www.who.int/wer/2013/wer8805.pdf. PMID:2342473023424730 [Google Scholar]
- [2].Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, Agocs M, Serhan F, De Oliveira L, Mwenda JM, Mihigo R, Ranjan Wijesinghe P, et al.. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis. 2016;62:S96-105. doi: 10.1093/cid/civ1013. PMID:27059362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lundgren O, Svensson L. Pathogenesis of Rotavirus diarrhea. Microbes Infect. 2001;3:1145-56. doi: 10.1016/S1286-4579(01)01475-7. PMID:11709295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bar-Zeev N, Kapanda L, Tate JE, Jere KC, Iturriza-Gomara M, Nakagomi O, Mwansambo C, Costello A, Parashar UD, Heyderman RS, et al.. Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: An observational and case-control study. Lancet Infect Dis. 2015;15:422-8. doi: 10.1016/S1473-3099(14)71060-6. PMID:25638521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Munos MK, Fischer Walker CL, Black RE. The effect of oral rehydration solution and recommended home fluids on diarrhoea mortality. Int J Epidemiol. 2010;39:75-87. doi: 10.1093/ije/dyq025. PMID:20348131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Diop A, Atherly D, Faye A, Lamine Sall F, Clark AD, Nadiel L, Yade B, Ndiaye M, Fafa Cissé M, Ba M. Estimated impact and cost-effectiveness of rotavirus vaccination in senegal: A country-led analysis. Vaccine. 2015;33:A119-25. Available from: https://doi.org/ 10.1016/j.vaccine.2014.12.065. doi: 10.1016/j.vaccine.2014.12.065. PMID:25919151 [DOI] [PubMed] [Google Scholar]
- [7].CNN What will make vaccines work better in developing countries? [Internet] Spec. to CNN Int. Ed. 2016; Available from: http://edition.cnn.com/2016/03/15/health/rotavirus-vaccine-bangladesh/. [Google Scholar]
- [8].Velazquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, Glass RI, Estes MK, Pickering KK, Ruiz-Palacios M. Rotavirus infection in infants as protection against subsequent infections. N Engl J Med. 1996;335:1022-8. Available from: http://www.nejm.org/doi/pdf/ 10.1056/NEJM199610033351404. doi: 10.1056/NEJM199610033351404. PMID:8793926 [DOI] [PubMed] [Google Scholar]
- [9].WHO Meeting of the immunization strategic advisory group of experts, April 2009 –conclusions and recommendations. Wkly Epidemiol Rec. 2009;84:220-36. PMID:19499606 [PubMed] [Google Scholar]
- [10].WHO Rotavirus vaccination. Wkly Epidemiol Rec. 2007. [cited 2017May26];84:232-6. Available from: http://www.who.int/immunization/stakeholders/mapping_vaccine_decision_making_networks.pdf. [Google Scholar]
- [11].Thiboonboon K, Santatiwongchai B, Chantarastapornchit V, Rattanavipapong W, Teerawattananon Y. A systematic review of economic evaluation methodologies between resource-limited and resource-rich countries: A case of rotavirus vaccines. Appl Health Econ Health Policy. 2016;14:1-14. doi: 10.1007/s40258-016-0265-y. PMID:26112982 [DOI] [PubMed] [Google Scholar]
- [12].ROTA Council National and regional rotavirus vaccine introductions [Internet] Rotavirus Organ. Tech. Allies. 2016. [cited 2016December1]. Available from: http://rotacouncil.org/toolkit/national-and-regional-rotavirus-introductions/. [Google Scholar]
- [13].Bar-Zeev N, Tate JE, Pecenka C, Chikafa J, Mvula H, Wachepa R, Mwansambo C, Mhango T, Chirwa G, Crampin AC, et al.. Cost-effectiveness of monovalent rotavirus vaccination of infants in Malawi: A postintroduction analysis using individual patient–level costing data. Clin Infect Dis. 2016;62:S220-8. doi: 10.1093/cid/civ1025. PMID:27059360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Postma MJ, Jit M, Rozenbaum MH, Standaert B, Tu H-A, Hutubessy RC. Comparative review of three cost-effectiveness models for rotavirus vaccines in national immunization programs; a generic approach applied to various regions in the world. BMC Med. 2011;9:84. Available from: http://www.biomedcentral.com/1741-7015/9/84. doi: 10.1186/1741-7015-9-84. PMID:21740545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fischer TK, Anh DD, Antil L, Cat NDL, Kilgore PE, Thiem VD, Rheingans R, Tho LH, Glass RI, Bresee JS. Health care costs of diarrheal disease and estimates of the cost-effectiveness of rotavirus vaccination in Vietnam. J Infect Dis. 2005;192:1720-6. doi: 10.1086/497339. PMID:16235169 [DOI] [PubMed] [Google Scholar]
- [16].Bhandari N, Rongsen-Chandola T, Bavdekar A, John J, Antony K, Taneja S, Goyal N, Kawade A, Kang G, Rathore SS, et al.. Efficacy of a monovalent human-bovine (116E) rotavirus vaccinein Indian children in the second year of life. Vaccine. 2014;32:A110-16. doi: 10.1016/j.vaccine.2014.04.079. PMID:25091663 [DOI] [PubMed] [Google Scholar]
- [17].Jit M, Brisson M. Modelling the epidemiology of infectious diseases for decision analysis: A primer. Pharmacoeconomics. 2011;29:371-86. doi: 10.2165/11539960-000000000-00000. PMID:21504239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Megiddo I, Colson AR, Nandi A, Chatterjee S, Prinja S, Khera A, Laxminarayan R. Analysis of the universal immunization programme and introduction of a rotavirus vaccine in India with IndiaSim. Vaccine. 2014;32:A151-61. Available from: https://doi.org/ 10.1016/j.vaccine.2014.04.080. doi: 10.1016/j.vaccine.2014.04.080. PMID:25091670 [DOI] [PubMed] [Google Scholar]
- [19].van Hoek AJ, Ngama M, Ismail A, Chuma J, Cheburet S, Mutonga D, Kamau T, Nokes DJ. A cost effectiveness and capacity analysis for the introduction of universal rotavirus vaccination in Kenya: Comparison between Rotarix and RotaTeq Vaccines. PLoS One. 2012;7:e47511. doi: 10.1371/journal.pone.0047511. PMID:23115650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ozawa S, Mirelman A, Stack ML, Walker DG, Levine OS. Cost-effectiveness and economic benefits of vaccines in low- and middle-income countries: A systematic review. Vaccine. 2012;31:96-108. Available from: https://doi.org/ 10.1016/j.vaccine.2012.10.103. doi: 10.1016/j.vaccine.2012.10.103. PMID:23142307 [DOI] [PubMed] [Google Scholar]
- [21].Isakbaeva ET, Musabaev E, Antil L, Rheingans R, Juraev R, Glass RI, Bresee JS. Rotavirus disease in Uzbekistan: Cost-effectiveness of a new vaccine. Vaccine. 2007;25:373-80. doi: 10.1016/j.vaccine.2006.07.029. PMID:16930784 [DOI] [PubMed] [Google Scholar]
- [22].Constenla DO, Linhares AC, Rheingans RD, Antil LR, Waldman EA, da Silva LJ. Economic impact of a rotavirus vaccine in Brazil. J Health Popul Nutr. 2008;26:388-96. PMID:19069617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lydon P, Gandhi G, Vandelaer J, Okwo-Bele JM. Health system cost of delivering routine vaccination in low- and lower-middle income countries: What is needed over the next decade? Bull World Health Organ. 2014;92:382-4. doi: 10.2471/BLT.13.130146. PMID:24839329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hutubessy R, Henao AM, Namgyal P, Moorthy V, Hombach J. Results from evaluations of models and cost-effectiveness tools to support introduction decisions for new vaccines need critical appraisal. BMC Med. 2011;9:55. Available from: http://bmcmedicine.biomedcentral.com/articles/10.1186/1741-7015-9-55. doi: 10.1186/1741-7015-9-55. PMID:21569407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jit M, Levin C, Brisson M, Levin A, Resch S, Berkhof J, Kim J, Hutubessy R. Economic analyses to support decisions about HPV vaccination in low- and middle-income countries: A consensus report and guide for analysts. BMC Med. 2013;11:23. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3582485&tool=pmcentrez&rendertype=abstract. doi: 10.1186/1741-7015-11-23. PMID:23363734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fox-Rushby JA. Disability adjusted life years (DAILY) for decision-making? An overview of the literature. London: (UK: ): Office of Health Economics; 2002. [Google Scholar]
- [27].Tan-Torres Edejer T, Baltussen R, Adam T, Hutubessy R, Acharya A, Evans DB, Murray CJL. WHO CEA guidelines. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- [28].Mara A, Morton A. Adjusting life for quality or disability: Stylistic difference or substantial dispute? Health Econ. 2009;18:1237-47. doi: 10.1002/hec.1424. PMID:19097040 [DOI] [PubMed] [Google Scholar]
- [29].Jauregui B, Janusz CB, Clark AD, Sinha A, Garcia AGF, Resch S, Toscano CM, Sanderson C, Andrus JK. ProVac global initiative: A vision shaped by ten years of supporting evidence-based policy decisions. Vaccine. 2015;33:A21-7. Available from: https://doi.org/ 10.1016/j.vaccine.2014.12.080. doi: 10.1016/j.vaccine.2014.12.080. PMID:25919164 [DOI] [PubMed] [Google Scholar]
- [30].Clark A, Jauregui B, Griffiths U, Janusz CB, Bolaños-Sierra B, Hajjeh R, Andrus JK, Sanderson C. TRIVAC decision-support model for evaluating the cost-effectiveness of Haemophilus influenzae type b, pneumococcal and rotavirus vaccination. Vaccine. 2013;31:15-7. doi: 10.1016/j.vaccine.2013.05.045. PMID:23777686 [DOI] [PubMed] [Google Scholar]
- [31].Glassman A, Cañón O, Silverman R. How to get cost-effectiveness analysis right? The case of vaccine economics in Latin America. Value Health. 2016;19:913-20. Available from: https://doi.org/ 10.1016/j.jval.2016.04.014. doi: 10.1016/j.jval.2016.04.014. PMID:27987640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hipgrave DB, Alderman KB, Anderson I, Soto EJ. Health sector priority setting at meso-level in lower and middle income countries: Lessons learned, available options and suggested steps. Soc Sci Med. 2014;102:190-200. Available from: https://doi.org/ 10.1016/j.socscimed.2013.11.056. doi: 10.1016/j.socscimed.2013.11.056. PMID:24565157 [DOI] [PubMed] [Google Scholar]
- [33].Brooks A, Cutts F, Justice J, Walt G. Policy study of factors influencing the adoption of new and underutilized vaccines in developing countries. Washingt USAID. 1999; Available from: http://pdf.usaid.gov/pdf_docs/PNACK648.pdf. [Google Scholar]
- [34].Bryson M, Duclos P, Jolly A, Bryson J. A systematic review of national immunization policy making processes. Vaccine. 2010;28:A6-12. Available from: https://doi.org/ 10.1016/j.vaccine.2010.02.026. doi: 10.1016/j.vaccine.2010.02.026. PMID:20413000 [DOI] [PubMed] [Google Scholar]
- [35].Haas M, Ashton T, Blum K, Christiansen T, Conis E, Crivelli L, Lim MK, Lisac M, MacAdam M, Schlette S. Drugs, sex, money and power: An HPV vaccine case study. Health Policy. 2009;92:288-95. Available from: https://doi.org/ 10.1016/j.healthpol.2009.05.002. doi: 10.1016/j.healthpol.2009.05.002. PMID:19505744 [DOI] [PubMed] [Google Scholar]
- [36].Lin V. Competing rationalities: Evidence-based health policy? In: Lin V, Gibson B, Evidence-based health policy: Problems and possibilities. Oxford: Oxford University Press; 2003. [Google Scholar]
- [37].Revill P, Walker S, Madan J, Ciaranello A, Mwase T, Gibb DM, Claxton K, Sculpher MJ. Using cost-effectiveness thresholds to determine value for money in low- and middle-income country healthcare systems: Are current international norms fit for purpose? University of York, York: Centre for Health Economics; 2004. [Google Scholar]
- [38].Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: Alternative approaches. Bull World Health Organ. 2015;93:118-24. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid =4339959&tool=pmcentrez&rendertype=abstract. doi: 10.2471/BLT.14.138206. PMID:25883405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jeuland M, Lucas M, Clemens J, Whittington D. A cost–benefit analysis of Cholera vaccination programs in Beira, Mozambique. World Bank Econ Rev. 2009;23:1-33. [Google Scholar]
- [40].Bärnighausen T, Bloom DE, Cafiero-Fonseca ET, O'Brien JC. Valuing vaccination. Proc Natl Acad Sci U S A. 2014;111:12313-9. Available from: http://www.scopus.com/inward/record.url?eid =2-s2.0-84906691708&partnerID=tZOtx3y1. doi: 10.1073/pnas.1400475111. PMID:25136129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bärnighausen T, Bloom DE, Canning D, Friedman A, Levine OS, O'Brien J, Privor-Dumm L, Walker D. Rethinking the benefits and costs of childhood vaccination: The example of the Haemophilus influenzae type b vaccine. Vaccine. 2011;29:2371-80. doi: 10.1016/j.vaccine.2010.11.090. PMID:21159324 [DOI] [PubMed] [Google Scholar]
- [42].Deogaonkar R, Hutubessy R, van der Putten I, Evers S, Jit M. Systematic review of studies evaluating the broader economic impact of vaccination in low and middle income countries. BMC Public Health. 2012;12:878. Available from: http://www.biomedcentral.com/1471-2458/12/878. doi: 10.1186/1471-2458-12-878. PMID:23072714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jauregui B, Sinha A, Clark AD, Bolanos BM, Resch S, Toscano CM, Matus CR, Andrus JK. Strengthening the technical capacity at country-level to make informed policy decisions on new vaccine introduction: Lessons learned by PAHO's ProVac Initiative. Vaccine. 2011;29:1099-106. Available from: https://doi.org/ 10.1016/j.vaccine.2010.11.075. doi: 10.1016/j.vaccine.2010.11.075. PMID:21144916 [DOI] [PubMed] [Google Scholar]
- [44].DeRoeck D, Clemens JD, Nyamete A, Mahoney RT. Policymakers' views regarding the introduction of new-generation vaccines against typhoid fever, shigellosis and cholera in Asia. Vaccine. 2005;23:2762-74. doi: 10.1016/j.vaccine.2004.11.044. PMID:15780724 [DOI] [PubMed] [Google Scholar]
- [45].Jit M, Yuzbashyan R, Sahakyan G, Avagyan T, Mosina L. The cost-effectiveness of rotavirus vaccination in Armenia. Vaccine. 2011;29:9104-11. Available from: https://doi.org/ 10.1016/j.vaccine.2011.08.127. doi: 10.1016/j.vaccine.2011.08.127. PMID:21945959 [DOI] [PubMed] [Google Scholar]
- [46].Smith ER, Rowlinson EE, Iniguez V, Etienne KA, Rivera R, Mamani N, Rheingans R, Patzi M, Halkyer P, JS L. Cost effectiveness of rotavirus vaccination in Bolivia from the state perspective. Vaccine. 2011;29:6704-11. doi: 10.1016/j.vaccine.2011.05.038. PMID:21624421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ortega O, El‐Sayed N, Sanders JW, Abd‐Rabou Z, Antil L, Bresee J, Mansour A, Adib I, Nahkla I, Riddle MS. Cost‐benefit analysis of a rotavirus immunization program in the Arab Republic of Egypt. J Infect Dis. 2009;200:S92-8. Available from: https://academic.oup.com/jid/article-lookup/doi/10.1086/605057. doi: 10.1086/605057. PMID:19817621 [DOI] [PubMed] [Google Scholar]
- [48].Abbott C, Tiede B, Armah G, Mahmoud A. Evaluation of cost-effectiveness of live oral pentavalent reassortant rotavirus vaccine introduction in Ghana. Vaccine. 2012;30:2582-7. Available from: https://doi.org/ 10.1016/j.vaccine.2012.01.076. doi: 10.1016/j.vaccine.2012.01.076. PMID:22321664 [DOI] [PubMed] [Google Scholar]
- [49].Tate JE, Chitambar S, Esposito DH, Sarkar R, Gladstone B, Ramani S, Raghava MV, Sowmyanarayanan TV, Gandhe S, Arora R, et al.. Disease and economic burden of rotavirus diarrhoea in India. Vaccine. 2009;27:F18-24. doi: 10.1016/j.vaccine.2009.08.098. PMID:19931713 [DOI] [PubMed] [Google Scholar]
- [50].Flem ET, Latipov R, Nurmatov ZS, Xue Y, Kasymbekova KT, Rheingans RD. Costs of diarrheal disease and the cost‐effectiveness of a rotavirus vaccination program in Kyrgyzstan. J Infect Dis. 2009;200:S195-202. Available from: https://academic.oup.com/jid/article-lookup/doi/10.1086/605040. doi: 10.1086/605040. PMID:19817600 [DOI] [PubMed] [Google Scholar]
- [51].Patel HD, Roberts ET, Constenla DO. Cost-effectiveness of a new rotavirus vaccination program in Pakistan: A decision tree model. Vaccine. 2013;31:6072-8. Available from: https://doi.org/ 10.1016/j.vaccine.2013.10.022. doi: 10.1016/j.vaccine.2013.10.022. PMID:24176497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Jeuland M, Cook J, Poulos C, Clemens J, Whittington D. Cost-effectiveness of new-generation oral cholera vaccines: A multisite analysis. Value Health. 2009;12:899-908. doi: 10.1111/j.1524-4733.2009.00562.x [DOI] [PubMed] [Google Scholar]
- [53].Ministry of Health and Family Welfare Government of the People's Republic of Bangladesh Comprehensive Multi-year Plan: 2011–2016 expanded programme on immunization (EPI) Bangladesh [Internet] Dhaka: (Bangladesh: ): Directorate General of Health Services of Bangladesh; 2010. Available from: http://www.nationalplanningcycles.org/sites/default/files/country_docs/Bangladesh/bangladesh_cmyp2011-2016.pdf. [Google Scholar]
- [54].Bangladesh Bureau of Statistics (BBS) Poulation projection of Bangladesh- dynamics and trends [Internet] Dhaka: (Bangladesh: ): Ministry of Planning, Government of The People's Republic Of Bangladesh; 2015. Available from: www.bbs.gov.bd. [Google Scholar]
- [55].Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost- effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- [56].Atherly D, Dreibelbis R, Parashar UD, Levin C, Wecker J, Rheingans RD. Rotavirus vaccination: Cost-effectiveness and impact on child mortality in developing countries. J Infect Dis. 2009;200(Suppl):S28-38. doi: 10.1086/605033. PMID:19817610 [DOI] [PubMed] [Google Scholar]
- [57].World Health Organization (WHO) Making choices in health: WHO guide to costeffectiveness analysis. Geneva: (Switzerland: ): World Health Organization; 2003. [Google Scholar]
- [58].Murray CJL, Lopez AD. The global burden of disease: A comprehensive assessment of mortality and disability from disease, injuries and risk factors in 1990 and projected to 2020 Cambridge: Harvard University Press; 1996. [Google Scholar]
- [59].McKenna MT, Michaud CM, Murray CJL, Marks JS. Assessing the burden of disease in the United States using disability-adjusted life years. Am J Prev Med. 2005;28:415-23. doi: 10.1016/j.amepre.2005.02.009. PMID:15894144 [DOI] [PubMed] [Google Scholar]
- [60].Lindstrand A, Bergstrom S, Rosling H, Rubenson B, Stenson B, Tylleskar T. Global health: An introductory textbook. Copenhagen: Narayana Press; 2008. [Google Scholar]
- [61].Cook J, Jeuland M, Whittington D, Poulos C, Clemense J, Sur D, Anh DD, Agtini M, Bhutta Z, DOMI Typhoid Economics Study Group . The cost-effectiveness of typhoid Vi vaccination programs: Calculations for four urban sites in four Asian countries. Vaccine. 2008;26:6305-16. doi: 10.1016/j.vaccine.2008.09.040. PMID:18835415 [DOI] [PubMed] [Google Scholar]
- [62].Chowdhury F, Khan IA, Patel S, Siddiq AU, Saha NC, Khan AI, Saha A, Cravioto A, Clemens J, Qadri F, et al.. Diarrheal illness and healthcare seeking behavior among a population at high risk for diarrhea in Dhaka, Bangladesh. PLoS One. 2015;10:1-14. doi: 10.1371/journal.pone.0130105. PMID:26121650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zaman K, Yunus M, Faruque ASG, El Arifeen S, Hossain I, Azim T, Rahman M, Podder G, Roy E, Luby S, et al.. Surveillance of rotavirus in a rural diarrhoea treatment centre in Bangladesh, 2000–2006. Vaccine. 2009;27:F31-4. doi: 10.1016/j.vaccine.2009.08.063. PMID:19931715 [DOI] [PubMed] [Google Scholar]
- [64].Tanka G, Faruque A, Luby S, Glass RI, Parashar UD. Deaths From rotavirus disease in Bangladeshi children: Estimates from hospital-based surveillance. Pediatr Infect Dis J. 2007;26:1014-8. doi: 10.1097/INF.0b013e318125721c. PMID:17984808 [DOI] [PubMed] [Google Scholar]
- [65].Sarker AR, Sultana M, Mahumud RA, Sheikh N, Van Der Meer R, Morton A. Prevalence and health care – seeking behavior for childhood diarrheal disease in Bangladesh. Glob Pediatr Health. 2016;3:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Morris SK, Awasthi S, Khera A, Bassani DG, Kang G, Parashar UD, Kumar R, Shet A, Glass RI, Jha P. Rotavirus mortality in India: Estimates based on a nationally representative survey of diarrhoeal deaths. Bull World Health Organ. 2012;90:720-7. doi: 10.2471/BLT.12.101873. PMID:23109739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Breuer T, Clemens SC, Cheuvart B, Ph D, Espinoza F, Gillard P, Innis BL, Cervantes Y, Linhares AC, López P, et al.. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11-22. doi: 10.1056/NEJMoa052434. PMID:16394298 [DOI] [PubMed] [Google Scholar]
- [68].Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al.. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23-33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16394299. doi: 10.1056/NEJMoa052664. PMID:16394299 [DOI] [PubMed] [Google Scholar]
- [69].CDC Vaccines for children program (VFC). http://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html.
- [70].Nelson EAS, De Quadros CA, Santosham M, Parashar UD, Steele D. Overcoming perceptions of financial barriers to rotavirus vaccine introduction in Asia. Hum Vaccines Immunother. 2013;9:2418-26. doi: 10.4161/hv.26107. PMID:23955246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Linhares AC, Velázquez FR, Pérez-Schael I, Sáez-Llorens X, Abate H, Espinoza F, López P, Macías-Parra M, Ortega-Barría E, Rivera-Medina DM, et al.. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: A randomised, double-blind, placebo-controlled phase III study. Lancet (London, England). 2008;371:1181-9. Available from: http://www.sciencedirect.com/science/article/pii/S0140673608605243. doi: 10.1016/S0140-6736(08)60524-3. PMID:18395579 [DOI] [PubMed] [Google Scholar]
- [72].Madhi S, Cunliffe N, Steele D, Witte D, Kirsten M, Cheuvart B, Han HH, Neuzil KM. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289-98. doi: 10.1056/NEJMoa0904797. PMID:20107214 [DOI] [PubMed] [Google Scholar]
- [73].Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, Meurice F, Han HH, Damaso S, Bouckenooghe A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: Randomised, double-blind controlled study. Lancet (London, England). 2007;370:1757-63. Available from: http://www.sciencedirect.com/science/article/pii/S0140673607617449. doi: 10.1016/S0140-6736(07)61744-9. PMID:18037080 [DOI] [PubMed] [Google Scholar]
- [74].Zaman K, Anh DD, Victor JC, Shin S, Yunus M, Dallas MJ, Podder G, Thiem VD, Mai LTP, Luby SP, et al.. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: A randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615-23. Available from: https://doi.org/ 10.1016/S0140-6736(10)60755-6. doi: 10.1016/S0140-6736(10)60755-6. PMID:20692031 [DOI] [PubMed] [Google Scholar]
- [75].Pareek S. India launches its first indigenous rotavirus vaccine. At $1, it is the cheapest in the world! [Internet]. The better India. 2015. [cited 2016November11]; Available from: http://www.thebetterindia.com/20337/india-launches-first-indigenous-and-the-cheapest-rotavirus-vaccine-1/.
- [76].Sarker AR, Islam Z, Khan IA, Saha A, Chowdhury F, Khan AI, Cravioto A, Clemens JD, Qadri F, Khan JAM. Estimating the cost of cholera-vaccine delivery from the societal point of view: A case of introduction of cholera vaccine in Bangladesh. Vaccine. 2015;33:4916-21. Available from: https://doi.org/ 10.1016/j.vaccine.2015.07.042. doi: 10.1016/j.vaccine.2015.07.042. PMID:26232545 [DOI] [PubMed] [Google Scholar]
- [77].Anderson EJ, Shippee DB, Weinrobe MH, Davila MD, Katz BZ, Reddy S, Cuyugan MGKP, Lee SY, Simons YM, Yogev R, et al.. Indirect protection of adults from rotavirus by pediatric rotavirus vaccination. Clin Infect Dis. 2013;56:755-60. doi: 10.1093/cid/cis1010. PMID:23349228 [DOI] [PubMed] [Google Scholar]
- [78].Pitzer VE, Atkins KE, de Blasio BF, van Effelterre T, Atchison CJ, Harris JP, Shim E, Galvani AP, Edmunds WJ, Viboud C, et al.. Direct and indirect effects of rotavirus vaccination: Comparing predictions from transmission dynamic models. PLoS One. 2012;7:e42320. doi: 10.1371/journal.pone.0042320. PMID:22912699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].National Institute of Population Research and Training (NIPORT), Mitra and Associates and II Bangladesh demographic and health survey 2014: Key Indicators. Dhaka, Bangladesh, and Rockville, Maryland, USA: NIPORT, Mitra and Associates, and ICE International; 2014. [Google Scholar]
- [80].Qadri F, Ali M, Chowdhury F, Khan A. Feasibility and eff ectiveness of oral cholera vaccine in an urban endemic setting in Bangladesh: A cluster randomised open-label trial. Lancet. 2015;386:1362-71. doi: 10.1016/S0140-6736(15)61140-0. PMID:26164097 [DOI] [PubMed] [Google Scholar]
- [81].Satter SM, Gastanaduy PA, Islam K, Rahman M, Rahman M, Luby SP, Heffelfinger JD, Parashar UD, Gurley ES. Hospital-based surveillance for rotavirus gastroenteritis among young children in Bangladesh: Defining the potential impact of a rotavirus vaccine program. Pediatr Infect Dis J. 2016;36(2):168-72. doi: 10.1097/INF.0000000000001381. PMID:27798545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Sarker AR, Sultana M, Mahumud RA, Van Der Meer R, Morton A. Economic costs of hospitalized diarrheal disease in Bangladesh: A societal perspective. Dhaka, Bangladesh: Icddrb; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Arifeen S, Zaman K, Santosham M, Saha S. Protect children from rotavirus illness and death [Internet]. Dly Star. 2016; Available from: http://www.thedailystar.net/health/protect-children-rotavirus-illness-and-death-1280035. [Google Scholar]
- [84].Ahmeti A, Preza I, Simaku A, Nelaj E, Clark AD, Felix Garcia AG, Lara C, Hoestlandt C, Blau J, Bino S. Cost-effectiveness of rotavirus vaccination in Albania. Vaccine. 2015;33:A201-8. Available from: https://doi.org/ 10.1016/j.vaccine.2014.12.075. doi: 10.1016/j.vaccine.2014.12.075. PMID:25919162 [DOI] [PubMed] [Google Scholar]
- [85].World Health Organization (WHO) Country specific life table- Bangladesh [Internet] Glob Heal Obs data Repos. 2016. [cited 2016November14]; Available from: http://apps.who.int/gho/data/?theme=main&vid=60120. [Google Scholar]
- [86].Ahmed S, Kabira RML, Rahman A, Hussain M, Phil M, Khatoon S. Severity of rotavirus diarrhea in children: One year experience in a children hospital of Bangladesh. Iran J Peidatr. 2009;19:108-16. [Google Scholar]
- [87].Cortese M, Immergluck L, Held M, Jain S, Chan T, Grizas A, Khizer S, Barrett C, Quaye O, Rustempasic J, et al.. Effectiveness of monovalent and pentavalent rotavirus vaccine. Pediatrics. 2013;132:25-33. Available from: http://www.cocukenfeksiyon.org/eng/sayilar/33/13-20.pdf%5Cnhttp://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE =reference&D =emed11&NEWS=N&AN=2013216342. doi: 10.1542/peds.2012-3804. PMID:23776114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Phua KB, Lim FS, Lau YL, Nelson EAS, Huang LM, Quak SH, Lee BW, van Doorn LJ, Teoh YL, Tang H, et al.. Rotavirus vaccine RIX4414 efficacy sustained during the third year of life: A randomized clinical trial in an Asian population. Vaccine. 2012;30:4552-7. Available from: https://doi.org/ 10.1016/j.vaccine.2012.03.030. doi: 10.1016/j.vaccine.2012.03.030. PMID:22497874 [DOI] [PubMed] [Google Scholar]
- [89].Naghavi M, Wang H, Lozano R, Davis A, Liang X, Zhou M, Vollset SE, Abbasoglu Ozgoren A, Abdalla S, Abd-Allah F, et al.. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet. 2015;385:117-71. Available from: https://doi.org/ 10.1016/S0140-6736(14)61682-2. doi: 10.1016/S0140-6736(14)61682-2. PMID:25530442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Independent Online Desk GDP growth 7.05% in current fiscal year [Internet] Indep. 2016; Available from: http://www.theindependentbd.com/post/39661. [Google Scholar]


