ABSTRACT
Background: In Quebec, Canada, a school-based HPV vaccination for girls has been offered since 2008. The vaccine used in the program targets HPV16/18, responsible for ∼70% of cervical cancers and HPV6/11, responsible for the majority of anogenital warts. The objective of this study was to assess the prevalence of HPV in vaccinated and unvaccinated women. Methods: Women aged 17–29 years were eligible to participate. Participants' age, vaccination status and diverse risk factors were assessed by a computer-assisted questionnaire. Biological specimens were obtained by self-sampling. HPV genotyping was performed by Linear Array. Results: A total of 2,118 women were recruited. 2,042 completed the questionnaire and 1,937 provided a vaginal sample. Vaccination coverage varied from 83.5% in women aged 17–19 to 19.1% in those aged 23–29. The overall prevalence of HPV in sexually active women was 39.4% (95%CI: 37.0-41.7) and 56.7% of infected women had multiple type infections. The prevalence of vaccine HPV types varied by age and vaccination status except for women aged 23–29 for whom similar results were observed. Vaccine HPV types were detected in 0.3%, 1.4% and 10.5% of vaccinated women aged 17–19, 20–23, and 23–29 (p<0.05), respectively. HPV16 or HPV18 were detected in 10 women having received at least one dose of vaccine. Nine of these women were already sexually active at the time of vaccination. Conclusion: Infections with HPV types included in the vaccine are rare in women aged less than 23 years and are virtually absent in those who received at least one dose of vaccine before sexual debut.
KEYWORDS: Human papillomavirus, HPV vaccine, HPV prevalence, self-sampling
Introduction
Human papillomavirus infections (HPV) are associated with the vast majority of cervical cancers1,2, 40% to 90% of other anogenital cancers, as well as a subset of head and neck cancers.3–7 Since 2006, prophylactic HPV vaccines targeting 2, 4 and more recently 9 HPV types, have been introduced in a number of countries, including Canada. The quadrivalent vaccine (4vHPV), used in the province of Quebec (population ≈8.2 M), targets 2 oncogenic types, HPV 16 and 18, responsible for about 70% of cervical cancers,8 the majority of HPV-positive cancers from other sites, as well as HPV 6 and HPV 11, responsible for 85–90% of anogenital warts.9,10 In clinical trials, HPV vaccines have shown high levels of efficacy against persistent infections and diseases caused by targeted HPV genotypes, particularly when given to HPV naive individuals, prior to sexual debut.11,12 Monitoring vaccine effectiveness in the real world is important to guide policy decisions. Because of the long delay between infection acquisition and the development of cancer, shorter term effectiveness indicators have to be considered. So far, a number of studies have reported post-licensure favourable impacts on outcomes such as genital HPV infections, anogenital warts and cervical cancer precursors.13 Moreover, within a few years after the implementation of vaccination programs, some studies have shown herd immunity effects.14–16
A publicly-funded school-based HPV vaccination has been offered in the province of Quebec since 2008. Grade 4 girls (9-10 years old) were eligible for routine vaccination. A catch-up vaccination was offered to grade 9 girls (14-15 years old) during 2008–2013 and to 15–17 years old girls during the 2008–2009 school year. Since the launch of the program, annual vaccination uptake in grade 4 and 9 varied from 74% to 81%, with some variation between regions (from 61% to 96% in 2014–2015).17 The 4vHPV vaccine was used from 2008 to 2015. Since 2016, the nonavalent vaccine (9vHPV), which contains the same 4 HPV types as the 4vHPV vaccine and 5 additional oncogenic types (31, 33, 45, 52, 58) is offered. The objective of this study was to assess the prevalence of genital HPV infections in vaccinated and unvaccinated young women 5–6 years after HPV vaccination implementation.
Results
Participants' characteristics and vaccination status
The initial study sample consisted of 2,118 women aged 17–29 years recruited during 184 recruitment sessions which took place between March 2013 and July 2014. The great majority of recruited women (2,042; 96.4%) completed the questionnaire. Participants' characteristics are shown in Table 1. Overall, 62.3% of the participants were vaccinated. The proportion of those vaccinated was 83.5%, 65.7% and 19.1% among those aged 17–19, 20–22 and 23–29 years, respectively. The proportion of those who were sexually active at the time of vaccination was 17.3%, 39.4% and 83.7% in 17–19, 20–22 and 23–29 years old, respectively.
Table 1.
Study participants characteristics.
| Participants |
||
|---|---|---|
| Caracteristic | N* | % |
| Recruitment site | ||
| College | 701 | 34.3 |
| University | 501 | 24.5 |
| Vocational school | 412 | 20.2 |
| Youth employment centre | 111 | 5.4 |
| Workers recruited through community pharmacies, hospitals, others | 317 | 15.5 |
| Region of residence | ||
| Montreal region | 600 | 29.4 |
| Capitale-Nationale (Québec) region | 129 | 6.3 |
| Other regions of Québec | 1296 | 63.5 |
| Outside province or unknown | 17 | 0.8 |
| Migrant status | ||
| Born in Canada, with both parents born in Canada | 1532 | 77.2 |
| Born in Canada, with 1 or 2 parents born outside Canada | 245 | 12.3 |
| Born outside Canada | 207 | 10.4 |
| Past sexual activity | ||
| 17–19 years old | 729 | 80.3 |
| 20–22 years old | 581 | 93.0 |
| 23–29 years old | 475 | 96.4 |
| Sexual orientation | ||
| Heterosexual | 1769 | 87.7 |
| Homosexual | 48 | 2.4 |
| Bisexual | 124 | 6.2 |
| Other/unknown | 76 | 3.7 |
The total number (N) varies depending on the availability of answers to a given question
HPV prevalence
A genital specimen was collected from 1,987 (93.8%) participants. After the exclusion of 50 β-globin-negative specimens (2.6% of samples), the analysed population consisted of 1,937 women, of whom 1,715 (88.5%) were sexually active.
The overall HPV prevalence irrespective of types was 35.7% (95% CI: 33.5-37.8) in the total study population and 39.4% (95% CI: 37.0-41.7) in sexually active participants. HPV prevalence in those who declared never had sex was 4%. The following analyses are restricted to sexually active women. In this group, 17% had a single type infection and 22.3% had multiple type infections (56.7% of those infected). The number of HPV types per infected woman varied from 1 to 11. The prevalence of HPV types by category is presented in Fig. 1 . Among oncogenic types, the most prevalent types were HPV 51, 52 and 59. Among the possibly oncogenic types, HPV 53 and 66 were the most common. Non-oncogenic types 89, 84, 62, 42 and 54 were the most frequent of that category.
Figure 1.
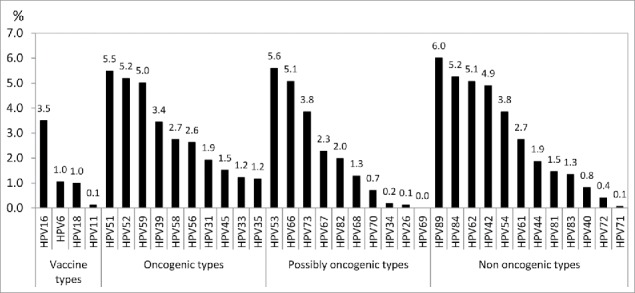
HPV prevalence in sexually active participants by virus type.
The overall HPV prevalence in women aged 17–19, 20–22, and 23–29 years was 31.7%, 42.1%, and 47.6% (p < 0.017 for each comparison). The same trend was observed for HPV 16 alone and four HPV vaccine types altogether (p < 0.01). Women aged 17–19 years had also a lower prevalence of HPV types 31, 33, 45 taken separately or as a group when compared with older age groups (Table 2 ).
Table 2.
HPV prevalence in sexually active participants by type and age group.
| 17–19 years (n = 691) |
20–22 years (n = 566) |
23–29 years (n = 458) |
All (n = 1715) |
|||||
|---|---|---|---|---|---|---|---|---|
| HPV type/category | n | % | n | % | n | % | N | % |
| HPV 16* | 3 | 0.4† | 20 | 3.5† | 37 | 8.1† | 60 | 3.5 |
| HPV 6/11/16/18* | 7 | 1.0† | 24 | 4.2† | 54 | 11.8† | 85 | 5.0 |
| HPV 31* | 8 | 1.2 | 9 | 1.6 | 16 | 3.5 | 33 | 1.9 |
| HPV 33* | 3 | 0.4† | 9 | 1.6 | 9 | 2.0 | 21 | 1.2 |
| HPV45* | 3 | 0.4† | 14 | 2.5 | 9 | 2.0 | 26 | 1.5 |
| HPV 31/33/45* | 13 | 1.9† | 27 | 4.8 | 32 | 7.0 | 72 | 4.2 |
| At least 1 oncogenic type* | 124 | 18.0† | 148 | 26.2 | 135 | 29.5 | 407 | 23.7 |
| At least 1 oncogenic type except 16/18 | 120 | 17.4 | 127 | 22.4 | 88 | 19.2 | 335 | 19.5 |
| At least 1 possibly oncogenic type | 108 | 15.6 | 94 | 16.6 | 74 | 16.2 | 276 | 16.1 |
| At least 1 non oncogenic type* | 128 | 18.5† | 151 | 26.7 | 132 | 28.8 | 411 | 24.0 |
| At least 1 non oncogenic type except 6/11* | 125 | 18.1† | 147 | 26.0 | 119 | 26.0 | 391 | 22.8 |
p < 0.05 for the global test
p < 0.01 for all group comparisons
HPV prevalence by vaccination status and number of lifetime sexual partners
In unvaccinated women, the prevalence of vaccine types shows little difference among age groups. In contrast, in vaccinated women, there is a significant gradual increase of vaccine types prevalence: 0.3%, 1.4% and 10.5% in those aged 17–19, 20–22 and 23–29 years, respectively. In the 23–29 age group, the prevalence of vaccine 4vHPV types was similar in vaccinated (10.5%) and unvaccinated women (11.9%). The prevalence of at least one oncogenic type except 16 and 18 types was not different among age groups (Table 3 ).
Table 3.
HPV prevalence in sexually active participants by vaccination status and age group.
| 17–19 years |
20–22 years |
23–29 years |
||||
|---|---|---|---|---|---|---|
| Vaccination status | Any HPV | Vaccine types | Any HPV | Vaccine types | Any HPV | Vaccine types |
| Unvaccinated n = 511 | 34.7 % | 8.2 % | 48.6 % | 9.9 % | 48.4 % | 11.9 % |
| Vaccinated* n = 1039 | 32.7 % | 0.3 % | 39.4 % | 1.4 % | 45.4 % | 10.5 % |
| Unknown n = 124 | 26.7 % | 2.2 % | 43.6 % | 10.3 % | 57.5 % | 15.0 % |
Global test of significance: p < 0.05
In vaccinated women aged 17–19 years, an increase in the prevalence of any HPV infection with the number of lifetime sexual partners was observed (from 6.4% with one partner to 75.5% with 7 or more partners). The vaccine type infections in this age group were almost absent (Fig. 2 ). The same pattern was observed for women aged 20–22 but not for women aged 23–29 years (data not shown). HPV 16 or 18 were detected in 10 women having received at least one dose of the vaccine. Nine of these women were already sexually active at the time of vaccination.
Figure 2.
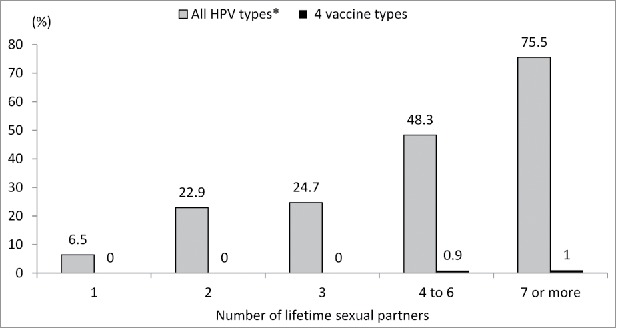
HPV prevalence in vaccinated 17–19 years old participants by the number of lifetime sexual partners.
Discussion
This is the first population-based study conducted in Québec to estimate the prevalence of HPV infection in young women shortly (5 to 6 years) after the implementation of HPV immunization with a relatively high coverage. Although baseline estimates of HPV infection prevalence prior to the immunization program that would have allowed to calculate a relative reduction of vaccine types between the two periods is lacking, results of the comparisons done by age group and vaccination status show that vaccine types are very rare in young women aged less than 23 and particularly in those aged 17–19 years old. The latter age group was eligible for school-based vaccination, reported the highest vaccine uptake, and the majority of these participants were vaccinated before sexual debut. It is also of mention that the prevalence of vaccine type infections in 17–19 years old participants does not seem to be impacted by the number of lifetime sexual partners, although the number of sexual partners is known to be the strongest determinant for HPV infection.18–23 The low prevalence of HPV 16 and HPV 18 is likely to translate into important cancer reduction. These two HPV genotypes, especially HPV 16, are known to have lower clearance rates and higher probability of progression toward pre-cancerous lesions and cancer.24 In Canada, a reduction in cervical precancerous lesions has already been reported in vaccinated populations in British Columbia.25
Another finding is that in all age groups, vaccine type infections occurred mainly in women who were already sexually active at the time of vaccination. This strengthens the recommendation of starting vaccination before sexual debut, when the risk of being infected is minimal. Our results are in line with studies undertaken in other settings that reported a decreased prevalence of HPV 16 and HPV 18 infections within a few years after the introduction of vaccination. The results of a meta-analysis showed that the prevalence of HPV 16 and HPV 18 is sensitive to vaccine coverage.13 These results have been reinforced in more recent clinic-based studies from Australia,14,15,26 Sweden.27 and the UK.28 as well as by population-based studies conducted in the USA.29 the UK and Scotland.16,30,31
The observed lower prevalence of HPV 31, 33 and 45 in 17–19 years old participants with high vaccination coverage when compared to older age groups with lower vaccination coverage could be explained by cross-protection, as reported by several previous clinical trials and surveillance studies.13,14,16,30,32 The 9vHPV vaccine has been licensed in Canada after the end of this study and its use cannot explain these findings.
Our study has some limitations. First, although efforts were applied to recruit a large number of subjects from several settings, the study sample is not a randomly selected one. Young adults are highly mobile, often without residential phone lines and there is not a unique database to determine a denominator by category of occupation. Without such information, weighing was not possible. As a result, extrapolating these results to the general population should be done cautiously. However, the almost 200 recruitment sessions in diverse institutions and regions, should have minimized the potential selection bias.
Second, because the questionnaire and the biologic specimens were anonymous, vaccination status was self-declared and could not be validated against medical records or vaccine registry. To minimize the risk of information bias, questions were added concerning the number of doses received and the year of vaccination. This information helped us to better categorized study participants by vaccination status.
Third, most of the study participants were vaccinated during a catch-up vaccination and were aged 14 to 18 years at the time of vaccination. Most routine vaccination programs are targeting 9–14 years old, an age group that is less exposed to HPV, and in which one can expect a greater impact of vaccination on the prevalence of HPV infection. The short period between vaccination program implementation and this study conduction preclude us from enrolling girls which were vaccinated in the routine vaccination program which covers Grade 4 students (9-10 years old) in Quebec.
Fourth, although the HPV self-collected sampling method has been previously validated and has been shown to be as sensitive as samples collected by a physician.33,34 the presence of ß-globin DNA sequence may not completely ascertain the source of the sample. However, the study protocol allowed informing the participants regarding Chlamydia trachomatis and Neisseria gonorrhea test-results. This is expected to be a strong incentive to participate in the study and to provide adequate specimens. Thus, we think the probability of having an inadequate source of sampling is low.
And finally, we cannot exclude some differences in the prevalence of HPV infection among the 7 birth cohorts included in the 23–29 year-old group. We were unable to split this age group because of the limited number of vaccinated participants. Such a split would make inconclusive the comparison of the HPV prevalence in vaccinated versus unvaccinated participants.
The strengths of the study are the high number of participants, which diminishes the risk of information bias, the face to face approach on site that allowed a high rate for obtaining biologic specimens (> 90%) among the respondents to the questionnaire, and the use of validated laboratory procedures for HPV genotyping.
Conclusion
This study adds to the growing evidence that HPV immunization is effective in reducing vaccine-type infections within a few years after the implementation of vaccination. In Quebec, HPV infections covered by the 4vHPV vaccine are very rare in vaccinated women aged less than 23 years and virtually absent in those having received at least one dose of vaccine before sexual debut. These findings reinforce the importance of vaccinating before sexual debut.
Material and methods
This study was nested in a larger study on sexual health in young adults aged 17–29 years. Participants' age, vaccination status and diverse risk factors were assessed by a computer-assisted questionnaire. Biological specimens were obtained by self-sampling and were tested for the presence of Chlamydia trachomatis, Neisseria gonorrhoea and HPV genital and oral infections. A complete study methodological report was published elsewhere.35 Here, we summarize the relevant elements regarding the prevalence of HPV genital infections in women.
Study sample
The study sample size calculation was based on HPV prevalence reported in previous studies.36,37 A sample size of 2,000 17–29 years old women was estimated to be sufficient to assess the overall prevalence of HPV infection as well as specific rates of 4vHPV vaccine types. Participants were recruited mainly from post-secondary educational institutions (college, university, and vocational schools) located in 9 different regions representing close to 85% of the Quebec population. A multistage sampling design was used to take into account age, region of residence and type of institution for students. It was complemented by a convenience sample of young workers according to their estimated weight in this age group.
Data collection
The questionnaire was administered on site and documented socio-demographic characteristics, sexual behaviour and health seeking experience, including questions about HPV vaccination (ever had, year of vaccination and number of doses received). The 4vHPV vaccine was used before and during the recruitment period. Sexually active was defined as ever had oral, vaginal or anal sex in their lifetime. Women were then instructed to provide a self-collected vaginal specimen using a dry Dacron swab, whether they were sexually active or not. Women who were pregnant or thought they could be pregnant were exempt. A written informed consent was obtained from all participants. All data were collected anonymously with codes that allowed matching the laboratory result with the questionnaire. HPV results were not disclosed to participants. This a priori decision was based on the fact that there is no treatment recommended for these infections and that the great majority of infections will disappear spontaneously. On the internet site of the study (www.portrait-pixel.ca), information about cervical cancer screening and HPV vaccination was offered as ways to prevent the consequences of HPV infections. A small incentive was offered to participants (20$CA for university students and workers or participation in the drawing of 100$CA gift cards for other students).
Laboratory analyses
Lyzed cervicovaginal specimens were first tested for the presence of HPV DNA using a generic HPV assay as described elsewhere.38 HPV-negative lysates were analyzed for the presence of ß-globin DNA sequence with PCR and gel electrophoresis to ensure they were adequate for PCR analysis.39 ß-globin-negative samples were further processed with Master Pure as reported previously.37 Purified DNA was then tested for ß-globin and, if ß-globin-positive, was tested with a generic HPV assay.
Samples that were ß-globin-positive and HPV-negative were considered negative for HPV. ß-globin-negative samples after Master Pure were considered inadequate for PCR analysis. Genotyping was performed on samples positive for HPV with the generic HPV assay.38 using the Linear Array HPV genotyping assay (LA-HPV) (Roche Molecular Systems) for 36 mucosal HPV genotypes.40 Samples reactive in the Linear Array with the cross-reactive probe for HPV 52 were further tested with a validated HPV52-specific real-time PCR assay.41
For HPV sub-groups analyses, categories were based on the International Agency for Research on Cancer (IARC) classification.42 oncogenic (HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59), probably oncogenic (HPV 68) and possibly oncogenic (26, 30, 34, 53, 66, 67, 68, 69, 70, 73, 82, 85, 97) together, and non-oncogenic (all others). Vaccine types refer to HPV 6, 11, 16 and 18.
Statistical analysis
Statistical analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC). Univariate analyses were based on Chi-2 test or Fischer exact test as appropriate. Bonferroni correction was applied in case of multiple comparisons. Overall prevalence rates of HPV infection are presented with their 95% confidence intervals (CI). Trend analyses were based on Cochran-Armitage test. Statistical significance was based on p<0.05 (2 sided).
Disclosure of potential conflicts of interests
F. Coutlée has received grants through his institution from Merck Sharp and Dome and Roche, as well as honoraria from Merck and Roche for lectures on human papillomavirus (HPV).
PG, CS, VG, FD, GL and SMC declare no conflicts of interest.
Ethics
Ethics approval for this study was received from the research ethics boards of the Agence de la Santé et des Services sociaux de Montréal and the Centre hospitalier de l'Université de Montréal.
Funding
This study was funded by the Ministère de la Santé et des Services Sociaux du Québec.
Acknowledgments
The authors thank Anne-Marie Bérard, who coordinated the data collection. Sampling kits were provided free of charge by BD Diagnostics. Neither the ministry nor the company had any involvement in study design, data collection, results analysis or interpretation.
References
- 1.Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–65. doi: 10.1136/jcp.55.4.244. PMID:11919208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F. PMID:10451482 [DOI] [PubMed] [Google Scholar]
- 3.Thibaudeau E, Fortin B, Coutlee F, Nguyen-Tan P, Weng X, Audet ML, Abboud O, Guertin L, Christopoulos A, Tabet J, et al.. HPV Prevalence and Prognostic Value in a Prospective Cohort of 255 Patients with Locally Advanced HNSCC: A Single-Centre Experience. Int J Otolaryngol. 2013;2013:437815. doi: 10.1155/2013/437815. PMID:23710185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, Steinau M, Watson M, Wilkinson EJ, Hopenhayn C, et al.. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst. 2015;107:djv086. doi: 10.1093/jnci/djv086. PMID:25925419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolesc Health Off Publ Soc Adolesc Med. 2010;46:S20–6. doi: 10.1016/j.jadohealth.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Tota JE, Chevarie-Davis M, Richardson LA, Devries M, Franco EL. Epidemiology and burden of HPV infection and related diseases: implications for prevention strategies. Prev Med. 2011;53(Suppl 1):S12–21. doi: 10.1016/j.ypmed.2011.08.017. PMID:21962466 [DOI] [PubMed] [Google Scholar]
- 7.Giuliano AR, Tortolero-Luna G, Ferrer E, Burchell AN, de Sanjose S, Kjaer SK, Munoz N, Schiffman M, Bosch FX. Epidemiology of human papillomavirus infection in men, cancers other than cervical and benign conditions. Vaccine. 2008;26(Suppl 10):K17–28. doi: 10.1016/j.vaccine.2008.06.021. PMID:18847554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coutlee F, Ratnam S, Ramanakumar AV, Insinga RR, Bentley J, Escott N, Ghatage P, Koushik A, Ferenczy A, Franco EL. Distribution of human papillomavirus genotypes in cervical intraepithelial neoplasia and invasive cervical cancer in Canada. J Med Virol. 2011;83:1034–41. doi: 10.1002/jmv.22081. PMID:21503917 [DOI] [PubMed] [Google Scholar]
- 9.Aubin F, Pretet JL, Jacquard AC, Saunier M, Carcopino X, Jaroud F, Pradat P, Soubeyrand B, Leocmach Y, Mougin C, et al.. Human papillomavirus genotype distribution in external acuminata condylomata: a Large French National Study (EDiTH IV). Clin Infect Dis. 2008;47:610–5. doi: 10.1086/590560. PMID:18637758 [DOI] [PubMed] [Google Scholar]
- 10.Garland SM, Steben M, Sings HL, James M, Lu S, Railkar R, Barr E, Haupt RM, Joura EA. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199:805–14. doi: 10.1086/597071. PMID:19199546 [DOI] [PubMed] [Google Scholar]
- 11.Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(Suppl 5):F123–38. doi: 10.1016/j.vaccine.2012.04.108. PMID:23199956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu B, Kumar A, Castellsague X, Giuliano AR. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review & meta-analysis. BMC Infect Dis. 2011;11:13. doi: 10.1186/1471-2334-11-13. PMID:21226933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drolet M, Benard E, Boily MC, Ali H, Baandrup L, Bauer H, Beddows S, Brisson J, Brotherton JM, Cummings T, et al.. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2015;15:565–80. doi: 10.1016/S1473-3099(14)71073-4. PMID:25744474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Liu B, Bateson D, McNamee K, Garefalakis M, Phillips S, Cummins E, et al.. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect Dis. 2014;14:958–66. doi: 10.1016/S1473-3099(14)70841-2. PMID:25107680 [DOI] [PubMed] [Google Scholar]
- 15.Chow EP, Danielewski JA, Fehler G, Tabrizi SN, Law MG, Bradshaw CS, Garland SM, Chen MY, Fairley CK. Human papillomavirus in young women with Chlamydia trachomatis infection 7 years after the Australian human papillomavirus vaccination programme: a cross-sectional study. Lancet Infect Dis. 2015;15:1314–23. doi: 10.1016/S1473-3099(15)00055-9. PMID:26201300 [DOI] [PubMed] [Google Scholar]
- 16.Cameron RL, Kavanagh K, Pan J, Love J, Cuschieri K, Robertson C, Ahmed S, Palmer T, Pollock KG. Human Papillomavirus Prevalence and Herd Immunity after Introduction of Vaccination Program, Scotland, 2009–2013. Emerg Infect Dis. 2016;22:56–64. doi: 10.3201/eid2201.150736. PMID:26692336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markowski F, Toth E, Landry M, Goggin P, Sauvageau C, Auger D, Mercier M. Vaccination contre les VPH. Flash Vigie. 2015;10:1. [Google Scholar]
- 18.Manhart LE, Holmes KK, Koutsky LA, Wood TR, Kenney DL, Feng Q, Kiviat NB. Human papillomavirus infection among sexually active young women in the United States: Implications for developing a vaccination strategy. Sex Transm Dis. 2006;33:502–8. doi: 10.1097/01.olq.0000204545.89516.0a. PMID:16572039 [DOI] [PubMed] [Google Scholar]
- 19.Hariri S, Unger ER, Sternberg M, Dunne EF, Swan D, Patel S, Markowitz LE. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003–2006. J Infect Dis. 2011;204:566–73. doi: 10.1093/infdis/jir341. PMID:21791659 [DOI] [PubMed] [Google Scholar]
- 20.Lenselink CH, Melchers WJ, Quint WG, Hoebers AM, Hendriks JC, Massuger LF, Bekkers RL. Sexual behaviour and HPV infections in 18 to 29 year old women in the pre-vaccine era in the Netherlands. PloS One. 2008;3:e3743. doi: 10.1371/journal.pone.0003743. PMID:19011683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlsson R, Jonsson M, Edlund K, Evander M, Gustavsson A, Boden E, Rylander E, Wadell G. Lifetime number of partners as the only independent risk factor for human papillomavirus infection: a population-based study. Sex Transm Dis. 1995;22:119–27. doi: 10.1097/00007435-199503000-00008. PMID:7624813 [DOI] [PubMed] [Google Scholar]
- 22.Roteli-Martins CM, de Carvalho NS, Naud P, Teixeira J, Borba P, Derchain S, Tyring S, Gall S, Diaz A, Blatter M, et al.. Prevalence of human papillomavirus infection and associated risk factors in young women in Brazil, Canada, and the United States: a multicenter cross-sectional study. Int J Gynecol Pathol. 2011;30:173–84. doi: 10.1097/PGP.0b013e3181f38dfe. PMID:21293281 [DOI] [PubMed] [Google Scholar]
- 23.Giambi C, Donati S, Carozzi F, Salmaso S, Declich S, Atti ML, Ronco G, Alibrandi MP, Brezzi S, Collina N, et al.. A cross-sectional study to estimate high-risk human papillomavirus prevalence and type distribution in Italian women aged 18–26 years. BMC Infect Dis. 2013;13:74. doi: 10.1186/1471-2334-13-74. PMID:23390953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaisamrarn U, Castellsague X, Garland SM, Naud P, Palmroth J, Del Rosario-Raymundo MR, Wheeler CM, Salmeron J, Chow SN, Apter D, et al.. Natural history of progression of HPV infection to cervical lesion or clearance: analysis of the control arm of the large, randomised PATRICIA study. PloS One. 2013;8:e79260. doi: 10.1371/journal.pone.0079260. PMID:24260180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogilvie GS, Naus M, Money DM, Dobson SR, Miller D, Krajden M, van Niekerk DJ, Coldman AJ. Reduction in cervical intraepithelial neoplasia in young women in British Columbia after introduction of the HPV vaccine: An ecological analysis. Int J Cancer. 2015;137:1931–7. doi: 10.1002/ijc.29508. PMID:25754686 [DOI] [PubMed] [Google Scholar]
- 26.Osborne SL, Tabrizi SN, Brotherton JM, Cornall AM, Wark JD, Wrede CD, Jayasinghe Y, Gertig DM, Pitts MK, Garland SM. Assessing genital human papillomavirus genoprevalence in young Australian women following the introduction of a national vaccination program. Vaccine. 2015;33:201–8. doi: 10.1016/j.vaccine.2014.10.045. PMID:25444787 [DOI] [PubMed] [Google Scholar]
- 27.Soderlund-Strand A, Uhnoo I, Dillner J. Change in population prevalences of human papillomavirus after initiation of vaccination: the high-throughput HPV monitoring study. Cancer Epidemiol Biomark Prev. 2014;23:2757–64. doi: 10.1158/1055-9965.EPI-14-0687. [DOI] [PubMed] [Google Scholar]
- 28.Mesher D, Panwar K, Thomas SL, Beddows S, Soldan K. Continuing reductions in HPV 16/18 in a population with high coverage of bivalent HPV vaccination in England: an ongoing cross-sectional study. BMJ Open. 2016;6:e009915. doi: 10.1136/bmjopen-2015-009915. PMID:26868944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markowitz LE, Liu G, Hariri S, Steinau M, Dunne EF, Unger ER. Prevalence of HPV After Introduction of the Vaccination Program in the United States. Pediatrics. 2016;137:1–9. doi: 10.1542/peds.2015-1968. [DOI] [PubMed] [Google Scholar]
- 30.Cuschieri K, Kavanagh K, Moore C, Bhatia R, Love J, Pollock KG. Impact of partial bivalent HPV vaccination on vaccine-type infection: a population-based analysis. Br J Cancer. 2016;114:1261–4. doi: 10.1038/bjc.2016.97. PMID:27115467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhatia R, Kavanagh K, Cubie HA, Serrano I, Wennington H, Hopkins M, Pan J, Pollock KG, Palmer TJ, Cuschieri K. Use of HPV testing for cervical screening in vaccinated women-Insights from the SHEVa (Scottish HPV Prevalence in Vaccinated Women) study. Int J Cancer. 2016;138:2922–31. doi: 10.1002/ijc.30030. PMID:26845632 [DOI] [PubMed] [Google Scholar]
- 32.Malagon T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, Brisson M. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:781–9. doi: 10.1016/S1473-3099(12)70187-1. PMID:22920953 [DOI] [PubMed] [Google Scholar]
- 33.Petignat P, Faltin DL, Bruchim I, Tramèr MR, Franco EL, Coutlée F. Are self-collected samples comparable to physician-collected cervical specimens for human papillomavirus DNA testing? A systematic review and meta-analysis. Gynecol Oncol. 2007;105:530–5. doi: 10.1016/j.ygyno.2007.01.023. PMID:17335880 [DOI] [PubMed] [Google Scholar]
- 34.Ogilvie GS, Patrick DM, Schulzer M, Sellors JW, Petric M, Chambers K, White R, FitzGerald JM. Diagnostic accuracy of self collected vaginal specimens for human papillomavirus compared to clinician collected human papillomavirus specimens: a meta-analysis. Sex Transm Infect. 2005;81:207–12. doi: 10.1136/sti.2004.011858. PMID:15923286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambert G, Mathieu-Chartier S, Goggin P, Maurais E, Coutlée F, Bérard A-M. Étude PIXEL – Portrait de la santé sexuelle des jeunes adultes au Québec, 2013–2014 – Rapport méthodologique [On line]: https://www.inspq.qc.ca/pdf/publications/2138_etude_pixel_rapport_methodologique.pdf (Page accessed June22, 2016).
- 36.Richardson H, Kelsall G, Tellier P, Voyer H, Abrahamowicz M, Ferenczy A, Coutlee F, Franco EL. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomark Prev. 2003;12:485–90. [PubMed] [Google Scholar]
- 37.Burchell AN, Tellier PP, Hanley J, Coutlee F, Franco EL. Human papillomavirus infections among couples in new sexual relationships. Epidemiology. 2010;21:31–7. doi: 10.1097/EDE.0b013e3181c1e70b. PMID:19907332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Legault V, Burchell A, Goggin P, Nicolau B, Brassard P, Guenoun J, Forest P, Mayrand MH, Franco EL, Coutlee F. Generic microtiter plate assay for triaging clinical specimens prior to genotyping of human papillomavirus DNA via consensus PCR. J Clin Microbiol. 2011;49:3977–9. doi: 10.1128/JCM.05646-11. PMID:21940471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aho J, Kornegay JR, Healey S, Roger M, Dion F, Gaudreault D, Shepard AP, Franco EL, Coutlee F. Evaluation of a convenient enzyme immunoassay to assess the quality of genital specimens submitted for the detection of human papillomavirus DNA by consensus PCR. J Clin Virol. 2004;29:127–33. doi: 10.1016/S1386-6532(03)00114-8. PMID:14747032 [DOI] [PubMed] [Google Scholar]
- 40.Coutlee F, Rouleau D, Petignat P, Ghattas G, Kornegay JR, Schlag P, Boyle S, Hankins C, Vezina S, Cote P, et al.. Enhanced detection and typing of human papillomavirus (HPV) DNA in anogenital samples with PGMY primers and the Linear array HPV genotyping test. J Clin Microbiol. 2006;44:1998–2006. doi: 10.1128/JCM.00104-06. PMID:16757590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coutlee F, Rouleau D, Ghattas G, Hankins C, Vezina S, Cote P, Macleod J, de Pokomandy A, Money D, Walmsley S, et al.. Confirmatory real-time PCR assay for human papillomavirus (HPV) type 52 infection in anogenital specimens screened for HPV infection with the linear array HPV genotyping test. J Clin Microbiol. 2007;45:3821–3. doi: 10.1128/JCM.01145-07. PMID:17898159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, et al.. A review of human carcinogens–Part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/S1470-2045(09)70096-8. PMID:19350698 [DOI] [PubMed] [Google Scholar]


