ABSTRACT
Cathelicidin has been reported to be multifunctional. The current study aimed to investigate the influences of exogenous cathelicidin-related antimicrobial peptide (CRAMP) on inflammatory responses in different disease models. In OVA-induced allergic airway inflammation, CRAMP significantly enhanced the infiltration of inflammatory cells and accumulation of proinflammatory Th2 cytokine IL-13 and IL-33 in bronchial alveolar lavage fluid (BALF), exacerbated lung tissue inflammation and airway goblet cell hyperplasia, and elevated OVA-specific IgE level in serum. In oxazolone-induced intestinal colitis, the expression levels of CRAMP and its receptor FPR2 significantly increased in comparison with those of TNBS-induced mice, vesicle and normal controls. Exogenous CRAMP significantly prevented the development of ulcerative colitis, evidenced by improved body weight regain, decreased colons weight/length ratio, elevated epithelial integrity, and ameliorated colon tissue inflammation. In addition, pro-inflammatory cytokines TNF-α, IL-1β, IL-4 and IL-13, as well as chemokines CXCL2 and CXCL5 for neutrophils recruitment were significantly decreased in CRAMP-treated mice, and epithelial repair-related factors MUC2 and Claudin1 were increased, determined by real time-PCR and ELISAs. The results indicated that although CRAMP has pro-inflammatory effects in airway, local application of exogenous CRAMP might be a potential approach for the treatment of ulcerative colitis.
KEYWORDS: allergic airway inflammation, CRAMP, colitis, oxazolone, mouse model
Introduction
Cathelicidin is a class of host defense peptides. LL-37 is the only cathelicidin identified in human to date, and CRAMP (cathelin-related antimicrobial peptide) is its orthologue found in mouse.1,2 Cathelicidin has broad antimicrobial spectrum, and early studies focused on their protective effects against bacteria, fungi, viruses and parasites. In recent years, more studies were interested in their effects of immune regulation. Cathelicidin enhances dendritic cell maturation and its recruitment through the formylpeptide receptor 2 (FPR2),3,4 facilitates macrophage activation and differentiation toward proinflammatory form,5,6 intervenes dendritic cell-induced T cell polarization,7 and modulates neutrophil effector functions.8 It has been reported that cathelicidin modulated autoimmune and inflammatory diseases,9 participated in wound healing,10 and had critical roles in the growth of malignant tumor.11 Furthermore, cathelicidin was found to show varied roles under specific pathological conditions. In contrast to its proinflammatory effects, it is considered as an anti-inflammatory role when it downregulates TLR4 signaling via binding LPS.12 In addition, while it suppresses colon cancer and prostate cancer, cathelicidin is required for lung tumor growth.13-15
It was reported that LL-37 level was dysregulated in asthmatic patients.16 However, the role of LL-37 in asthma is largely unknown, except that there are indirect evidences showing that LL-37 may be involved into the pathogenesis of asthma. LL-37 has chemotactic effects on effector cells in asthma including eosinophils,17 neutrophils,18 T cells,18 monocyte-derived dendritic cells,4 and mast cells via FPR2.19 And, LL-37 triggers release of histamine and PGD2 from mast cells,19 induces MUC5AC mucin (a major mucin component of mucus) production by airway epithelial cells via TACE-TGF-α-EGFR pathway,20 activates neutrophils, and promotes release of LTB421 which is a potent chemoattractant for many effector cells in the development of asthma.22 In addition, a direct evidence was presented by Sun and colleagues’ study.23 They demonstrated that LL-37 was an eosinophil activator and triggered intracellular trafficking and activation of cys-LT-synthesizing enzymes and subsequent release of proinflammatory mediators of importance in asthma. Further, by comparing samples from asthmatic patients and healthy controls, they showed that eosinophils isolated from asthmatics expressed and released significantly higher levels of LL-37, suggesting that the peptide and its signaling pathway represent potential therapeutic targets for this disease
It has also been reported that cathelicidin mediated immune responses in intestinal colitis.24 The expression of LL-37 was elevated in colon mucosa from ulcerative colitis (UC) patients but not in those from Crohn's disease (CD) patients25; and, LL-37 can directly stimulate mucus synthesis through activation of MUC1 and MUC2 expression and MAP kinase pathway in human colonic cells,26 indicating the possible relevance of CRAMP in the pathogenesis of ulcerative colitis. Intrarectal administration of CRAMP ameliorated DSS-induced colitis, significantly reduced the increased number of fecal microflora, reversed the decline of colonic mucus thickness through upregulation of the expression of mucin genes, and prevented colitis development by suppressing the induction of apoptosis by DSS.27 Similar results were obtained by the administration with CRAMP-transformed Lactococcus lactis in murine DSS-induced UC.28 And more, intrarectal administration with cathelicidin-BF, a cathelicidin peptide found in reptiles, improved the morphology of the colon epithelium, significantly attenuated apoptosis and infiltration of inflammatory cells in colonic epithelium, effectively inhibited phosphorylation of NF-κB (p65), and indicating cathelicidin-BF effectively attenuated inflammation and improved disrupted barrier function in DSS-induced colitis.29 On the other hand, cathelicidin-knockout mice had more severe symptoms and mucosal disruption than the wild-type mice in response to DSS challenge, including increased colonic levels of IL-1β and TNF-α, myeloperoxidase activity and the number of apoptotic cells, as well as impaired mucus secretion and mucin gene expression, all of which were reversed by the intrarectal administration of CRAMP or CRAMP-encoding plasmid.27,30 Interestingly, in TNBS-exposed mice, intrarectal CRAMP administration or intravenous delivery of lentivirus-overexpressing CRAMP gene significantly reduced colonic col1α mRNA expression; and, LL-37 reduced TGF-β1 and/or IGF-1 induced collagen protein and mRNA expression in human primary intestinal fibroblasts and human colonic CCD-18Co fibroblasts, indicating that LL-37 can reverse intestinal fibrosis by directly inhibiting collagen synthesis in colonic fibroblasts.31 Increased expression of cathelicidin in monocytes and experimental models of colitis involves activation of TLR9-ERK signaling by bacterial DNA.32 Recently, Yi et al. reported Cathelicidin-WA, which is derived from the endemic genera Bungarus fascia, can improves intestinal epithelial barrier function in enterohemorrhagic Escherichia coli- induced colitis.33 The results indicated that CRAMP might be a potential new drug for the clinical treatment of intestinal colitis. However, considering CRAMP has potent pro-inflammatory effects, its potential application need to be carefully assessed under different disease conditions and mechanisms.
In the current study, mouse models of ovalbumin (OVA)-induced allergic airway inflammation and hapten oxazolone-induced murine colitis in which the immuno-pathogensis is dominated by Th2 responses and resembles clinical UC, were employed to investigate the possible effects of exogenous CRAMP on the diseases process.
Results
Exogenous CRAMP promotes the recruitment of monocytes and neutrophils and the expression of pro-inflammatory cytokines in airway
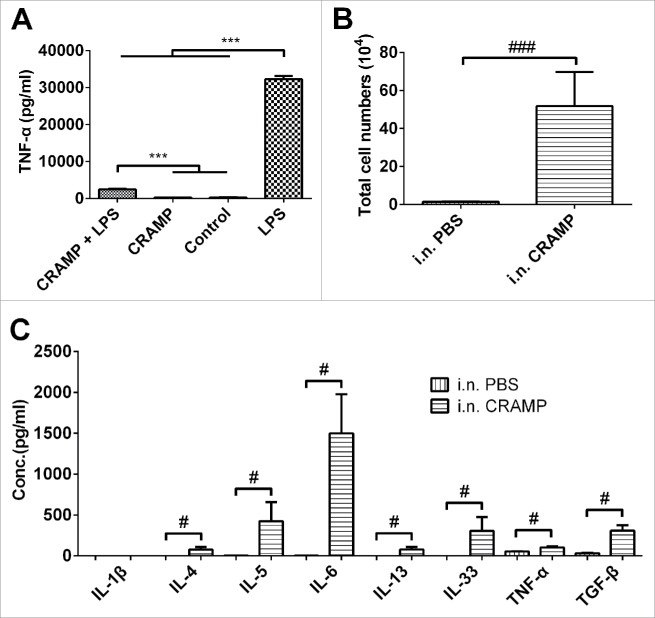
To identify the activity of synthetic CRAMP peptides, RAW264.7 cells were cultured and incubated with CRAMP alone or in combination with LPS, and the level of TNF-α in medium was measured with ELISA. As a positive control, LPS significantly induced the expression of TNF-α by RAW264.7 cells, and CRAMP significantly suppressed LPS-induced TNF-α expression. There was no change found in CRAMP alone – treated cells as compared to the control receiving medium only (Fig. 1A). The results were well consistent with what reported elsewhere, indicating synthesized peptides have the activity of CRAMP. To evaluate the activity of synthetic CRAMP in vivo, mice were administered with CRAMP intranasally for four consecutive days. Total immune cells number in BALF was profoundly increased in the mice receiving CRAMP as compared to the mice administrated by PBS (Fig. 1B). The recruited cells in BALF were mainly neutrophils and monocytes. In addition, the levels of pro-inflammatory cytokines IL-4, IL-5, IL-6, IL-13, IL-33, TNF-α and TGF-β in BALF were elevated significantly in CRAMP-administrated mice (Fig. 1C).
Figure 1.
Exogenous CRAMP promotes the recruitments of monocytes and neutrophils and the expression of pro-inflammatory cytokines. (A) Synthetic CRAMP significantly suppressed the expression of TNF-α in LPS-stimulated Raw264.7 cells. (B) Intranasal administration with CRAMP induced immune cell accumulation in airway (majored by neutrophils and monocytes) and (C) promoted the expression of pro-inflammatory cytokines. Statistical significance was analyzed by one-way ANOVA followed by Newman-keuls multiple comparisons test, ***p < 0.001, n = 4 (A), and by unpaired Student's t-test, ###p < 0.001, #p < 0.05, n = 4 (B and C).
Exogenous CRAMP significantly exacerbates Ovalbumin-induced airway inflammation
To clarify the possible effects of CRAMP on allergen-induced airway inflammatory responses, an acute mouse model induced by OVA was employed. In the OVA-challenged mice, exogenous CRAMP significantly enhanced the accumulation of inflammatory cells in BALF (Fig 2A), which was dominated by eosinophils, neutrophils and monocytes. OVA-specific IgE in serum was elevated significantly by CRAMP administration (Fig 2B). The levels of Th2 associated cytokines IL-33 and IL-13 in mice receiving CRAMP administration were significantly higher than those in mice receiving only OVA-challenge (Fig 2C). Histology analyses showed that OVA challenges significantly induced the peribronchial and perivascular infiltration of inflammatory cells as well as airway goblet cell hyperplasia, comparing mice subjected to OVA challenge and those receiving PBS; and, the administration with CRAMP significantly exacerbated airway goblet cell hyperplasia and lung tissue inflammation, as compared to challenge only with OVA. The representative pictures were shown (Fig. 2D, left), and goblet cells with overproduced mucus were stained in purplish red by PAS staining. Semi-quantitative analyses based on scoring systems were performed (Fig. 2D, right).
Figure 2.
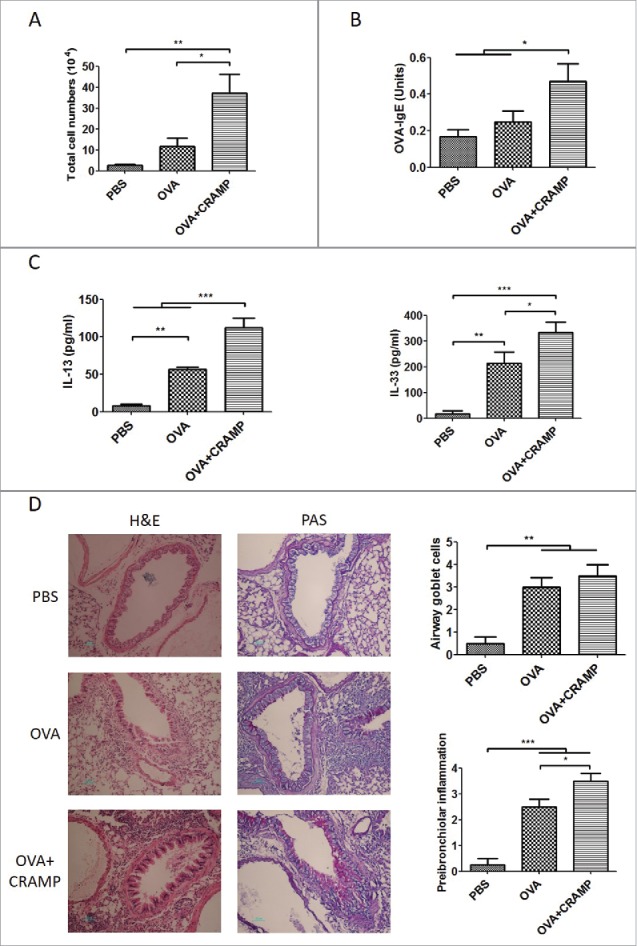
Exogenous CRAMP significantly exacerbates the ovalbumin-induced allergic airway inflammation. In OVA-challenged mice, administration with 5 mg/kg CRAMP increased (A) inflammatory cells accumulation, (B) OVA-specific IgE levels in serum, (C) Th2 cytokine levels in BALF and (D) peribronchial and perivascular inflammatory cells infiltration and airway goblet cells hyperplasia (original magnification is 200X), with the results of semi-quantitative analyses according to a scoring system shown at the right panel. Statistical significance was analyzed by one-way ANOVA followed by Newman-keuls multiple comparisons test, ***p < 0.001, **p < 0.01, *p < 0.05, n = 4.
The expression levels of CRAMP and its receptor FPR2 significantly increase in oxazolone-induced colitis model
The colon tissues were collected from mice challenged with TNBS or oxazolone. The mRNA and protein expression levels of CRAMP and its reported receptor FPR2 were quantitatively detected by real-time PCR and immunoblot. The results showed that CRAMP and FPR2 were significantly increased at both the mRNA and protein levels in oxazolone-challenged mice in comparison with those in normal mice and the mice receiving ethanol as a vehicle control. In TNBS-challenged mice, the expressions of CRAMP and FPR2 were also elevated as compared to those of the controls, but significantly lower than those in oxazolone-challenged mice (Fig. 3A and 3B). The results were basically consistent with the finding in clinic that the level of human LL-37 was elevated in UC patients but not in CD patients.25
Figure 3.
Treatment with CRAMP suppresses the development of oxazolone-induced colitis in mice. (A) The RNA and (B) protein levels of CRAMP and its receptor FPR2 increased more significantly in oxazolone-induced ulcerative colitis than those in TNBS-induced colitis. Gene expression levels in colon tissue were determined by real-time PCR and normalized with GAPDH. Mice were sensitized and administrated intrarectally with 1% oxazolone or 2.0 mg TNBS in 100 μL 50% Ethanol. Colon samples were collected 3 days later. (C) In a treatment experiment, mice were sensitized and challenged intrarectally with oxazolone, and CRAMP was administered intrarectally for six consecutive days. (D) The survival rate of mice, and (E) body weight regain were improved in the mice receiving 20 mg/kg CRAMP. (F) Representative pictures of colon were presented, and (G) reduced ratios of colon weight to length were obtained in 20 mg/kg of CRAMP-treated mice. Statistical significance was analyzed by one-way ANOVA followed by Newman-keuls multiple comparisons test, ***p < 0.001, **p < 0.01; n = 4 (A and B) and n = 5–6 (F); ###p < 0.001, comparing 20 mg/kg (n = 6) with 5 mg/kg (n = 5) of CRAMP by unpaired Student's t-test, (F); n = 6–11 (D). Repeated-measures two-way ANOVA with Bonferroni correction was used to compare measurements of mouse bodyweight, **p < 0.01, n = 12 – 22, comparing the mice receiving 20 mg/kg CRAMP and the mice receiving 0 mg/kg CRAMP (E).
Exogenous CRAMP improves the regain of bodyweight loss caused by oxazolone challenge
CRAMP was administered intrarectally for six consecutive days at doses of 5 mg/kg and 20 mg/kg respectively in oxazolone-challenged mice (Fig. 3C). At day 7 from the intrarectal challenge with oxazolone, there were 5 of 10, 2 of 9, and 4 of 11 mice died in the groups receiving the doses of 5 mg/kg, 20 mg/kg or 0 mg/kg CRAMP respectively (Fig. 3D). In the results of bodyweight change, mice receiving 20 mg/kg of CRAMP showed significant regain of bodyweight loss as compared to those receiving 0 or 5 mg/kg of CRAMP (Fig. 3E). Further, the analyses on the ratios of colon weight to length were performed to indicate the severity of colon colitis and inflammation. The representative pictures of colons were shown (Fig. 3F). The results showed that the ratio of 20 mg/kg group was similar to those of ethanol control and normal control, while 0 and 5 mg/kg groups had dramatically higher ratio of colon weight to length than 20 mg/kg group and the negative controls (Fig. 3G).
Exogenous CRAMP reduces the expression of proinflammatory cytokines and enhances the expression of mediators promoting epithelial integrity in oxazolone-induced colitis
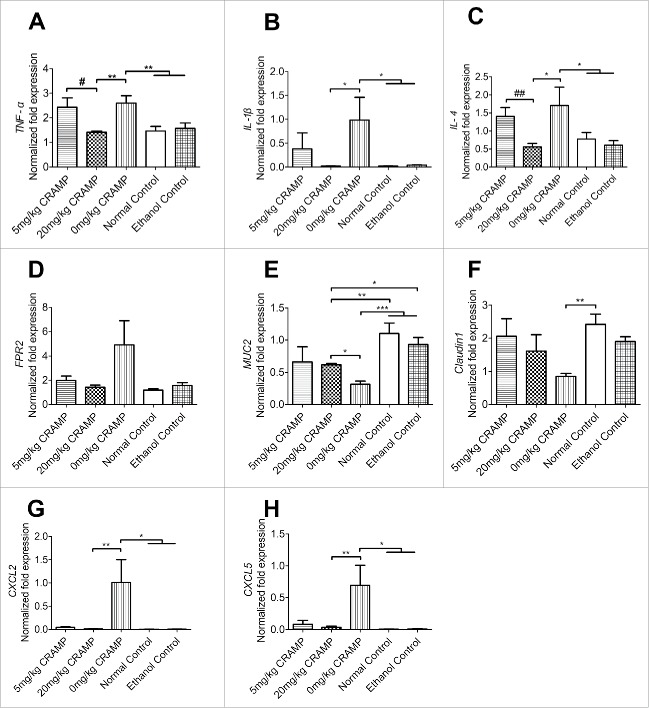
The mRNA expression levels of proinflammatory cytokines in colon tissues were quantified by real-time PCR. Two of the most typical proinflammatory cytokines TNF-α and IL-1β as well as a dominant Th2 cytokine IL-4 which is a critical driver of intestinal inflammation in UC,39 were increased in oxazolone-challenged mice receiving no CRAMP as compared to those in the negative controls receiving the vehicle ethanol or PBS. And the increases were suppressed significantly in mice receiving 20 mg/kg of CRAMP but not in those receiving 5 mg/kg of CRAMP. There were no significant differences found between the groups receiving no CRAMP and 5 mg/kg of CRAMP (Fig. 4A-C). Interestingly, the mRNA level of CRAMP receptor FPR2 in 20 mg/kg of CRAMP group decreased also nearly to those of the controls at the checkpoint (Fig. 4D). MUC2 and Claudin1 were detected as key markers indicating epithelial integrity and tissue repairs. Oxazolone challenged mice showed significantly reduced expression of MUC2 and Claudin1, as compared to those receiving the vehicle or the normal mice. It was found that the reduction of MUC2 and Claudin1 levels were suppressed in mice receiving 20 mg/kg or 5 mg/kg dose of CRAMP as compared with those in model mice receiving no CRAMP (Fig. 4E-F). CXCL2 and CXCL5 were important chemokines for neutrophils recruitment,40 and their levels were significantly increased in oxazolone-challenged mice, however, the treatment with 20 mg/kg or 5 mg/kg dose of CRAMP significantly suppressed the increases of CXCL2 and CXCL5 induced by oxazolone (Fig. 4G and H).
Figure 4.
Exogenous CRAMP reduces the expression of proinflammatory cytokines and chemokines, and increased those of epithelial repair-related genes. (A) The expression of TNF-α, (B) IL-1β, (C) IL-4, (D) FPR2, and chemokines (G) CXCL2 and (H) CXCL5 were reduced in mice receiving CRAMP treatment at a dose of 20 mg/kg bodyweight, and the levels of tissue repair-related genes (E) MUC2 and (F) Claudin1 were increased, as compared to those in oxazolone challenged control mice. Gene expression levels in colon tissue were determined by real-time PCR and standardized with GAPDH. Statistical significance was analyzed by one-way ANOVA followed by Newman-keuls multiple comparisons test, ***p < 0.001, **p < 0.01, *p < 0.05, n = 6; ##p < 0.01, #p < 0.05, comparing 20 mg/kg (n = 6) with 5 mg/kg CRAMP (n = 5) by unpaired Student's t-test.
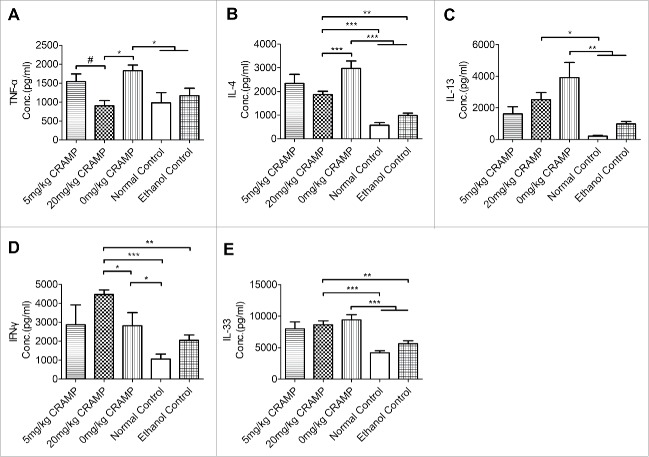
The significantly reduced expression of TNF-α and Th2 cytokines IL-4 and IL-13 was found in mice receiving 20 mg/kg but not 5 mg/kg of CRAMP at a protein level measured with ELISAs (Fig. 5A-C). Th1 cytokine IFN-γ in 20 mg/kg CRAMP group had a marked increased level as compared to the other groups (Fig. 5D). Th2 responses-associated cytokine IL-33 was elevated in oxazolone-challenged model mice but there was no significant effects produced by CRAMP treatment (Fig. 5E).
Figure 5.
Treatment with CRAMP reduces protein levels of pro-inflammatory cytokines in colon tissues. (A) TNF-α, (B) IL-4, and (C) IL-13 were reduced and (D) IFN-γ level was increased by CRAMP administration at a dose of 20 mg/kg bodyweight. (E) The level of IL-33 was elevated by oxazolone challenge but wasn't affected by CRAMP administration. Cytokines levels in the homogenates of colon tissues were measured by ELISA and standardized with total protein concentrations. Statistical significance was analyzed by one-way ANOVA followed by Newman-keuls multiple comparisons test, ***p < 0.001, **p < 0.01, *p < 0.05, n = 5 – 6; #p < 0.05, comparing 20 mg/kg (n = 6) with 5 mg/kg CRAMP (n = 5) by unpaired Student's t-test.
Exogenous CRAMP ameliorates colonic epithelial damage and tissue inflammation in mice receiving oxazolone challenge
Histological analyses on H&E staining sections of colon tissues showed that oxazolone challenge in mice induced clear symptoms resembling human ulcerative colitis, including distortion of crypt architecture, inflammation of crypts, crypt abscesses, and hemorrhage or inflammatory cells in the lamina propria. Administration with exogenous CRAMP at a dose of 20 mg/kg but not 5 mg/kg significantly increased the integrity of the colonic epithelium and decreased tissue inflammation. The representative microphotographs of H&E staining sections of colons were shown (Fig. 6A-E). The conclusions were further supported by a semiquantitaive analysis according to a histological scoring system (Fig. 6F). To further demonstrate whether CRAMP facilitated the preservation of gut barrier integrity, an experiment with FITC-dextran was performed. The results indicated that the increase of intestinal permeability caused by oxazolone challenge was reduced significantly by exogenous CRAMP administration at both 5 mg/kg and 20 mg/kg doses (Fig. 6G).
Figure 6.
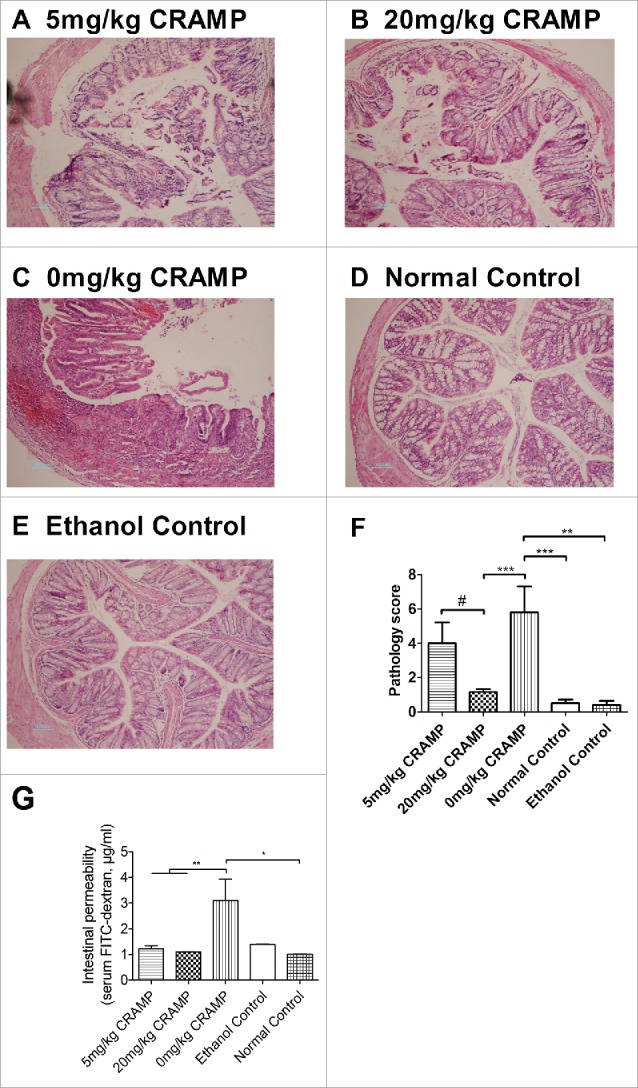
Treatment with exogenous CRAMP reduces colonic epithelial damage and tissue inflammation. Representative pictures of H&E stained colon tissue sections were presented (original magnification is 100X):(A) 5 mg/kg CRAMP, (B) 20 mg/kg CRAMP, (C) 0 mg/kg CRAMP, (D) Normal control, (E) Ethanol control. (F) Semiquantitative analyses based on a histological scoring system were performed. Colonic tissues were fixed in formalin, embedded in wax, and sliced at a thickness of 4 µm, and then stained with H&E solution. Statistical significance was analyzed by one-way ANOVA followed by Newman-keuls multiple comparisons test, ***p < 0.001, **p < 0.01, *p < 0.05, n = 6. ##p < 0.01, comparing 20 mg/kg (n = 6) with 5 mg/kg CRAMP (n = 5) by unpaired Student's t-test. (G) Intestinal permeability through quantitative analysis of FITC-dextran in serum (n = 5 – 7). Statistical significance was analyzed by one-way ANOVA followed by Newman-keuls multiple comparisons test, **p < 0.01, *p < 0.05, n = 4.
Discussion
The etiology of inflammatory bowel diseases (IBD) is still not quite clear till now. However, accumulated data indicated that intestinal flora were associated with the development and maintenance of IBD. The speculation is based on the findings like that, adherent bacteria and increased bacterial number were found in intestinal specimens clinically collected from IBD patients, germ-free condition suppressed the development of experimental colitis in animal model, and antibiotic application also reduced DSS-induced colitis.41,42 Therefore, a deduced possible mechanism of IBD pathogenesis is that bacteria invade into damaged intestinal epithelium in genetically susceptible individuals, and caused inflammatory responses. Considering cathelicidin is a wide-spectrum antimicrobial agent and resistance to cathelicidin is not easy to develop, employing exogenous cathelicidin or cathelicidin-related peptides to suppress intestinal flora and thus intervene in IBD development is becoming an attractive therapeutic strategy. This idea was supported by studies of concept approval in a DSS-induced animal model of IBD.27 Intrarectal administration with either exogenous CRAMP, or CRAMP expressing recombinant Lactococcus lactis, or plasmid encoding CRAMP successfully ameliorated DSS-induced colitis.27,28,30 The likely mechanisms were attributed to reduced intestinal flora, promoted epithelial integrity, and suppressed LPS-elicited inflammatory responses, which were caused by increased local concentration of exogenous CRAMP. However, cathelicidin has also proven to have significant pro-inflammatory effects through chemoattractant immune cells such as eosinophils, neutrophils, monocytes, and lymphocytes, and promoting the release of inflammatory mediators.9 In previous studies, the potential therapeutic application of exogenous cathelicidin was investigated mainly in DSS-induced colitis. In the model, DSS is believed to cause intestinal epithelial damage and allow bacteria to invade epithelium and then cause inflammation. Although the model resembles some clinical symptoms of UC, the development of colitis is fast and isn't dependent on adaptive immune, making it be used mainly for studying epithelial repair and innate immune.43,44 As known, clinical UC was considered as a Th2-type responses-driven chronic intestinal inflammatory disease. Therefore, in this current study, an oxazolone-induced colitis model, in which adaptive Th2-dominant immune was considered the main pathogenesis mechanism,45,46 was employed to further investigate the therapeutic potentials of exogenous CRAMP. The importance of Th2 responses in the model was demonstrated by the finding that a monoclonal antibody against the key Th2 cytokine IL-4 significantly suppressed oxazolone-induced intestinal inflammation.39,47
By using macrophage cell line RAW264.7, TNF-α production is induced by LPS whereas CRAMP suppresses the induction by binding to LPS, we confirmed at first the activity of synthesized CRAMP peptides in vitro to ensure the appropriate interpretation of the results using CRAMP to intervene in disease processes in vivo. Our results were consistent to that previously reported, CRAMP at a concentration of 10 μg/mL suppressed significantly LPS-stimulated expression of TNF-α. Further, considering CRAMP is a potent chemoattractant for granulocytes and monocytes, we deduced that exogenous CRAMP alone might have the capability of initiating and exacerbating inflammation responses. In the current study, intranasal administration with CRAMP did cause inflammatory cells accumulation in BALF, accompanied by increased pro-inflammatory cytokines. Considering the development of asthma may associate with bacterial, fungi, and virus infections, which also elicited CRAMP expression by epithelial cells, we speculated that CRAMP might be involved in airway allergic inflammation, especially in chronic asthma and airway remodeling. Briefly, in an ovalbumin-induced allergic airway inflammation model, simultaneous intranasal instillation of CRAMP significantly enhanced accumulation of inflammatory cells and expression of pro-inflammatory cytokines in BALF. Further, histology analyses showed exogenous CRAMP stimulate lung tissue inflammation and airway goblet cells hyperplasia. The data of these studies strongly indicated the pro-inflammation effects of exogenous CRAMP, and were presented in this study to put an emphasis on that, when administration with exogenous CRAMP or targeting CRAMP signaling is considered as a possible therapeutic approach for diseases such as in colitis, the actual outcome might be related to the disease condition and treatment manipulation.
CRAMP has proven to be effective in DSS-induced colitis, however, whether it is still effective or not in adaptive immune-driven oxazolone-induced colitis hasn't been demonstrated. CRAMP and its possible receptor FPR2 were found to be highly elevated in oxazolone-induced colitis. Although they were elevated in TNBS-induced colitis which is driven by Th1/Th17 responses, the extent was significantly lower than that of oxazolone colitis. The result is sort of consistent with what found in clinic that CRAMP elevated in UC patients but not CD patients.25 Accordingly, we deduced at first that CRAMP associated with T cell immune responses, and might have different roles in oxazolone or TNBS-induced colitis, which are Th2 or Th1 responses-driven respectively. However, it was reported that CRAMP suppressed inflammation and fibrosis in a TNBS-induced chronic model,31 implying distinct T cell immune pathogenesis may not affect exogenous CRAMP's protective effects on intestinal colitis. Considering that CRAMP is not found elevated in clinical CD, in this current study we focus on oxazolone-induced colitis. We employed a sensitization/challenge protocol to induce intestinal colitis, allowing adaptive immune to be well developed, and, 24h after oxazolone challenge, CRAMP was administrated once a day for continuous 6 days to provide a persistent intervention. Well consistent with the findings from DSS model, 20 mg/kg dose of CRAMP protected mice from oxazolone-induced colitis, which were evidenced by improved regain of body weight, increased ratio of colon length/weight, and ameliorated epithelial damage and inflammatory cell infiltration. The results in the current study support the therapeutic application of exogenous CRAMP in intestinal colitis. The effects of exogenous CRAMP in this study might be mainly attributed to the preservation of gut epithelial integrity and /or the modulation of inflammation. Intrarectal administration with exogenous CRAMP may have directly reduced intestinal flora and promoted epithelial integrity, and thus prevented bacterial invasion. In the other hand, CRAMP may bind to endotoxin and suppress LPS-elicited inflammatory responses, or directly modulate the functions of inflammatory cells. Further analyses showed that 20mg/kg dose of CRAMP administration suppressed significantly pro-inflammatory cytokines at mRNA and protein levels. In contrary, the expression of MUC2 and Claudin1, which were key mediators reflecting epithelium integrity,48,49 were significantly elevated. Furthermore, the expression of important chemokines CXCL2 and CXCL5 for the recruitment of neutrophils were significantly reduced in mice receiving CRAMP. In comparison, 5 mg/kg dose of CRAMP failed to reduce bodyweight loss, weight/length ration of colon, and tissue pathology scores. We found that 20mg/kg but not 5mg/kg dose significantly reduced TNF-α, IL-1, and IL-4, and increased INF-γ levels; and interestingly, both 5 mg/kg and 20 mg/kg groups showed decreased chemokines and increased MUC2 and Claudin1 levels. In a recent review, Piktel et al. summarized the pleiotropic properties of LL-37 in relation to the different cells and tissues, and presented the different doses requirement for a specific function. The required dose of cathelicidin is 1–5 μg/ml for tissue repairing, 0.1-70 μg/ml for antimicrobial activity, and 0.5-50 μg/ml for immunomodulation, respectively.11 It indicates that the effective mechanisms are tissue specific, complex, and depend mostly on the ability of LL-37 to act as a ligand for different membrane receptors whose expression varies on different cells. The results might imply that administration with high dose of CRAMP produced more effective suppression of inflammatory responses than the low dose, which contributed to the significantly ameliorated oxazolone-induced colitis symptoms.
In a summary, exogenous CRAMP exacerbated allergen-induced airway inflammation, but significantly protected mice from oxazolone-induced colitis probably through both promoting the process of epithelial repairs and suppressing inflammation responses. The results strongly indicated that, although CRAMP has pro-inflammation functions, application of exogenous CRAMP might be a potential approach for the treatment of ulcerative colitis.
Materials and methods
Animals and induction of allergic airway inflammation and intestinal colitis
Female BALB/c mice (6-8 weeks of age) were purchased from Vital River Company (Beijing, China), and maintained under specific pathogen-free conditions in the Central Animal Care Services of Institute of Medical Biology, CAMS & PUMC (Kunming, China).
Induction of allergic airway inflammation with Ovalbumin (OVA) (grade IV; Sigma-Aldrich) was performed as previously described.34 Mice were subjected to one intraperitoneal sensitization with 10 μg OVA and 2 mg Alum adjuvant mixed in 500 μL PBS and 14 days later 3 continuous everyday intranasal (i.n.) challenges with 50 μg OVA in 40 μL PBS. One day after OVA challenge, mice were anaesthetized and euthanized by Isoflurane inhaled anesthesia and cardiac puncture for blood collection.
Induction of colitis with oxazolone (Sigma-Aldrich) was performed as previously described.35 Briefly, mice were first sensitized with 100 μL of 3% (w/v) oxazolone in 100% ethanol on shaved abdominal skin, and five days later, mice were anesthetized with isoflurane inhalation and then administrated intrarectally with 100 μL of 1% oxazolone in 50% ethanol using a 3.5 F catheter equipped with a 1 mL syringe. The catheter was advanced into the rectum until the tip was 4 cm proximal to the anal verge. To ensure distribution of oxazolone within the entire colon and cecum, mice were held in a vertical position for 50 seconds after the intrarectal administration. TNBS-induced colitis was induced as preciously described.36 Briefly, mice were administered intrarectally 2.0 mg of TNBS (Sigma-Aldrich) in 50% ethanol (100 μL).
CRAMP identification and mouse treatment
Synthesized peptide CRAMP was provided by GL Biotech, Co., Ltd. (ShangHai, China. Purification: 97.15%). Murine macrophage cell line RAW264.7 cells were cultured in DMEM/HIGH glucose (HyClone) with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. To identify the activity of synthesized peptide CRAMP, the cells at 1 × 105/well were incubated with CRAMP (10 μg/mL) alone, CRAMP plus LPS, or LPS (100 ng/mL) as a positive control. After 24 h of incubation, TNF concentration in the cultured medium was determined by sandwich enzyme-linked immunosorbent assay (ELISA).34
To display the possible pathophysiological functions of CRAMP, mice were administered intranasally with 20 mg/kg of CRAMP in 40 μL phosphate buffered saline (PBS, pH 7.2) for four consecutive days after isoflurane anaesthetization. Cytokines and total cell numbers in bronchial alveolar lavage fluid (BALF) were evaluated.
To investigate the possible effects of CRAMP on OVA-induced airway inflammation, 5 mg/kg CRAMP was combined with OVA when the intranasal challenges were performed. The total volume is 40 μL.
To investigate the possible effects of CRAMP on oxazolone-induced intestinal colitis and inflammation, 24 hour after the intrarectal challenge of oxazolone, mice were administered intrarectally with CRAMP in 100 μL PBS at a dose of 20 mg/kg/day or 5 mg/kg/day for six consecutive days. Mice received only PBS served as controls. There were 4 independent partially or completely repeated experiments were conducted, and the data presented in this manuscript was obtained from one of these experiments.
BALF collection and cytospinning
BALF samples were collected after three repeated washing of the excised lungs using 1 mL PBS. After cytospinning, the slides were stained with HEMA 3 (Fisher Diagnostics, Pittsburg, PA, USA), and the cell types in BALF were analyzed.
Quantization of mRNA expression by real-time PCR
Total RNA was isolated from colon tissues with RNAiso Plus reagent (Takara) according to the manufacturer's instruction, and used as templates to synthesize cDNA by using RT reagent kit with gDNA eraser (Takara). Obtained cDNA was amplified and quantitatively analyzed using SYBR select Master Mix (Invitrogen) on the CFX Connect Real-Time System (Bio-Rad). The mRNA levels of target molecules were standardized with that of a housekeeping gene GAPDH.
Cytokine measurement by ELISA
Colon tissues were suspended in ice-cold PBS (at 10 mL per gram of tissue) containing protease inhibitor cocktail (Sigma-Aldrich) and then homogenized briefly. After the homogenates were centrifuged at 12000 rpm for 10 min at 4°C, the supernatants were collected. The cytokine levels in colon and BALF samples were measured by sandwich ELISAs. ELISAs were performed by using paired capture and biotinylated detection antibodies purchased from eBioscience and following the supplier's instruction. Avidin-alkaline phosphatase and substrate system (Sigma-Aldrich) was used to develop the reactions. The protein standards were purchased from Peprotech, Inc. The cytokine levels for colonic samples were normalized by the concentration of total protein detected by Bradford's method (Bio-Rad).
Immunobloting assays
For immunoblotting assays, mice were sacrificed 3 days after Oxazolone or TNBS administration. Mice colons were collected and homogenate were prepared. Protein sample was separated by using 12% SDS-PAGE and transferred onto PVDF membranes. After 5% non-fatty milk blocking 1h, membranes were incubated with primary Abs overnight at 4°C. Secondary Abs incubated 1h at room temperature. Primary Abs goat anti-CRAMP, mouse anti-Tublin were purchased from SantaCruz. Primary Ab rabbit anti-FPR2 were purchased from proteintech.
Histology analysis
The left lung was fixed in formalin, and then embedded in paraffin and sectioned. Specimens were stained either with hematoxylin and eosin (H&E) or with periodic acid Schiff (PAS). As previously described,37 peribronchiolar and perivascular inflammation in the H&E-stained slides were assessed using an indexed scale according to rings of inflammatory cells. Goblet-cell abundance was measured as the percentage of PAS-positive cells in the total airway epithelia of medium-sized airways.
Colons fixed in formalin were embedded in paraffin, cut, and stained with hematoxylin and eosin (H&E). For inflammation assessment, histological scoring was based on severity of inflammation (based on inflammatory cells infiltration, 0–3: none, slight, moderate, severe), depth of injury (0-3: none, mucosal, mucosal and submucosal, transmural), crypt damage (none, basal one-third damaged, basal two-third damaged, only surface epithelium intact, entire crypt and epithelium lost). Histological examination was performed by a pathologist blinded to the source of treatment.
Fluorescein isothiocyanate-dextran 4000 (FITC-D4000) test
Intestinal permeability assay with FITC-dextran was performed as previously described.38 Briefly, 24 hour after the intrarectal challenge of oxazolone, mice were administered intrarectally with CRAMP in PBS at a dose of 20 mg/kg or 5 mg/kg once a day for two days. 24 hour after the last CRAMP administration, FITC-D4000 (Sigma-Aldrich) was administered to the mice by gavage (600 mg/kg, 300 μL). And then, mice were fasting for 1–4 hours until they were sacrificed. Blood was collected by heart puncture under isoflurane inhalation and centrifuged at 12,000 g for 10 min at 4°C. Isolated plasma was diluted with equal volume of PBS and analyzed with multi-detection microplate reader (Synergy 4, BioTek), with excitation at 485 nm and emission at 528 nm.
Statistical analyses
Differences between experimental groups were assessed by one-way analysis of variance (ANOVA), followed by the Newman-Keuls multiple-comparison test. Unpaired Student's t test was used to compare the effects of 5 mg/kg and 20 mg/kg doses of CRAMP. Repeated-measures two-way ANOVA with Bonferroni correction was used to compare measurements of mouse body weight. The values are reported as the mean ± standard error of the mean. (GraphPad Prism 5.01, GraphPad Software, Inc., San Diego, CA, USA)
Funding Statement
This work was financially supported by the National Natural Science Foundation of China (81273299, 81773270), the CAMS Initiative for Innovative Medicine (2016-I2M-1-019), and Yunnan Provincial Science and Technology Department (2013IA005, 2016FA049).
Ethical considerations
The animal experimental procedures were approved by the Ethics Committee of Animal Care and Welfare, Institute of Medical Biology, Chinese Academy of Medical Sciences (CAMS) & Peking Union Medical College (PUMC) (Kunming, China), in accordance with the animal ethics guidelines of the Chinese National Health and Medical Research Council (NHMRC) and the Office of Laboratory Animal Management of Yunnan Province, China. All efforts were made to minimize animal sufferings.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Mr. Jiahong Gao and Ms. Wanpu Wang for assistance with the histological analyses.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
Acquisition, analysis and interpretation of data; drafting of the manuscript; statistical analysis: Y.L., Y.M. Technical or material support: X.C., L.C., Y.X., Q.L., W.H., K.L., P.S., X.Y., W.S., H.B. Study concept and design: Y.M. Critical revision of the manuscript for important intellectual content; obtained funding; study supervision: Y.M.
References
- 1.Gallo RL, Kim KJ, Bernfield M, Kozak CA, Zanetti M, Merluzzi L, Gennaro R. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem. 1997;272(20):13088–93. doi: 10.1074/jbc.272.20.13088. PMID:9148921. [DOI] [PubMed] [Google Scholar]
- 2.Gudmundsson GH, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem. 1996;238(2):325–32. doi: 10.1111/j.1432-1033.1996.0325z.x. PMID:8681941. [DOI] [PubMed] [Google Scholar]
- 3.Chen K, Xiang Y, Huang J, Gong W, Yoshimura T, Jiang Q, Tessarollo L, Le Y, Wang JM. The formylpeptide receptor 2 (Fpr2) and its endogenous ligand cathelin-related antimicrobial peptide (CRAMP) promote dendritic cell maturation. J Biol Chem. 2014;289(25):17553–63. doi: 10.1074/jbc.M113.535674. PMID:24808174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen K, Liu M, Liu Y, Wang C, Yoshimura T, Gong W, Le Y, Tessarollo L, Wang JM. Signal relay by CC chemokine receptor 2 (CCR2) and formylpeptide receptor 2 (Fpr2) in the recruitment of monocyte-derived dendritic cells in allergic airway inflammation. J Biol Chem. 2013;288(23):16262–73. doi: 10.1074/jbc.M113.450635. PMID:23603910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakagawa Y, Gallo RL. Endogenous intracellular cathelicidin enhances TLR9 activation in dendritic cells and macrophages. J Immunol. 2015;194(3):1274–84. doi: 10.4049/jimmunol.1402388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Does AM, Beekhuizen H, Ravensbergen B, Vos T, Ottenhoff TH, van Dissel JT, Drijfhout JW, Hiemstra PS, Nibbering PH. LL-37 directs macrophage differentiation toward macrophages with a proinflammatory signature. J Immunol. 2010;185(3):1442–9. doi: 10.4049/jimmunol.1000376. [DOI] [PubMed] [Google Scholar]
- 7.Davidson DJ, Currie AJ, Reid GS, Bowdish DM, MacDonald KL, Ma RC, Hancock RE, Speert DP. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J Immunol. 2004;172(2):1146–56. doi: 10.4049/jimmunol.172.2.1146. [DOI] [PubMed] [Google Scholar]
- 8.Woloszynek JC, Hu Y, Pham CT. Cathepsin G-regulated release of formyl peptide receptor agonists modulate neutrophil effector functions. J Biol Chem. 2012;287(41):34101–9. doi: 10.1074/jbc.M112.394452. PMID:22879591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahlenberg JM, Kaplan MJ. Little peptide, big effects: the role of LL-37 in inflammation and autoimmune disease. J Immunol. 2013;191(10):4895–901. doi: 10.4049/jimmunol.1302005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otte JM, Zdebik AE, Brand S, Chromik AM, Strauss S, Schmitz F, Steinstraesser L, Schmidt WE. Effects of the cathelicidin LL-37 on intestinal epithelial barrier integrity. Regul Pept. 2009;156(1–3):104–17. doi: 10.1016/j.regpep.2009.03.009. PMID:19328825. [DOI] [PubMed] [Google Scholar]
- 11.Piktel E, Niemirowicz K, Wnorowska U, Watek M, Wollny T, Gluszek K, Góźdź S, Levental I, Bucki R. The role of Cathelicidin LL-37 in cancer development. Arch Immunol Ther Exp (Warsz). 2015;64(1):33–46. doi: 10.1007/s00005-015-0359-5. PMID:26395996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenfeld Y, Papo N, Shai Y. Endotoxin (lipopolysaccharide) neutralization by innate immunity host-defense peptides. Peptide properties and plausible modes of action. J Biol Chem. 2006;281(3):1636–43. [DOI] [PubMed] [Google Scholar]
- 13.Hensel JA, Chanda D, Kumar S, Sawant A, Grizzle WE, Siegal GP, Ponnazhagan S. LL-37 as a therapeutic target for late stage prostate cancer. Prostate. 2011;71(6):659–70. doi: 10.1002/pros.21282. PMID:20957672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li D, Beisswenger C, Herr C, Schmid RM, Gallo RL, Han G, Zakharkina T, Bals R. Expression of the antimicrobial peptide cathelicidin in myeloid cells is required for lung tumor growth. Oncogene. 2014;33(21):2709–16. doi: 10.1038/onc.2013.248. PMID:23812430. [DOI] [PubMed] [Google Scholar]
- 15.Ren SX, Cheng AS, To KF, Tong JH, Li MS, Shen J, Wong CC, Zhang L, Chan RL, Wang XJ, et al. Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer. Cancer Res. 2012;72(24):6512–23. doi: 10.1158/0008-5472.CAN-12-2359. PMID:23100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao W, Hsu YP, Ishizaka A, Kirikae T, Moss RB. Sputum cathelicidin, urokinase plasminogen activation system components, and cytokines discriminate cystic fibrosis, COPD, and asthma inflammation. Chest. 2005;128(4):2316–26. doi: 10.1378/chest.128.4.2316. PMID:16236890. [DOI] [PubMed] [Google Scholar]
- 17.Tjabringa GS, Ninaber DK, Drijfhout JW, Rabe KF, Hiemstra PS. Human cathelicidin LL-37 is a chemoattractant for eosinophils and neutrophils that acts via formyl-peptide receptors. Int Arch Allergy Immunol. 2006;140(2):103–12. doi: 10.1159/000092305. PMID:16557028. [DOI] [PubMed] [Google Scholar]
- 18.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192(7):1069–74. doi: 10.1084/jem.192.7.1069. PMID:11015447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I. Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur J Immunol. 2001;31(4):1066–75. doi: 10.1002/1521-4141(200104)31:4%3c1066::AID-IMMU1066%3e3.0.CO;2-. PMID:11298331. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Zhu M, Yang Z, Pan X, Jiang Y, Sun C, Wang Q, Xiao W. The human Cathelicidin LL-37 induces MUC5AC mucin production by airway epithelial cells via TACE-TGF-alpha-EGFR pathway. Exp Lung Res. 2014;40(7):333–42. doi: 10.3109/01902148.2014.926434. PMID:24901072. [DOI] [PubMed] [Google Scholar]
- 21.Wan M, Sabirsh A, Wetterholm A, Agerberth B, Haeggstrom JZ. Leukotriene B4 triggers release of the cathelicidin LL-37 from human neutrophils: novel lipid-peptide interactions in innate immune responses. FASEB J. 2007;21(11):2897–905. doi: 10.1096/fj.06-7974com. PMID:17446260. [DOI] [PubMed] [Google Scholar]
- 22.Hallstrand TS, Henderson WR Jr. An update on the role of leukotrienes in asthma. Curr Opin Allergy Clin Immunol. 2010;10(1):60–6. doi: 10.1097/ACI.0b013e32833489c3. PMID:19915456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J, Dahlen B, Agerberth B, Haeggstrom JZ. The antimicrobial peptide LL-37 induces synthesis and release of cysteinyl leukotrienes from human eosinophils–implications for asthma. Allergy. 2013;68(3):304–11. doi: 10.1111/all.12087. PMID:23330796. [DOI] [PubMed] [Google Scholar]
- 24.Ho S, Pothoulakis C, Koon HW. Antimicrobial peptides and colitis. Curr Pharm Des. 2013;19(1):40–7. doi: 10.2174/1381612811306010040 . PMID:22950497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schauber J, Rieger D, Weiler F, Wehkamp J, Eck M, Fellermann K, Scheppach W, Gallo RL, Stange EF. Heterogeneous expression of human cathelicidin hCAP18/LL-37 in inflammatory bowel diseases. Eur J Gastroenterol Hepatol. 2006;18(6):615–21. doi: 10.1097/00042737-200606000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Tai EK, Wong HP, Lam EK, Wu WK, Yu L, Koo MW, Koo MW, Cho CH. Cathelicidin stimulates colonic mucus synthesis by up-regulating MUC1 and MUC2 expression through a mitogen-activated protein kinase pathway. J Cell Biochem. 2008;104(1):251–8. doi: 10.1002/jcb.21615. PMID:18059019. [DOI] [PubMed] [Google Scholar]
- 27.Tai EK, Wu WK, Wong HP, Lam EK, Yu L, Cho CH. A new role for cathelicidin in ulcerative colitis in mice. Exp Biol Med. 2007;232(6):799–808. PMID:17526772. [PubMed] [Google Scholar]
- 28.Wong CC, Zhang L, Li ZJ, Wu WK, Ren SX, Chen YC, Ng TB, Cho CH. Protective effects of cathelicidin-encoding Lactococcus lactis in murine ulcerative colitis. J Gastroenterol Hepatol. 2012;27(7):1205–12. doi: 10.1111/j.1440-1746.2012.07158.x. PMID:22507188. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Xia X, Han F, Jiang Q, Rong Y, Song D, Wang Y. Cathelicidin-BF, a novel antimicrobial peptide from bungarus fasciatus, attenuates disease in a dextran sulfate sodium model of colitis. Mol Pharm. 2015;12(5):1648–61. [DOI] [PubMed] [Google Scholar]
- 30.Tai EK, Wu WK, Wang XJ, Wong HP, Yu L, Li ZJ, Lee CW, Wong CC, Yu J, Sung JJ, et al. Intrarectal administration of mCRAMP-encoding plasmid reverses exacerbated colitis in Cnlp(-/-) mice. Gene Ther. 2013;20(2):187–93. doi: 10.1038/gt.2012.22. PMID:22378344. [DOI] [PubMed] [Google Scholar]
- 31.Yoo JH, Ho S, Tran DH, Cheng M, Bakirtzi K, Kukota Y, Ichikawa R, Su B, Tran DH, Hing TC, et al. Anti-fibrogenic effects of the anti-microbial peptide cathelicidin in murine colitis-associated fibrosis. Cell Mol Gastroenterol Hepatol. 2015;1(1):55–74 e1. doi: 10.1016/j.jcmgh.2014.08.001. PMID:25729764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koon HW, Shih DQ, Chen J, Bakirtzi K, Hing TC, Law I, Ho S, Ichikawa R, Zhao D, Xu H, et al. Cathelicidin signaling via the Toll-like receptor protects against colitis in mice. Gastroenterology. 2011;141(5):1852–63 e1–3. doi: 10.1053/j.gastro.2011.06.079. PMID:21762664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi H, Hu W, Chen S, Lu Z, Wang Y. Cathelicidin-WA improves intestinal epithelial barrier function and enhances host defense against enterohemorrhagic escherichia coli O157:H7 infection. J Immunol. 2017;198(4):1696–705. doi: 10.4049/jimmunol.1601221. [DOI] [PubMed] [Google Scholar]
- 34.Long Q, Huang W, Yao Y, Yang X, Sun W, Jin X, Li Y, Chu X, Liu C, Peng Z, et al. Virus-like particles presenting interleukin-33 molecules: immunization characteristics and potentials of blockingIL-33/ST2 pathway in allergic airway inflammation. Hum Vaccin Immunother. 2014;10(8):2303–11. doi: 10.4161/hv.29425. PMID:25424936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17(5):629–38. doi: 10.1016/S1074-7613(02)00453-3. PMID:12433369. [DOI] [PubMed] [Google Scholar]
- 36.Guan Q, Ma Y, Hillman CL, Qing G, Ma AG, Weiss CR, Zhou G, Bai A, Warrington RJ, Bernstein CN, et al. Targeting IL-12/IL-23 by employing a p40 peptide-based vaccine ameliorates TNBS-induced acute and chronic murine colitis. Mol Med. 2011;17(7–8):646–56. doi: 10.2119/molmed.2010.00252. PMID:21424108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma Y, Halayko AJ, Basu S, Guan Q, Weiss CR, Ma AG, HayGlass KT, Becker AB, Warrington RJ, Peng Z. Sustained suppression of IL-13 by a vaccine attenuates airway inflammation and remodeling in mice. Am J Respir Cell Mol Biol. 2013;48(5):540–9. doi: 10.1165/rcmb.2012-0060OC. PMID:23470628. [DOI] [PubMed] [Google Scholar]
- 38.Volynets V, Reichold A, Bardos G, Rings A, Bleich A, Bischoff SC. Assessment of the intestinal barrier with five different permeability tests in healthy C57BL/6J and BALB/cJ Mice. Dig Dis Sci. 2016;61(3):737–46. doi: 10.1007/s10620-015-3935-y. PMID:26520109. [DOI] [PubMed] [Google Scholar]
- 39.Kasaian MT, Page KM, Fish S, Brennan A, Cook TA, Moreira K, Zhang M, Jesson M, Marquette K, Agostinelli R, et al. Therapeutic activity of an interleukin-4/interleukin-13 dual antagonist on oxazolone-induced colitis in mice. Immunology. 2014;143(3):416–27. doi: 10.1111/imm.12319. PMID:24831554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu C, Zhang S, Wang Y, Zhang S, Luo L, Thorlacius H. Platelet-derived CCL5 regulates CXC chemokine formation and neutrophil recruitment in acute experimental colitis. J Cell Physiol. 2015. [DOI] [PubMed] [Google Scholar]
- 41.Sheehan D, Moran C, Shanahan F. The microbiota in inflammatory bowel disease. J Gastroenterol. 2015;50(5):495–507. doi: 10.1007/s00535-015-1064-1. PMID:25808229. [DOI] [PubMed] [Google Scholar]
- 42.Nagalingam NA, Kao JY, Young VB. Microbial ecology of the murine gut associated with the development of dextran sodium sulfate-induced colitis. Inflamm Bowel Dis. 2011;17(4):917–26. doi: 10.1002/ibd.21462. PMID:21391286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawada M, Arihiro A, Mizoguchi E. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J Gastroenterol. 2007;13(42):5581–93. doi: 10.3748/wjg.v13.i42.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2(3):541–6. doi: 10.1038/nprot.2007.41. PMID:17406617. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi K, Imaeda H, Fujimoto T, Ban H, Bamba S, Tsujikawa T, Sasaki M, Fujiyama Y, Andoh A. Regulation of eotaxin-3/CC chemokine ligand 26 expression by T helper type 2 cytokines in human colonic myofibroblasts. Clin Exp Immunol. 2013;173(2):323–31. doi: 10.1111/cei.12117. PMID:23607908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boirivant M, Fuss IJ, Chu A, Strober W. Oxazolone colitis: A murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998;188(10):1929–39. doi: 10.1084/jem.188.10.1929. PMID:9815270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoving JC, Kirstein F, Nieuwenhuizen NE, Fick LC, Hobeika E, Reth M, Brombacher F. B cells that produce immunoglobulin E mediate colitis in BALB/c mice. Gastroenterology. 2012;142(1):96–108. doi: 10.1053/j.gastro.2011.09.044. PMID:21983080. [DOI] [PubMed] [Google Scholar]
- 48.Saeedi BJ, Kao DJ, Kitzenberg DA, Dobrinskikh E, Schwisow KD, Masterson JC, Kendrick AA, Kelly CJ, Bayless AJ, Kominsky DJ, et al. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol Biol Cell. 2015;26(12):2252–62. doi: 10.1091/mbc.E14-07-1194. PMID:25904334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Büller HA, Dekker J, Van Seuningen I, Renes IB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131(1):117–29. doi: 10.1053/j.gastro.2006.04.020. PMID:16831596. [DOI] [PubMed] [Google Scholar]