ABSTRACT
With maternal and infant vaccines against respiratory syncytial virus (RSV) in development, it is timely to consider how the deployment of these vaccines might vary according to local RSV disease seasonality. In temperate regions RSV infection is predictably limited to a period of 3 to 5 months, while in tropical regions disease seasonality is often both more variable and more prolonged. Accordingly, in tropical regions a year-round immunisation schedule for both maternal and infant immunisation might be appropriate. In contrast, in temperate regions the benefit of year-round maternal immunisation would be heavily dependent on the duration of protection this provided, potentially necessitating a strategy directed at children due to be born in the months immediately prior to the RSV season. This review will consider the impact of seasonality on maternal and infant immunisation strategies against RSV, and the potential of an alternative approach of passive immunisation for all infants immediately prior to the RSV season.
KEYWORDS: Respiratory syncytial virus, immunisation, public health, seasonality, maternal immunisation
Introduction
Respiratory Syncytial Virus (RSV) has been recognised as the second most common cause of death in infants from 1 months to 1 year of age and each year is responsible for 34 million new episodes of lower respiratory tract infection (LRTI) worldwide.1 An estimated 199,000 deaths were caused by RSV in 2005 in children younger than 5 years, 99% of which occurred in low-resource settings. In high income countries such as the UK, the average rate of hospital admissions due to RSV bronchiolitis has increased by an average of 1.8% per year since 20042 and RSV bronchiolitis accounts for 12% of PICU admissions.2 These data highlight the significant health, financial and social impact of RSV in both high- and low-income countries.
Developing interventions to prevent RSV disease has been complex. Not only has the field been working in the shadow of the vaccine-enhanced disease following formalin inactivated vaccine in the 1960's,3,4 but it is clear that the immune response of the target population (RSV-naïve infants under 6 months of age) differs greatly from older age groups who have had multiple prior RSV exposures, making it difficult to predict vaccine effectiveness from clinical trials in older children and adults.5 There are 13 candidate RSV vaccines designed to prevent paediatric disease in phase 1 to 3 clinical trials. These have focussed on two, potentially complementary, strategies: maternal immunisation (to provide passive immunisation to infants) and active infant immunisation.6 In addition, 2 new monoclonal antibodies for passive immunisation against RSV recently entered phase 2 and 3 clinical trials, one of which was stopped prematurely due to lack of efficacy.7,8,9
Of relevance to these prevention strategies is that in temperate regions the burden of RSV disease is concentrated in a predictable annual 3 to 5 month season,10 while in tropical regions RSV season is less predictable and more prolonged,11,12,13 potentially necessitating region or country specific immunisation strategies. In this paper we will discuss the implications of RSV seasonality on the different preventive strategies currently proposed.
RSV seasonality around the globe
RSV is one of the most contagious human pathogens, with over 80% of children experiencing RSV infections by 2 years of age.14 Reinfection occurs throughout life and can occur more than once in the same season.15 RSV seasonality is highly dependent on geographic location and climate. In northern and southern hemisphere temperate regions an annual seasonal pattern is predictably limited to 3–5 months during winter and autumn (Fig. 1).12,16 Numerous explanations for this have been proposed, including the possibility that inclement climate modifies human behaviour, reducing outdoor activities and increasing indoor crowding enhancing exposure and transmission of RSV,13,17,18 or that the low temperatures present during winter prolong the stability of RSV in fomites.19 Other authors have found that low absolute humidity during this season increases the risk of RSV disease,20 however none of these associations have been proven to be causal. In the tropics, humidity and temperature play a different role than the one observed in temperate regions, with studies in tropical regions suggesting that higher levels of humidity and stable temperatures allows large aerosol droplets to sustain RSV transmission all year around.18,21 Nonetheless, the correlations between RSV incidence, temperature and relative humidity are particularly variable and inconsistent amongst the tropical regions22; for example one Colombian study demonstrated that RSV cases were distributed throughout the year with different epidemic trends amongst different cities around the country.23 Interestingly, RSV has been found to be perennially present mostly in coastal areas, islands or along the equator.24 Regardless of the cause, identifying seasonal patterns is important when planning strategies for prevention such as vaccination.
Figure 1.
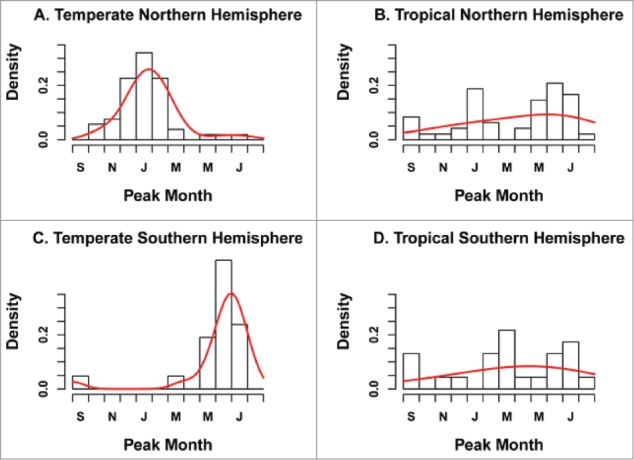
Distribution of RSV peak month by geographic zone (n = 96 locations). The black histogram represents observations while the red curve illustrates the fit of a Gaussian density kernel. Reproduced from: Bloom-Feshbach K, Alonso WJ, Charu V, Tamerius J, Simonsen L, Miller MA, Viboud C. Latitudinal Variations in Seasonal Activity of Influenza and Respiratory Syncytial Virus (RSV): A Global Comparative Review. PLOS ONE 2013;8:e54445.
Maternal immunisation strategies
Inspired by the success of the maternal tetanus elimination program and the influenza and pertussis vaccination strategies,25,27 manufacturers and policy makers are exploring the potential for maternal vaccination against RSV to significantly reduce the burden of RSV disease. While this approach potentially offers significant benefits over active infant immunisation in terms of protection in the first 2 to 4 months of life, it has a fundamental limitation in that any vaccine-induced protection will subsequently wane. Understanding the nature and duration of this protection will be critical in determining how such a strategy could be best employed, especially in light of the seasonality of RSV infections.
Support for a potentially protective role for trans-placental protection antibodies against RSV was provided by a US study of pregnant women previously experiencing natural RSV infection, demonstrating that higher titers of neutralising maternal antibodies near the onset of illness were correlated with partial protection against RSV severe infection in infants, and high titers at birth delayed the age of first RSV infection.28 In a Danish cohort a clear temporal association was found between the incidence of RSV hospitalisations in infants younger than 6 months and the mean maternal RSV neutralising antibodies titers in cord blood.29 In addition, the proven protective effect of passive immunisation via palivizumab,30,31 a monoclonal antibody targeting a neutralising epitope at the antigenic site A of RSV F protein,32 provides direct evidence of the protective effect of passively acquired neutralising antibodies; it is in light of this that vaccine-induced antibodies that compete with palivizumab to bind at this site (palivizumab competitive antibodies, PCA) have been adopted as a potential correlate of protection.
At present two phase II trials have evaluated maternal RSV immunisation in pregnant women. Munoz ET AL. randomised 35 US women in the third trimester of pregnancy to receive either a vaccine based on purified fusion protein (RSV-PFP-2 subunit) or placebo. The study raised no safety concerns, with no enhanced disease observed in either population.33 In the second trial investigators randomised 50 pregnant women in their third trimester to receive one dose of an RSV F protein nanoparticle vaccine or placebo. There were no safety concerns in the vaccine group, and no enhanced disease was observed in the infants of vaccinated mothers.34 Although some concerns have been raised by disappointing efficacy data for RSV prevention in the elderly for a non-adjuvanted version of this vaccine, an ongoing phase 3 efficacy trial will address the efficacy of this vaccine for prevention of infant RSV.35
With potentially effective maternal vaccines against RSV in the pipeline, the appropriate timing of any such immunisation campaign needs to be identified. While superficially there would be some similarities with seasonal maternal influenza vaccination campaigns,36,37 the little data that are available does not suggest a significant burden of RSV disease in pregnancy.38,39 On that basis, the overriding objective of maternal RSV vaccination would be protection of the neonate, in contrast to influenza immunisation that aims to protect both mother and infant.
Maternal RSV immunisation in temperate climates
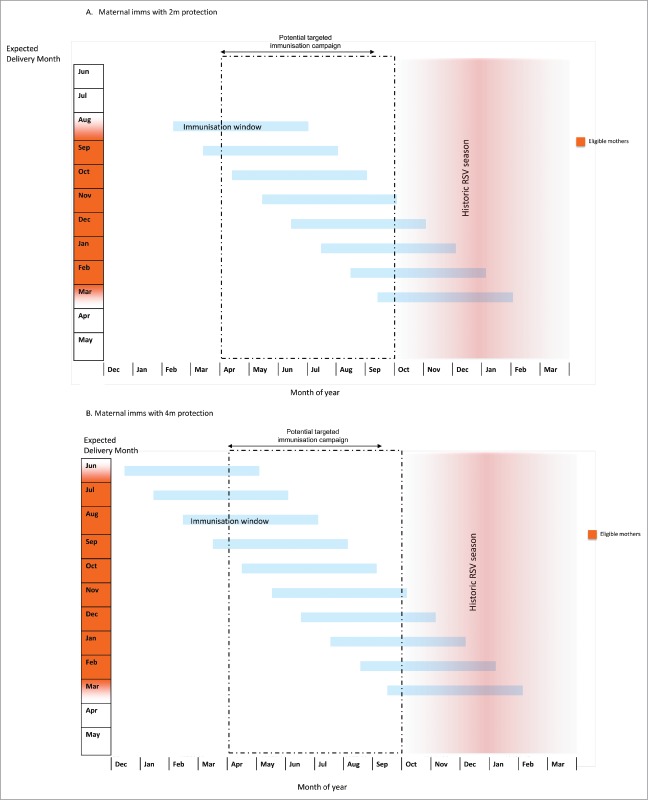
In temperate climates, babies due to be born during, or shortly before, the RSV season could potentially benefit from maternal RSV immunisation, while babies due to be born outside this period are unlikely to do so (Fig. 2). As one example, the United Kingdom has a RSV season which typically starts in late October and has mostly finished by the beginning of March. Accordingly, English children less than 1 year of age with respiratory infections who were born in September to November were more than twice as likely to have RSV detected than those born in December to May.40 The ‘expected due date’ is therefore likely to be a key determinant of which babies born in a temperate climate could potentially benefit from maternal immunisation against RSV, but this will also be influenced by the duration of immune protection afforded. Furthermore, the optimal gestational timing for immunisation will influence the nature of any maternal RSV immunisation campaign.
Figure 2.
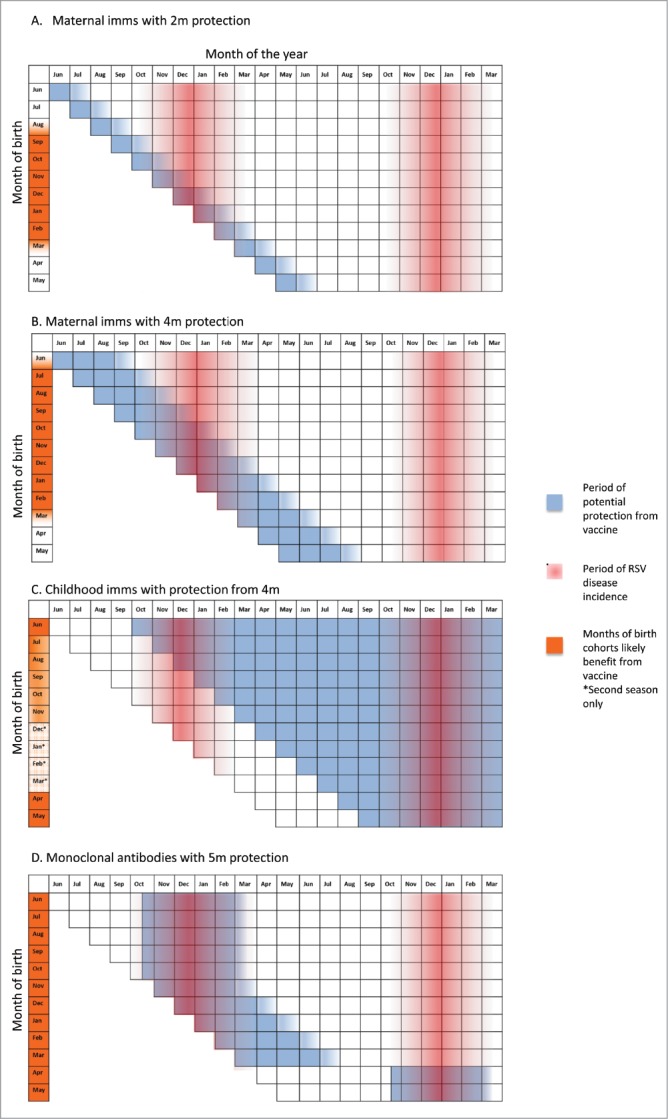
Period of potential protection from RSV infection using different vaccine strategies, using UK as an example of temperate countries assuming RSV season 16th October- 12th March.
Length of protection offered by naturally induced transplacental antibodies against RSV was explored by Brandenburg ET AL., who studied 45 Dutch children with detectable natural maternally derived neutralising antibodies at birth and found that these antibodies decline steadily over the first 3 months of life, with a mean half-life of 26 days; at 6 months of age these antibodies were detectable in only 4.5% of the infants.41 Ochola ET AL. studied 635 Kenyan mother-child pairs and identified that 97% of infants had RSV specific maternal antibodies at birth, with 50% of the children remaining seropositive at 4–5 months of age, with a mean half-life of 2.5 months.42 Chu ET AL. in Bangladesh found a strong correlation between the titers of naturally induced RSV neutralising antibodies in the third trimester and those of infant cord blood, with higher cord blood titers being associated with protection against infection. The half-life of neutralizing antibodies in this study was 38 days, and the median time to fall below a proposed correlate of protection of titers ≥1:256 was 17 weeks (95% CI: 14 to 20 weeks).43
While these studies provide important information about the duration of protection from natural maternal infection, they do not necessarily apply to antibodies induced by maternal immunisation, which aims to surpass naturally induced immunity. In the Munoz study, infants born to vaccine recipients had higher concentrations of anti-F IgG binding antibody to RSV at birth, 2 and 6 months of life than those born to placebo recipients. RSV A and B neutralising antibodies were reported as being higher in infants of vaccine recipients than placebo at 0 and 2 months, although no specific data were provided for this or the statement that the half-life of ‘maternal antibodies’ in infants was ≥3 weeks.33 In the study of the nano-particle vaccine, the half-life of palivizumab competitive antibodies in the infant was 41 days, with a predicted persistence ≥30 μg/mL to 16 weeks; or 14 μg/mL up to 22 weeks.34 Interpretation of these data remains difficult in the absence of an established correlate of protection and this will potentially be one of the most important outcomes of the ongoing efficacy study.44 Of possible relevance to this is a recent study of maternal influenza immunisation in South Africa suggesting little infant protection against influenza infections beyond 2 months of age.45 Regardless, protection from maternal immunisation is unlikely to be an ‘all-or-none’ phenomenon; whatever impact is achieved will be at its greatest immediately after birth, and waning of efficacy is likely to be a progressive decline from this peak over months, rather than a sudden cessation at a given time.
Accordingly Fig. 2 and 3 display, using a descriptive approach, the likely impact of maternal immunisation on different birth cohorts and in different settings, adjusting for different potential durations of protection. If, like antenatal influenza immunisation, antenatal RSV immunisation provided 2 months of protection, then only babies born in January and February would both receive protection throughout their first RSV season in the UK (Fig. 2A). Babies born earlier than this (e.g. in November), would receive protection during the peak of the RSV season, but become vulnerable at 3 months of age in February, when RSV is still circulating. Babies born in September may receive some benefit in their second month of life (October), but would still become vulnerable during the peak of the RSV season. Babies born in April to August in the UK would receive no benefit from maternal RSV immunisation.
Figure 3.
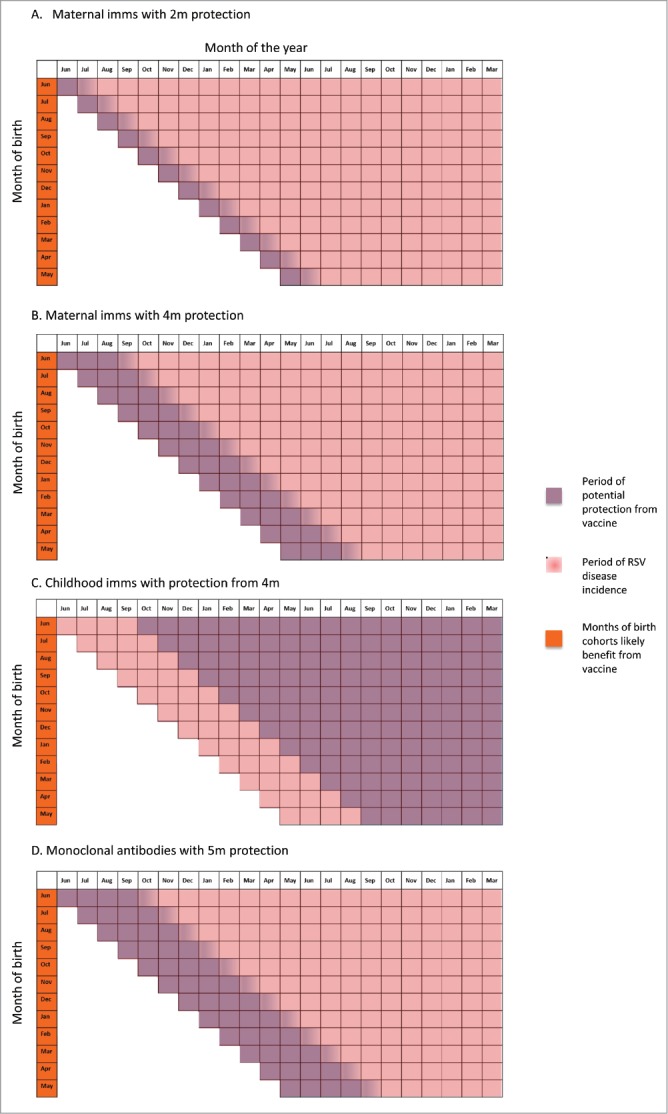
Period of potential protection from RSV infection using different vaccine strategies, tropical countries.
If, however, the vaccine was to provide 4 months protection, then babies born in the UK between October and February would receive protection throughout the peak of their first RSV season (with the greatest potential reduction of disease compared to unimmunised children occurring in those born in October to November) (Fig. 2B). Babies born in June to November may be protected during the early stages of their first RSV season but become susceptible later, while those born in December to February would be protected against the latter stages of the RSV season but have less overall benefit in terms of RSV infections prevented. UK children born after the season from 13th March to June (i.e. approximately 33% of an annual birth cohort) would be unlikely to receive any benefit as they are less likely to have an RSV infection before 4 months of age.
It is worth emphasising that, even though this suggests that only 66% of infants would benefit from a maternal RSV vaccine providing 4 months protection, with sufficient uptake and a highly effective vaccine such an intervention could delay the first RSV infection to the age of 4 months for 41% of the birth cohort (born end of June to November) with babies born in December to February (25% of the birth cohort) having an 8 to 10-month delay to their first RSV infection. Given the age of hospitalisation for RSV infection is skewed towards the first 3 months of life (Fig. 4),46 with a median age of hospital admission of 120 days47 this could potentially dramatically reduce hospital admissions for this illness. By contrast, a vaccine providing only 2 months protection would have less of an impact, with the babies born August to January (50% of the birth cohort) still becoming susceptible to circulating RSV at 2 months of age.
Figure 4.
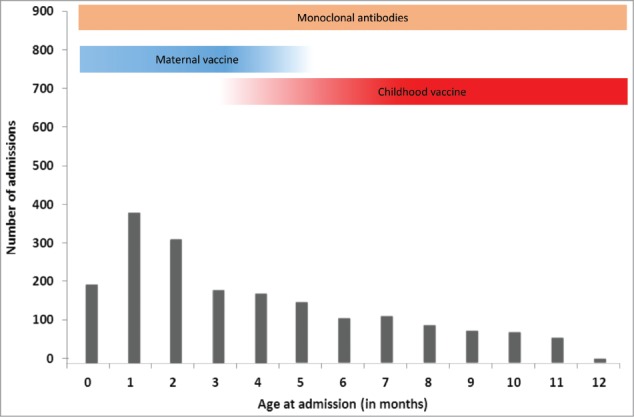
Period of benefit of vaccination strategies on hospital admissions for bronchiolitis in temperate countries (adapted from Parikh ET AL. 2017). Adapted from: Murray J, Bottle A, Sharland M, Modi N, Aylin P, Majeed A, Saxena S, Medicines for Neonates Investigator Group. Risk factors for hospital admission with RSV bronchiolitis in England: a population-based birth cohort study. PloS one. 2014 Feb 26;9(2):e89186.
Optimal gestational age for immunisation to induce trans-placental antibodies
The UK guidelines for maternal immunisation against pertussis were recently modified to reduce the minimum gestational age from 26 to 16 weeks in light of observational data suggesting that early second trimester immunisation with tetanus-diphtheria-acellular pertussis vaccine resulted in significantly higher vaccine-induced neonatal antibodies than third trimester immunisations.48,49 Potentially, an effective antenatal vaccine against RSV could also be given at this early gestational age, but regardless immunisation against RSV would ideally be administered well before 36 weeks gestation to allow sufficient time for the mother's immune system to respond prior to birth.
As shown in Fig. 5, a possible gestational window of 16 to 36 weeks suggests an RSV vaccine providing 4 months protection could be administered between January and June to women expecting to deliver in July, and between August and January to women expecting to deliver in February. Alternatively, the campaign could be focussed on the months of April to September, with all ‘eligible’ women being at an appropriate gestation within this period (i.e. women with expected due dates in August would be immunised from April, and those with expected due dates in March would be immunised in September).
Figure 5.
Suggested maternal RSV immunisation strategy by expected due date (EDD) in United Kingdom (Northern temperate climate), assuming (A) 2 and (B) 4-month period of protection from trans-placental antibodies. Assuming RSV season 16th October- 12th March. Dotted box represents potential targeted immunisation campaign for pregnant women between 16 weeks and 36 weeks gestation in the UK.
Antenatal RSV immunisation in a tropical/sub-tropical region
In contrast to temperate climates, in many tropical and subtropical areas prolonged RSV seasons have been identified. Taiwan is reported as having a 10 month RSV season,50 and similarly sustained RSV seasons are reported in Hawaii,51 Hong Kong,21 Lombok island, Indonesia,52 Florida (USA),53 Singapore,54 and crowded urban cities such as Dhaka,55 Bangkok,56 Benin City,57 and Kampala.58 In such regions maximal reduction in the burden of RSV disease could only be achieved by a year-round maternal RSV vaccination program, and even with this all infants are likely to experience ongoing exposure to RSV as their transplacental immunity wanes. Therefore, while a maternal immunisation programme with an effective RSV vaccine is likely to provide protection during the period of greatest vulnerability to severe RSV disease (i.e. the first 2 to 4 months of life),59,60 in countries with prolonged RSV seasons each monthly cohort will continue to experience RSV infections at the point their transplacental immunity wanes (Fig. 3).
Active immunisation in infants and children
Paediatric RSV vaccine development has been challenging, especially in light of the deaths and increased morbidity due to vaccine-enhanced-disease in seronegative infants receiving a formalin-inactivated RSV (FI-RSV) vaccine in the 1960s. Fortunately this is now an area of great activity, and there are currently seven vaccines being developed for use in infants in phase 1 to 2 clinical trials.61 Two of these candidate vaccines use viral vectors with genetic inserts coding for RSV antigens.62,63 Two candidate vaccines use particle-based technology, including an intranasal administration of RSV F protein carried on a bacterium-like-particle, and a folded RSV F nano-protein with aluminium adjuvant.64,65 Three live- attenuated vaccines, currently being trialled in RSV sero-negative children, contain genetic deletions coding for viral replication and protein downregulation.66,67 In January 2017, a phase II clinical trial was started of a gene-based vector vaccine against RSV to evaluate safety and immunogenicity in healthy RSV sero-positive children aged 12 to 17 months compared with placebo.62
Direct protection afforded by infant immunisation
The early peak of infant RSV disease burden creates an inherent challenge for direct prevention by infant immunisation, given most infant vaccine schedules commence at 6 to 8 weeks and require multiple doses to achieve full effectiveness. In this regard there are parallels with immunisation against pertussis disease, a disease that has also required a maternal immunisation campaign to maintain control in the absence of herd immunity.25 It is highly likely that an active vaccine would be used year-round as part of a routine schedule. Despite this, some children would remain susceptible during their first RSV season, and during their most vulnerable months of age. For example, a child born in the UK in October and immunised in a 2, 3, 4-month infant schedule (i.e. December, January, February) would remain susceptible during their first RSV season (Fig. 2C) and any benefit would depend on immune protection extending to their second season (with or without a booster dose at 12 months of age). In comparison, a child born in March is likely to receive protection upon their first exposure to RSV from the age of 7 months. While children experiencing RSV disease at 9 to 12 months are less likely to experience severe disease or be hospitalised, preventing RSV infection would have other benefits including reduction of subsequent viral induced wheeze.68
An alternative approach for temperate countries would be to immunise all children under 1 year shortly before the RSV season (e.g. in August/September in the Northern Hemisphere), similar to influenza vaccines. However, this may be more challenging programmatically. By contrast, in countries with year-round RSV exposure, there would be a benefit to all birth cohorts, but all children born in these areas will still remain susceptible during their first few months of life while they develop protection from multiple dose active immunisation (Fig. 3C). Future data on immunogenicity of the paediatric RSV vaccines, currently in early clinical trial phases, will better inform the right immunisation strategy to be implemented in each setting.
An important consideration with active infant immunisation is the potential for this to be combined with maternal immunisation. This could achieve the most comprehensive protection from RSV infection during the child's first RSV season, and may be of particular benefit in countries with perennial RSV exposure. One important aspect to consider with such a combined approach is the potential for maternally derived antibodies to interfere with the responses to infant immunisation. This has been observed for maternal immunisation with a combination diphtheria, tetanus, acellular pertussis, polio and Haemophilus influenza type B vaccine, which has inhibited infant responses to pertussis, diphtheria and some CRM-conjugated vaccine antigens in the routine infant schedule.69 Should this be the case, this would further support the argument to limit maternal RSV immunisation to those children expected to be born in an ‘at-risk’ period.
Herd immunity
In addition to providing direct protection, active paediatric RSV immunisation could potentially provide benefits through reduced circulation of the RSV virus (herd immunity) if long-term immunity is induced. Children under 5 years of age are the group predominantly responsible for transmission,70 suggesting that by vaccinating this age group it is likely that a reduction in disease in unvaccinated age groups would also be seen. Modelling work by Yamin ET AL. and Poletti ET AL. suggest that immunisation strategies to ensure immunity across this age range could reduce RSV burden in infants and adults.70,71 Future studies using immunogenicity data from phase II and III trials, once available, should inform these models further.
Passive immunisation
Passive immunisation with monoclonal antibody (palivizumab) has been used widely in high risk populations as an effective method to protect such individuals from RSV infection, and this is the only approach to RSV prevention with proven efficacy. A seasonal course of monthly immunisations over five months is the preferred schedule given to high-risk preterms in most temperate climate countries. Two new monoclonal antibodies recently entered clinical trials. In 2015, a phase III clinical trial started comparing a one or two-dose schedule of a monoclonal antibody to placebo in healthy preterm children; unfortunately this has recently been stopped due to lack of efficacy and further development of this product has been halted.8,9 In 2016, a phase IIb trial started comparing a one –dose monoclonal antibody to placebo in healthy preterm children using altered Fc binding to generate an extended half life.7 If a single dose of the monoclonal antibody in development at the beginning of RSV season could provide 5 months protection,72 this could potentially provide the most direct way of preventing the seasonal peak of RSV disease in temperate countries, during the age of highest vulnerability and (depending on cost effectiveness) could be expanded from high risk infants to all children entering their first RSV season.
An effective annual, universal campaign of this nature could render unnecessary maternal and active infant immunisation in temperate climates. However, unlike active infant immunisation it could not provide herd immunity, as children would acquire RSV infections in their second RSV season. In contrast, in tropical regions children would become susceptible from 5 months of age, although this could still protect infants during the age of highest vulnerability. The use of monthly immunisations with palivizumab over a 6 month period to at risk preterm newborns in Taiwan was shown to be effective in reducing RSV related hospitalisations across their prolonged (10 month) RSV season by 86% within 6 months after discharge independently of the time of the year.50,73 Alternative schedules may need to be applied to cover the different patterns of RSV season. Other areas with perennial RSV season such as coastal areas in the sub/tropical regions face the same dilemma observed in Taiwan.13 The use of monoclonal antibodies to provide optimal reduction in the burden of RSV disease would require administration to all children within the first few weeks of life. The feasibility of this would, of course be heavily dependent on the cost of this product; which would need to be significantly lower than the estimated average 2016–2017 seasonal cost of palivizumab treatment per child which ranges from $3221 to $12,568, (£2500-£9600; €2725-€ 10625) for this to be affordable.74
Conclusion
The benefits of an RSV immunisation programme that selectively immunises children born at the periods of greatest risk for RSV disease are supported by a recent UK cost-effectiveness analysis suggesting that 9 of the 10 most cost-effective strategies focussed disease prevention on babies born in 4 months of the year or fewer.75 This analysis also emphasised the critical importance of prevention of disease in the first few months of life (whether by monoclonal antibodies or maternal immunisation) to a cost-effective RSV prevention programme, with an incremental direct benefit of active immunisation providing protection from 3 months onwards.
Nevertheless, RSV prevention policy needs to be adapted to the seasonal patterns of RSV, to protect the most vulnerable citizens from severe disease; in tropical regions with longer, more variable RSV seasons a year-round immunisation schedule for both maternal and infant immunisation might be appropriate. It is therefore critical that, in anticipation of the availability of effective maternal or paediatric RSV vaccines, each country obtain accurate epidemiological RSV surveillance data in order to inform future decisions about the most appropriate immunisation schedule for their local circumstances.
Abbreviations
- FI-RSV
formalin-inactivated respiratory syncytial virus
- LRTI
lower respiratory tract infection
- PCA
Palivizumab competitive antibodies
- RSV
Respiratory Syncytial Virus
- UK
United Kingdom
- US/USA
United States of America
Disclosure of potential conflicts of interest
Dr. Snape has acted as Principal Investigator on behalf of the University of Oxford for research studies funded by GlaxoSmithKline, Pfizer, Novartis Vaccines, Medimmune, Novavax and Johnson and Johnson. Prior to 2017 he received assistance from vaccine manufacturers to attend conferences, participated in advisory boards for vaccine manufacturers and spoke at industry-sponsored symposia. Payments for these activities were made to the University of Oxford and Dr. Snape received no personal financial benefit. The other authors disclose no conflict of interest.
Funding
This work received no grant funding.
References
- 1.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, ET AL.. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green CA, Yeates D, Goldacre A, Sande C, Parslow RC, McShane P, Pollard AJ, Goldacre MJ. Admission to hospital for bronchiolitis in England: trends over five decades, geographical variation and association with perinatal characteristics and subsequent asthma. Arch Dis Child. 2015;101:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89:449–63. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 4.Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969;89:435–48. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- 5.Murphy BR, Alling DW, Snyder MH, Walsh EE, Prince GA, Chanock RM, Hemming VG, Rodriguez WJ, Kim HW, Graham BS. Effect of age and preexisting antibody on serum antibody response of infants and children to the F and G glycoproteins during respiratory syncytial virus infection. J Clin Microbiol. 1986;24:894–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins D, Trujillo C, Keech C. Advances in RSV vaccine research and development - A global agenda. Vaccine. 2016;34:2870–75. doi: 10.1016/j.vaccine.2016.03.109. [DOI] [PubMed] [Google Scholar]
- 7.MedImmune A Phase 2b Randomized, Double-Blind, Placebo-controlled Study to Evaluate the Safety and Efficacy of MEDI8897, a Monoclonal Antibody With an Extended Half-life Against Respiratory Syncytial Virus, in Healthy Preterm Infants. ClinicalTrials.gov; 2016. Available at: https://clinicaltrials.gov/ct2/show/NCT02878330?term=rsv+medimmune&rank=19. [Google Scholar]
- 8.Regeneron pharmaceuticals. Regeneron to Discontinue Development of Suptavumab for Respiratory Syncytial Virus. Cision PR Newswire. 2017. Available at: https://www.prnewswire.com/news-releases/regeneron-to-discontinue-development-of-suptavumab-for-respiratory-syncytial-virus-300503716.html. [Google Scholar]
- 9.Regeneron A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Efficacy and Safety of a Human Monoclonal Antibody, REGN2222, for the Prevention of Medically Attended RSV Infection in Preterm Infants. ClinicalTrials.gov; 2014. Available at: https://clinicaltrials.gov/ct2/show/NCT02325791?term=rsv+regeneron&rank=1. [Google Scholar]
- 10.Terletskaia-Ladwig E, Enders G, Schalasta G, Enders M. Defining the timing of respiratory syncytial virus (RSV) outbreaks: an epidemiological study. BMC Infectious Diseases. 2005;5:20. doi: 10.1186/1471-2334-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber MW, Mulholland EK, Greenwood BM. Respiratory syncytial virus infection in tropical and developing countries. Trop Med Int Health. 1998;3:268–80. doi: 10.1046/j.1365-3156.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- 12.Hogan AB, Anderssen RS, Davis S, Moore HC, Lim FJ, Fathima P, Glass K. Time series analysis of RSV and bronchiolitis seasonality in temperate and tropical Western Australia. Epidemics. 2016;16:49–55. doi: 10.1016/j.epidem.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Stensballe LG, Devasundaram JK, Simoes EA. Respiratory syncytial virus epidemics: the ups and downs of a seasonal virus. Pediatr Infect Dis J. 2003;22:S21–32. doi: 10.1097/01.inf.0000053882.70365.c9. [DOI] [PubMed] [Google Scholar]
- 14.Public Health England Respiratory syncytial virus (RSV): symptoms, transmission, prevention, treatment; 2008. Available at: https://www.gov.uk/government/publications/respiratory-syncytial-virus-rsv-symptoms-transmission-prevention-treatment.
- 15.Hall CB, Long CE, Schnabel KC. Respiratory Syncytial Virus Infections in Previously Healthy Working Adults. Clin Infect Dis. 2001;33:792–6. doi: 10.1086/322657. [DOI] [PubMed] [Google Scholar]
- 16.Bloom-Feshbach K, Alonso WJ, Charu V, Tamerius J, Simonsen L, Miller MA, Viboud C. Latitudinal Variations in Seasonal Activity of Influenza and Respiratory Syncytial Virus (RSV): A Global Comparative Review. PLoS One. 2013;8:3–4. doi: 10.1371/journal.pone.0054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colosia AD, Masaquel A, Hall CB, Barrett AM, Mahadevia PJ, Yogev R. Residential crowding and severe respiratory syncytial virus disease among infants and young children: a systematic literature review. BMC Infect Dis. 2012;12:95. doi: 10.1186/1471-2334-12-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yusuf S, Piedimonte G, Auais A, Demmler G, Krishnan S, Van Caeseele P, Singleton R, Broor S, Parveen S, Avendano L, ET AL.. The relationship of meteorological conditions to the epidemic activity of respiratory syncytial virus. Epidemiol Infect. 2007;135:1077–90. doi: 10.1017/S095026880600776X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welliver RC. Temperature, humidity, and ultraviolet B radiation predict community respiratory syncytial virus activity. Pediatr Infect Dis J. 2007;26:S29–S35. doi: 10.1097/INF.0b013e318157da59. [DOI] [PubMed] [Google Scholar]
- 20.Lapeña S, Robles M, Castanon L. Climatic factors and lower respiratory tract infection due to respiratory syncytial virus in hospitalised infants in northern Spain. Eur J Epidemiol. 2005;20:271–6. doi: 10.1007/s10654-004-4539-6. [DOI] [PubMed] [Google Scholar]
- 21.Chan P, Sung R. Epidemiology of respiratory syncytial virus infection among paediatric patients in Hong Kong: seasonality and disease impact. Epidemiol. 1999;123:257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang JW, Loh T. Correlations between climate factors and incidence-a contributor to RSV seasonality. Reviews in Medical Virology. 2014;24:15–34. doi: 10.1002/rmv.1771. [DOI] [PubMed] [Google Scholar]
- 23.Pineros JG, Baquero H, Bastidas J, García J, Ovalle O, Patiño CM, Restrepo JC. Respiratory syncytial virus infection as a cause of hospitalization in population under 1 year in Colombia. J Pediatr (Rio. J). 2013;89:544–548. doi: 10.1016/j.jped.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Stensballe LG, Devasundaram JK, Simoes EA. Respiratory syncytial virus epidemics: The ups and downs of a seasonal virus. Pediatr Infect Dis J. 2003;22:S21–32. doi: 10.1097/01.inf.0000053882.70365.c9. [DOI] [PubMed] [Google Scholar]
- 25.Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Donegan K, Fry NK, Miller E, Ramsay M. Effectiveness of maternal pertussis vaccination in England: An observational study. Lancet. 2014;384:1521–8. doi: 10.1016/S0140-6736(14)60686-3. [DOI] [PubMed] [Google Scholar]
- 26.Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, Omer SB, Shahid NS, Breiman RF, Steinhoff MC. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–64. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 27.Roper MH, Vandelaer JH, Gasse FL. Maternal and neonatal tetanus. Lancet. 2007;370:1947–59. doi: 10.1016/S0140-6736(07)61261-6. [DOI] [PubMed] [Google Scholar]
- 28.Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981;98:708–15. doi: 10.1016/S0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- 29.Stensballe LG, Ravn H, Kristensen K, Meakins T, Aaby P, Simoes EA. Seasonal variation of maternally derived respiratory syncytial virus antibodies and association with infant hospitalizations for respiratory syncytial virus. J Pediatr. 2009;154:296–8. doi: 10.1016/j.jpeds.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 30.Wegzyn C, Toh LK, Notario G, Biguenet S, Unnebrink K, Park C, Makari D, Norton M. Safety and Effectiveness of Palivizumab in Children at High Risk of Serious Disease Due to Respiratory Syncytial Virus Infection: A Systematic Review. Infect Dis Ther. 2014;3:133–158. doi: 10.1007/s40121-014-0046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The IMpact-RSV Study Group Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Paediatrics. 1998;102:531–7. doi: 10.1542/peds.102.3.531. [DOI] [PubMed] [Google Scholar]
- 32.FDA Synagis (Palivizumab); 1996. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2002/palimed102302LB.pdf.
- 33.Munoz FM, Piedra PA, Glezen WP. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine. 2003;21:3465–67. doi: 10.1016/S0264-410X(03)00352-9. [DOI] [PubMed] [Google Scholar]
- 34.August A, Novavax. RSV F Vaccine: Phase 2 Clinical Trial to Protect Infants via Maternal Immunization. 2015. Available at: http://novavax.com/download/files/presentations/FIGO_7OCT2015_AA_P2_Data_10_14_15_FINAL(1).pdf. [Google Scholar]
- 35.GlaxoSmithKline An Observer-blind Safety and Reactogenicity Study to Assess GlaxoSmithKline (GSK) Biologicals' Investigational Respiratory Syncytial Virus (RSV) Vaccine (GSK3003891A) in Healthy Women. ClinicalTrials.gov; 2016. Available at: https://clinicaltrials.gov/ct2/show/NCT02753413?term=gsk+rsv&rank=1. [Google Scholar]
- 36.Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm SR, ET AL.. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 37.Rasmussen SA, Jamieson DJ, Uyeki TM. Effects of influenza on pregnant women and infants. Am J Obstet Gynecol. 2012;207:S3–S8. doi: 10.1016/j.ajog.2012.06.068. [DOI] [PubMed] [Google Scholar]
- 38.Chu HY, Katz J, Tielsch J, Khatry SK, Shrestha L, LeClerq SC, Magaret A, Kuypers J, Steinhoff MC, Englund JA. Clinical Presentation and Birth Outcomes Associated with Respiratory Syncytial Virus Infection in Pregnancy. PLoS One. 2016;11:e0152015. doi: 10.1371/journal.pone.0152015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wheeler SM, Dotters-Katz S, Heine RP, Grotegut CA, Swamy GK. Maternal effects of respiratory syncytial virus infection during pregnancy. Emerg Infect Dis. 2015;21:1951–55. doi: 10.3201/eid2111.150497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reeves RM, Hardelid P, Gilbert R, Warburton F, Ellis J, Pebody RG. Estimating the burden of respiratory syncytial virus (RSV) on respiratory hospital admissions in children less than five years of age in England, 2007-2012. Influenza Other Respi Viruses. 2017;11:122–29. doi: 10.1111/irv.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brandenburg AH, Groen J, van Steensel-Moll HA, Claas EC, Rothbarth PH, Neijens HJ, Osterhaus AD. Respiratory syncytial virus specific serum antibodies in infants under six months of age: limited serological response upon infection. J Med Virol. 1997;52:97–104. doi:. [DOI] [PubMed] [Google Scholar]
- 42.Ochola R, Sande C, Fegan G, Scott PD, Medley GF, Cane PA, Nokes DJ. The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLoS One. 2009;4:e8088. doi: 10.1371/journal.pone.0008088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chu HY, Steinhoff MC, Magaret A, Zaman K, Roy E, Langdon G, Formica MA, Walsh EE, Englund JA. Respiratory syncytial virus transplacental antibody transfer and kinetics in mother-infant pairs in Bangladesh. J Infect Dis. 2014;210:1582–89. doi: 10.1093/infdis/jiu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clinicaltrials.gov A Study to Determine the Safety and Efficacy of the RSV F Vaccine to Protect Infants Via Maternal Immunization - Full Text View - ClinicalTrials.gov. National Library of Medicine (U.S.). 2017. Available at: https://clinicaltrials.gov/ct2/show/NCT02624947. [Google Scholar]
- 45.Nunes MC, Cutland CL, Jones S, Hugo A, Madimabe R, Simões EA, Weinberg A, Madhi SA. Duration of Infant Protection Against Influenza Illness Conferred by Maternal Immunization. JAMA Pediatr. 2016;170:840. doi: 10.1001/jamapediatrics.2016.0921. [DOI] [PubMed] [Google Scholar]
- 46.Parikh RC, McLaurin KK, Margulis AV, Mauskopf J, Ambrose CS, Pavilack M, Candrilli SD. Chronologic Age at Hospitalization for Respiratory Syncytial Virus Among Preterm and Term Infants in the United States. Infect Dis Ther. 2017;6:477–486. doi: 10.1007/s40121-017-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murray J, Bottle A, Sharland M, Modi N, Aylin P, Majeed A, Saxena S. Risk Factors for Hospital Admission with RSV Bronchiolitis in England: A Population-Based Birth Cohort Study. PLoS One. 2014;9:e89186. doi: 10.1371/journal.pone.0089186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eberhardt CS, Blanchard-Rohner G, Lemaître B, Boukrid M, Combescure C, Othenin-Girard V, Chilin A, Petre J, de Tejada BM, Siegrist CA. Maternal immunization earlier in pregnancy maximizes antibody transfer and expected infant seropositivity against pertussis. Clin Infect Dis. 2016;62:829–36. doi: 10.1093/cid/ciw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.NHS Whooping cough vaccination in pregnancy. NHS choices; 2016. Available at: http://www.nhs.uk/conditions/pregnancy-and-baby/pages/whooping-cough-vaccination-pregnant.aspx#When. [Google Scholar]
- 50.Hsu C.-H, Lin CY, Chi H, Chang JH, Hung HY, Kao HA, Peng CC, Jim WT. Prolonged seasonality of respiratory syncytial virus infection among preterm infants in a subtropical climate. PLoS One. 2014;9:e110166. doi: 10.1371/journal.pone.0110166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reese PE, Marchette NJ. Respiratory syncytial virus infection and prevalence of subgroups A and B in Hawaii. J Clin Microbiol. 1991;29:2614–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Omer SB, Sutanto A, Sarwo H, Linehan M, Djelantik IG, Mercer D, Moniaga V, Moulton LH, Widjaya A, Muljati P, ET AL.. Climatic, temporal, and geographic characteristics of respiratory syncytial virus disease in a tropical island population. Epidemiol Infect. 2008;136:1319–27. doi: 10.1017/S0950268807000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bauman J, Eggleston M, Oquist N, Malinoski F. Respiratory syncytial virus: seasonal data for regions of Florida and implications for palivizumab. South Med J. 2007;100:669–76. doi: 10.1097/SMJ.0b013e318048589e. [DOI] [PubMed] [Google Scholar]
- 54.Chew FT, Doraisingham S, Ling AE, Kumarasinghe G, Lee BW. Seasonal trends of viral respiratory tract infections in the tropics. Epidemiology and Infection. 1998;121:121–8. doi: 10.1017/S0950268898008905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huq F, Rahman M, Nahar N, Alam A, Dhaka C. Acute Lower Respiratory Tract Infection Due to Virus among Hospitalized Children in Dhaka, Bangladesh. Rev Med Virol. 1990;12:982–7. [DOI] [PubMed] [Google Scholar]
- 56.Vathanophas K, Sangchai RA. Community-Based Study of Acute Respiratory Tract Infection in Thai Children. Rev Med Virol. 1990;12:957–65. [DOI] [PubMed] [Google Scholar]
- 57.Nwankwo M, Dym A, Schuit K, Offor E, Omene J. Seasonal variation in respiratory syncytial virus infections in children in Benin-City, Nigeria. Trop Geogr Med. 1988;40:309–13. [PubMed] [Google Scholar]
- 58.Sobĕslavský O, Sebikari SR, Harland PS, Skrtić N, Fayinka OA, Soneji AD. The viral etiology of acute respiratory infections in children in Uganda. Bull World Health Organ. 1977;55:625–31. [PMC free article] [PubMed] [Google Scholar]
- 59.Bardach A, Rey-Ares L, Cafferata ML, Cormick G, Romano M, Ruvinsky S, Savy V. Systematic review and meta-analysis of respiratory syncytial virus infection epidemiology in Latin America. Rev Med Virol. 2014;24:76–89. doi: 10.1002/rmv.1775. [DOI] [PubMed] [Google Scholar]
- 60.Johnson A.-W, Aderele WI, Osinusi K, Gbadero DA, Fagbami AH, Rotowa NA. Acute bronchiolitis in tropical Africa: A hospital-based perspective in Ibadan, Nigeria. Pediatr Pulmonol. 1996;22:236–47. doi:. [DOI] [PubMed] [Google Scholar]
- 61.PATH RSV Vaccine and mAb Snapshot - PATH; 2017. Available at: http://www.path.org/publications/files/CVIA_rsv_snapshot_final.pdf.
- 62.U.S. National Institutes of Health A Study to Evaluate Safety and Immunogenicity of GSK Biologicals' RSV Investigational Vaccine in RSV-seropositive Infants Aged 12 to 17 Months. ClinicalTrials.gov. 2017; Available at: https://www.clinicaltrials.gov/ct2/show/NCT02927873?term=rsv+vaccine+gsk&rank=6. [Google Scholar]
- 63.Widjojoatmodjo MN, Bogaert L, Meek B, Zahn R, Vellinga J, Custers J, Serroyen J, Radošević K, Schuitemaker H. Recombinant low-seroprevalent adenoviral vectors Ad26 and Ad35 expressing the respiratory syncytial virus (RSV) fusion protein induce protective immunity against RSV infection in cotton rats. Vaccine. 2015;33:5406–14. doi: 10.1016/j.vaccine.2015.08.056. [DOI] [PubMed] [Google Scholar]
- 64.Clinicaltrials.gov Phase I Study for SynGEM, an Intranasal Respiratory Syncytial Virus (RSV) Prefusion F Subunit Candidate Vaccine - Full Text View - ClinicalTrials.gov. National Library of Medicine (U.S.). 2017. Available at: https://clinicaltrials.gov/ct2/show/NCT02958540. [Google Scholar]
- 65.Glenn G. RSV F Recombinant Nanoparticle Vaccine: Summary of Clinical Data. Novavax.com. 2014. Available at: http://novavax.com/download/files/presentations/Glenn_RSV_F_Recombinant_Nanoparticle_Vaccine_JH.pdf. [Google Scholar]
- 66.Clinicaltrials.gov Infectivity, Safety and Immunogenicity of a Recombinant Live-Attenuated Respiratory Syncytial Virus Vaccine (D46/NS2/N/ΔM2-2-HindIII) in RSV-Seronegative Infants and Children 6 to 24 Months of Age - Full Text View - ClinicalTrials.gov. National Library of Medicine (U.S.). 2017. Available at: https://clinicaltrials.gov/ct2/show/NCT03099291. [Google Scholar]
- 67.Clinicaltrials.gov Evaluating the Infectivity, Safety, and Immunogenicity of the Recombinant Live-Attenuated Respiratory Syncytial Virus Vaccines RSV ΔNS2/Δ1313/I1314L or RSV 276 in RSV-Seronegative Infants 6 to 24 Months of Age - Full Text View - ClinicalTrials.gov. National Library of Medicine (U.S.). 2017. Available at: https://clinicaltrials.gov/ct2/show/NCT03227029. [Google Scholar]
- 68.Bont L, Ramilo O. The relationship between RSV bronchiolitis and recurrent wheeze: the chicken and the egg. Early Hum Dev. 2011;87 Suppl 1:S51–4. doi: 10.1016/j.earlhumdev.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 69.Ladhani SN, Andrews NJ, Southern J, Jones CE, Amirthalingam G, Waight PA, England A, Matheson M, Bai X, Findlow H, ET AL.. Antibody responses after primary immunization in infants born to women receiving a Pertussis-containing vaccine during pregnancy: Single arm observational study with a historical comparator. Clin Infect Dis. 2015;61:1637–44. doi: 10.1093/cid/civ695. [DOI] [PubMed] [Google Scholar]
- 70.Yamin D, Jones FK, DeVincenzo JP, Gertler S, Kobiler O, Townsend JP, Galvani AP. Vaccination strategies against respiratory syncytial virus. Proc Natl Acad Sci USA. 2016;113:13239–44. doi: 10.1073/pnas.1522597113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Poletti P, Merler S, Ajelli M, Manfredi P, Munywoki PK, Nokes D, Melegaro A. Evaluating vaccination strategies for reducing infant respiratory syncytial virus infection in low-income settings. BMC Med. 2015;13:49. doi: 10.1186/s12916-015-0283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Griffin MP, Khan AA, Esser MT, Jensen K, Takas T, Kankam MK, Villafana T, Dubovsky F. Safety, Tolerability, and Pharmacokinetics of MEDI8897, the Respiratory Syncytial Virus Prefusion F-Targeting Monoclonal Antibody with an Extended Half-Life, in Healthy Adults. Antimicrob. Agents Chemother. 2017;61:e01714–16. doi: 10.1128/AAC.01714-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chi H, Hsu CH, Chang JH, Chiu NC, Hung HY, Kao HA, Weng LC, Huang FY, Chiu YY, Chang LY, ET AL.. A novel six consecutive monthly doses of palivizumab prophylaxis protocol for the prevention of respiratory syncytial virus infection in high-risk preterm infants in Taiwan. PLoS One. 2014;9:e100981. doi: 10.1371/journal.pone.0100981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shahabi A, Peneva D, Incerti D, Mclaurin K. Assessing Variation in the Cost of Palivizumab for Respiratory Syncytial Virus Prevention in Preterm Infants. PharmacoEconomics - Open. 2017:1–9. doi: 10.1007/s41669-017-0042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cromer D, van Hoek AJ, Newall AT, Pollard AJ, Jit M. Burden of paediatric respiratory syncytial virus disease and potential effect of different immunisation strategies: a modelling and cost-effectiveness analysis for England. Lancet Public Health. 2017;2:e367–e374. doi: 10.1016/S2468-2667(17)30103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]