ABSTRACT
Staphylococcus aureus produces an antiphagocytic polysaccharide capsule to evade neutrophil-mediated killing. Many vaccines against encapsulated bacterial pathogens require generation of functional anti-capsular antibodies to mediate protection against infection and disease. Here it is shown that the generation of such antibody responses to S. aureus in vivo and in vitro requires the presence of O-acetyl modifications on the capsular polysaccharides. O-acetylation of S. aureus capsular polysaccharide therefore should be monitored carefully during vaccine development and production. This finding may provide additional insight into the previous failure of a S. aureus capsular polysaccharide conjugate vaccine.
KEYWORDS: Staphylococcus aureus, capsular polysaccharide, vaccine, antibody response, O-acetylation
Introduction
The medical need for a Staphylococcus aureus vaccine is clear. Although S. aureus is carried asymptomatically in the nares of 20–50% of the general population,1 colonization increases the risk of infection, ranging from relatively mild skin infections, such as impetigo, to life-threatening invasive disease. S. aureus is recognized as a leading cause of morbidity and mortality in both healthcare-associated and community settings. In particular in surgical patients, these infections carry high mortality rates and survivors of S. aureus surgical infections require an additional 13–17 days in the hospital, significantly increasing healthcare costs.2 The burden of S. aureus disease is exacerbated by the emergence of S. aureus isolates that are resistant to new classes of antibiotics, highlighting the need for alternative approaches such as a prophylactic vaccine.
The human host employs several mechanisms to prevent S. aureus infection. These include mucosal and epithelial layers that act as a barrier to infection, and engulfment and killing of bacteria by professional phagocytes, such as neutrophils. However, S. aureus exploits virulence mechanisms that counteract such host defenses, thus facilitating establishment of an infection. These virulence mechanisms include scavenging of essential nutrients from blood, adhesion to host tissues and immune evasion.3, 4 Therefore, a successful vaccine will need to neutralize such virulence mechanisms.
Capsular polysaccharides help bacteria evade immune-mediated killing through inhibiting phagocytosis.5, 6 Vaccine-induced antibodies against capsular polysaccharides can overcome this virulence mechanism by enabling the organism to be opsonized and subsequently phagocytosed. All invasive human S. aureus isolates encode the genes required to express either type 5 or type 8 capsule (denoted CP5 and CP8, respectively),7,7. and most adults have anticapsular antibodies, demonstrating that the capsule is expressed in the host environment.8 Bacterial polysaccharides often contain an array of substituents, such as O-acetyl, phosphate, sialic acid, which may constitute an important part of the immunodominant epitopes.
Both S. aureus CP5 and CP8 are comprised of 2-acetamido-2-deoxy-D-mannuronic acid (ManNAcA), 2-acetamido-2-deoxy-L-fucose (L-FucNAc), and 2-acetamido-2-deoxy-D-fucose. Both capsules are O-acetylated and differ in the stereochemical glycosidic linkages between the sugars and the site of O-acetylation. The sites of O-acetylation are the 3′OH moiety of L-FucNAc for CP5 and 4′OH substituent of ManNAcA for CP59. Here, it is shown that O-acetylation of capsular polysaccharides is critical for the induction of protective anticapsular antibody responses upon vaccination.
Materials and methods
Production of de-O-Acetylated S. aureus capsular polysaccharide conjugates
CP8 and CP5 were isolated as previously described10 from CP5 expresing strain BD285 and CP8 expressing strain BD287, then de-O-acetylated by incubation in 0.2 N NaOH or 0.2 N TBOH, respectively (Sigma) at 37ºC for 4 h. Samples were neutralized with HCl, washed with water, and resuspended at ∼4 mg/mL as determined with 4-hydroxybenzoic acid hydrazide (PAHBAH). Removal of O-acetyl groups was verified by size exclusion chromatography multi angle laser light scattering (SEC MALLS). De-O-acetylated CP5 and CP8 and native CP5 were conjugated to diphtheria toxin mutant CRM197 via reductive amidation.
Opsonophagocytic activity assay (OPA)
OPAs were performed as previously described.5 Baby rabbit complement (BRC) was used as a source of complement in the assay containing bacteria (either CP5-expressing S. aureus strain PFESA0158 or CP8-expressing S. aureus strain PFESA0186, both clinical isolates), serially diluted heat-treated serum and HL-60 cells (Pel-Freez, Cat. #: 31061 3). An OPA antibody titer was defined as the reciprocal of the highest serum dilution resulting in 50% reduction of the number of bacterial colonies after incubation for 60 min at 37ºC when compared to the background control from which serum was omitted. Samples without detectable activity at the lowest serum dilution of 100 were assigned OPA titer values of 50.
Murine pyelonephritis model
All animal studies were conducted under the guidance of the local and global institutional animal care and use committee at an AAALAC-accredited facility. Female CD-1 mice (20/group) were immunized three times subcutaneously on weeks 0, 3 and 6 with 1 μg of de-O-acetylated CP5-CRM197 +AlPO4, de-O-acetylated CP8-CRM197 +AlPO4, native CP5-CRM197 + AlPO4, or vehicle alone (AlPO4). Two weeks after the final immunization, mice were challenged with ∼3 × 108 CFU S. aureus Reynolds. Kidneys were harvested 48 h after infection, homogenized, and serial dilutions plated on tryptic soy agar. Bacterial colonies were enumerated after 24 h of incubation at 37ºC.
Statistical analysis
All statistical comparisons were performed using unpaired Student's t test with Welch's Correction by GraphPad Prism software v7.02.
Results and discussion
The presence of O-acetyl groups in capsular polysaccharides has been observed in a wide range of pathogenic bacteria, including N. meningitidis serogroup A and S. pneumoniae 9V. However, the role of O-acetylation in the immunogenicity and pathogenicity of microorganisms cannot be generalized. While capsular polysaccharide O-acetylation induces functional responses against N. meningitidis serogroup A,11 for S. pneumoniae 9V12 the presence of O-acetyl groups mask epitopes important for protective immune responses, thus misdirecting the immune response and allowing bacterial immune evasion.13
To determine if de-O-acetylated CP5-CRM197 elicited a protective immune response after immunization, a pyelonephritis model of disseminated end-organ infection was used. Female CD-1 mice were immunized three times with O-acetylated (OAc) or de-O-acetylated (deOAc) CP5-CRM197 adjuvanted with AlPO4, or AlPO4-containing vehicle alone, and subsequently challenged with S. aureus. Use of vehicle resulted in a high kidney burden of 106 CFU. Although immunization with de-O-acetylated CP5-CRM197 reduced S. aureus burden in the kidneys compared to vehicle alone, O-acetylated CP5-CRM197 reduced kidney bacterial burden by over 2 logs more than the de-O-acetylated CP5-CRM197, a statistically significant decrease (Figure 1, p < 0.0001). To test whether O-acetylation of S. aureus capsular polysaccharides impacts generation of functional immune responses, it was first established that a dose of 0.01 mcg of capsular polysaccharide conjugate was the lowest threshold dose level able to induce opsonophagocytic antibodies (data not shown). The use of a low threshold dose allowed for the sensitive discrimination of differences in elicitation of opsonophagocytic antibodies. Female CD-1 mice were immunized three times with either native or de-O-acetylated CP5-CRM197 or native or de-O-acetylated CP8-CRM197, each in the presence of AlPO4. Sera were collected one week after the last immunization and assessed for opsonophagocytic activity (OPA, Figure 2). Neither de-O-acetylated CP5-CRM197 nor de-O-acetylated CP8-CRM197 were able to elicit detectable OPA responses in all but one animal, while their native counterparts elicited OPA responses in the majority of animals (Figure 2). This result indicates that immunization with the de-O-acetylated polysaccharide was unable to protect in vivo due to the inability to induce functional antibodies that resulted in opsonophagocytic killing.
Figure 1.
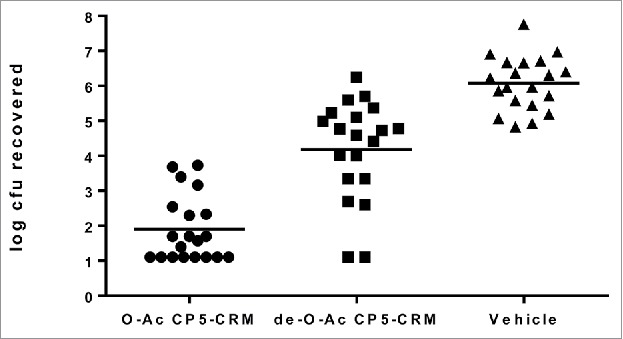
O-acetylation is necessary for an effective protective vaccine response in a murine pyelonephritis model. Female CD-1 mice (20/group) were immunized three times with 0.01 mcg of O-acetylated (native) CP5-CRM197 with AlPO4, de-O-acetylated CP5-CRM197 with AlPO4, or AlPO4 alone, then challenged with S. aureus. Bacterial burden in the kidneys was determined two days after challenge.
Figure 2.
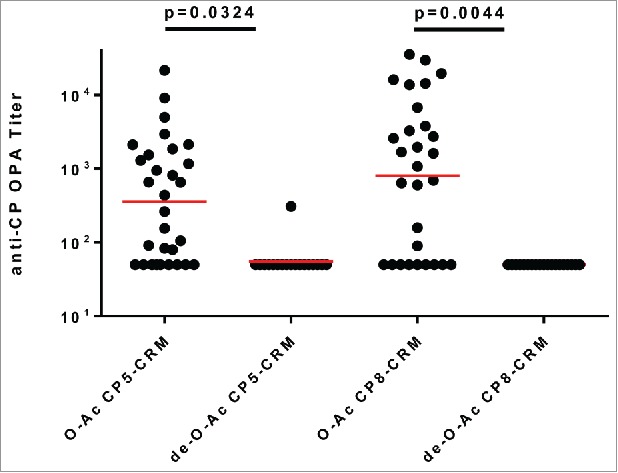
O-acetylation is necessary for capsular polysaccharide conjugates to induce effective opsonophagocytic killing responses. Female CD-1 mice (20/group) were immunized three times with 0.01 mcg of O-acetylated (native) CP5-CRM197 with AlPO4, de-O-acetylated CP5-CRM197 with AlPO4, O-acetylated (native) CP8-CRM197 with AlPO4, or de-O-acetylated CP8-CRM197 with AlPO4 and OPA titers from week 7 sera were assessed.
S. aureus CP-CRM197 conjugates have previously been shown to induce robust bacterial killing responses as measured in OPA (5, Figure 2) and capsular polysaccharide is known to be an important immune evasion mechanism for S. aureus. Studies by Fattom et al.14 suggested that antibodies to both OAc and non-OAc epitopes of S. aureus CP5 and CP8 opsonized O-acetylated isolates and are functional and efficient against variations in O-acetylation of capsules polysaccharides expressed by clinical isolates. In contrast, here we have demonstrated that O-acetylation is required to induce effective functional killing responses to S. aureus as measured both in vitro by OPA killing assay, and in vivo by protection in the murine pyelonephritis model. Although there were experimental differences between the studies, such as the carrier protein used for the conjugate (CRM197 vs Pseudomonas aeruginosa exotoxin A), and the details of the opsonophagocytic assays used, which could have contributed to the divergent observations in the studies, it should also be noted that the Fattom et al study conducted the OPAs with highly purified rabbit IgG. In vivo, immunization with non-O-acetylated CP5 did reduce recovered CFU compared to vehicle alone (Figure 1); likewise the Fattom et al study could have been observing this minor effect using highly purified rabbit IgG. Using a competitive luminex immunoassay, which monitors the ability of immunization-induced antibodies to compete with a functional monoclonal antibody, serum from animals immunized with de-O-acetylated CP5 and CP8 conjugates had very low titers compared to animals immunized with O-acetylated CP5 and CP8 conjugates (data not shown). Together, these results support the hypothesis that de-O-acetylated conjugates are poor functional immunogens.
A tetra-antigen S. aureus vaccine (SA4Ag) is currently in development, which contains capsular polysaccharide types 5 and 8 conjugated to CRM197 as well as clumping factor A (ClfA) and the manganese transport component MntC. Pivotal vaccine trials with an earlier capsular polysaccharide conjugate vaccine were not successful. While this capsular polysaccharide conjugate vaccine did show a 57% decrease in bacteremia versus controls in hemodialysis patients, no significant protection was observed in a larger phase 3 trial.15 These results sharply contrast the striking successes of capsular polysaccharide-based vaccines in protecting against disease caused by Hemophilus influenzae type B (HiB), Neisseria meningitidis types A, C, Y and W and Streptococcus pneumoniae. The reasons for this failure have been speculated about. One factor is that there may have been issues with consistency of manufacture of the phase 3 clinical trial material, due to changes in contract facilities used, although the specific impacts of these changes were not further explained.15 It is also possible that the assays used to monitor vaccine quality were not sufficiently sensitive to detect important critical quality attributes. Lack of access to the material used in the phase 3 study precludes a direct comparison between earlier results and the data presented here. The data presented here indicates that O-acetylation of the S. aureus capsular polysaccharide is critical for vaccine-induced functional immune responses in vitro and in vivo, and variations therein should be minimized to ensure consistent functional immune responses and efficacy.
Disclosure of potential conflicts of interest
All authors are employees of Pfizer, Inc., and as such may hold stock and/or stock options. This work was presented at the Staphylococcus aureus Gordon Conference (July 2013, Waterville Valley, NH).
Acknowledgments
The authors would like to thank Pfizer comparative medicine staff for excellent technical support, John Jay Buckley for preparation of the deO-acetylated capsular polysaccharide material, and Natalie Silmon de Monerri, Ed Buurman, Paul Liberator, David Cooper and Kathrin Jansen for critical review of the manuscript.
Funding
The work contained in this manuscript was funded by Pfizer.
References
- 1.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clinical microbiology reviews 1997;10:505–20. PMID:9227864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noskin GA, Rubin RJ, Schentag JJ, Kluytmans J, Hedblom EC, Jacobson C, et al.. National trends in Staphylococcus aureus infection rates: impact on economic burden and mortality over a 6-year period (1998-2003). Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 2007;45:1132–40. doi: 10.1086/522186. PMID:17918074 [DOI] [PubMed] [Google Scholar]
- 3.Anderson AS, Miller AA, Donald RG, Scully IL, Nanra JS, Cooper D, et al.. Development of a multicomponent Staphylococcus aureus vaccine designed to counter multiple bacterial virulence factors. Hum Vaccin Immunother 2012;8:1585–94. doi: 10.4161/hv.21872. PMID:22922765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dayan GH, Mohamed N, Scully IL, Cooper D, Begier E, Eiden J, et al.. Staphylococcus aureus: the current state of disease, pathophysiology and strategies for prevention. Expert review of vaccines 2016:1–20. [DOI] [PubMed] [Google Scholar]
- 5.Nanra JS, Buitrago SM, Crawford S, Ng J, Fink PS, Hawkins J, et al.. Capsular polysaccharides are an important immune evasion mechanism for Staphylococcus aureus. Human vaccines & immunotherapeutics 2012;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thakker M, Park JS, Carey V, Lee JC. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect Immun 1998;66:5183–9. PMID:9784520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy E, Lin SL, Nunez L, Andrew L, Fink PS, Dilts DA, et al.. Challenges for the evaluation of Staphylococcus aureus protein based vaccines: monitoring antigenic diversity. Hum Vaccin 2011;7 Suppl:51–9. doi: 10.4161/hv.7.0.14562. PMID:21245656 [DOI] [PubMed] [Google Scholar]
- 8.Nissen M, Marshall H, Richmond P, Shakib S, Jiang Q, Cooper D, et al.. A randomized phase I study of the safety and immunogenicity of three ascending dose levels of a 3-antigen Staphylococcus aureus vaccine (SA3Ag) in healthy adults. Vaccine 2015. doi: 10.1016/j.vaccine.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 9.Jones C. Revised structures for the capsular polysaccharides from Staphylococcus aureus Types 5 and 8, components of novel glycoconjugate vaccines. Carbohydrate research 2005;340:1097–106. doi: 10.1016/j.carres.2005.02.001. PMID:15797125 [DOI] [PubMed] [Google Scholar]
- 10.Fattom A, Schneerson R, Szu SC, Vann WF, Shiloach J, Karakawa WW, et al.. Synthesis and immunologic properties in mice of vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides conjugated to Pseudomonas aeruginosa exotoxin A. Infection and immunity 1990;58:2367–74. PMID:2114365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berry DS, Lynn F, Lee CH, Frasch CE, Bash MC. Effect of O acetylation of Neisseria meningitidis serogroup A capsular polysaccharide on development of functional immune responses. Infection and immunity 2002;70:3707–13. doi: 10.1128/IAI.70.7.3707-3713.2002. PMID:12065513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNeely TB, Staub JM, Rusk CM, Blum MJ, Donnelly JJ. Antibody responses to capsular polysaccharide backbone and O-acetate side groups of Streptococcus pneumoniae type 9V in humans and rhesus macaques. Infection and immunity 1998;66:3705–10. PMID:9673252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fusco PC, Farley EK, Huang CH, Moore S, Michon F. Protective meningococcal capsular polysaccharide epitopes and the role of O acetylation. Clinical and vaccine immunology: CVI 2007;14:577–84. doi: 10.1128/CVI.00009-07. PMID:17376859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fattom AI, Sarwar J, Basham L, Ennifar S, Naso R. Antigenic determinants of Staphylococcus aureus type 5 and type 8 capsular polysaccharide vaccines. Infection and immunity 1998;66:4588–92. PMID:9746554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matalon AM MBJ, Block G, Hohenboken M, Fattom A, Horwirth G, Rasmussen H, Damasco S, Boutriau D. Efficacy profile of a bivalent staphylococcus aureus glycoconjugate investigational vaccine in adults on haemodialysis: Phase III randomized study. International Symposium on Staphylococcal Infections Lyon, France, 2012. [Google Scholar]


