Abstract
Pattern recognition receptors (PRRs) are critical to the early detection and innate immune responses to pathogens. In particular, the TLR system and its associated adaptor proteins play essential roles in early host responses to infection. Epidemic keratoconjunctivitis, caused by the human adenovirus, is a severe ocular surface infection associated with corneal inflammation (stromal keratitis). We previously showed that adenovirus capsid was a key molecular pattern in adenovirus keratitis, with viral DNA playing a lesser role. We have now investigated the role of the adaptor molecule MyD88 in a mouse model of adenovirus keratitis in which there is no viral replication. In MyD88−/− mice infected with human adenovirus type 37, clinical keratitis was markedly reduced, along with infiltration of CD45+ cells, and expression of inflammatory cytokines. Reduction of inflammatory cytokines was also observed in infected primary human corneal fibroblasts pretreated with a MyD88 inhibitory peptide. Keratitis similar to wild type mice was observed in TLR2, TLR9, and IL-1R knockout mice, but was reduced in TLR2/9 double knockout mice, consistent with synergy of TLR2 and TLR9 in the response to adenovirus infection. MyD88 co-immunoprecipitated with Src kinase in mice corneas and in human corneal fibroblasts infected with adenovirus, and MyD88 inhibitory peptide reduced Src phosphorylation, linking MyD88 activation to inflammatory gene expression through a signaling cascade previously shown to be directed by Src. Our findings reveal a critical role for the PRRs TLR2 and 9, and their adaptor protein MyD88, in corneal inflammation upon adenovirus infection.
Introduction
Human adenoviruses (HAdVs) are nonenveloped, double-stranded DNA viruses that broadly infect mucosal epithelia and cause significant morbidity and mortality.1 Therapeutic measures against infection by HAdV are currently limited to the relatively toxic antiviral cidofovir and its derivatives, adenovirus-specific donor T cells in allogeneic stem cell transplant recipients,2 and vaccination directed against adenovirus pneumonia caused by HAdV types 4 and 7, in use only for US military personnel.3 Existing evidence in animal models of adenovirus disease points to a critical role for innate immunity in the most serious manifestations of infection,4 suggesting that therapeutics targeted at the host immune system might mitigate the morbidity of clinical disease. In the very first stage of infection, specific ligands on the surface of the adenovirus interact with both primary and secondary host cell receptors to mediate viral entry and subsequent intracellular trafficking. The same binding events can simultaneously trigger the expression of proinflammatory mediators. For example, the interaction between adenovirus capsid fiber protein of HAdV-C5 with the cellular coxsackie–adenovirus receptor in vitro activates intracellular signaling pathways leading to the expression of IL-6.5 A secondary interaction thought to be crucial to adenovirus entry involves binding of the Arg-Gly-Asp (RGD) motifs on adenovirus penton base capsomer with host cell integrins αvβ1, αvβ3, αvβ5, α5β1, and αMβ2.6–9 This results in integrin aggregation and intracellular signaling, which in turn mediate virus internalization,10, 11 protection from apoptosis,12 and expression of proinflammatory chemokines by the infected cell.13 Adenovirus capsid induces chemokine expression in vitro in the absence of viral DNA.14, 15 In vivo activation of splenic macrophages by the adenovirus protein capsid induced IL-1α expression in an integrin (β3) dependent fashion,16 and adenovirus capsid without viral DNA triggered innate immune responses in a mouse model of adenovirus keratitis in which viral replication is both absent and unnecessary for induction of inflammation.17, 18 Adenovirus nucleic acid also contributes directly to cytokine expression after adenovirus infection.19–21
In general terms, the earliest immune responses to microbial infection occur upon the recognition of microbial signatures known as pathogen-associated molecular patterns (PAMPs), typically located on the microbe’s surface or its nucleic acids, by host molecular pattern recognition receptors (PRRs).22 PAMPs tend to be broadly expressed across the microbial spectrum and are distinct from microbial antigens presented to lymphocytes by antigen presenting cells. Examples of PAMPs include lipopolysaccharide, peptidoglycan, flagellin, unmethlyated CpG DNA of bacteria and viruses, and double-stranded viral RNA. Stimulation of PRRs by PAMPs initiates highly conserved intracellular signaling cascades leading to antimicrobial innate immune responses. Central to molecular pattern recognition in humans are the TLRs.23 There are ten TLRs in humans, including the transmembrane molecules expressed within endosomes (TLR3, TLR7, and TLR9) and those on the cell surface (the remainder). MyD88, first identified as a myeloid differentiation primary response gene in 1990,24 is an adapter molecule recruited to dimerized TLRs upon activation by PAMPs. TLR signaling is either MyD88-dependent or independent.25 MyD88-independent TLR signaling occurs through the Toll/IL-1 receptor domain-containing adaptor, TRIF.26 All TLRs recruit MyD88 upon dimerization except TLR3, while TLR4 signals through both MyD88 and TRIF.26, 27 MyD88 also participates in signaling through several interleukin receptors, including IL-1R1, IL-18R, and IL-33R.28–30 Following its activation, MyD88 forms an oligomer with one or more IL-1R kinases, mediating the activation of various transcription factors, including IRF5 and IRF7, AP-1, and NF-kB, depending on the cell type and the cell surface receptor stimulation.31
The human cornea is an avascular tissue forming the outermost component of the eye, and consists of epithelial, stromal, and endothelial layers. The middle stromal layer contains predominantly extracellular matrix, interspersed by a highly interconnected web of fibroblast-like cells known as keratocytes.32 Smaller numbers of dendritic cells and macrophages, accounting for 5–10% of total corneal stromal cell content, also reside in the anterior and posterior stroma, respectively.33, 34 Corneal transparency is determined by a precise arrangement of collagen fibrils and other extracellular matrix components, but also by keratocyte crystalline expression.35 Keratocytes respond robustly to infection and mechanical trauma by expressing chemokines in significant quantities.36 Experimental infection of human keratocytes with adenoviruses induces chemokine expression well before viral gene expression,37 similar to the innate immune responses seen upon transduction by adenoviral vectors used in gene therapy.38
In this study, we show that MyD88 is required for adenovirus keratitis in a mouse model. MyD88 recruitment did not contribute to viral entry, but suppression of MyD88 activation significantly reduced adenoviral early gene expression. Although the individual effects of TLR2 and TLR9 knockout on adenovirus keratitis were modest, adenovirus keratitis was markedly reduced in TLR2/9−/− mice, suggesting a synergistic activation of TLR2 and TLR9. Moreover, MyD88 appeared to regulate inflammatory responses in adenovirus keratitis through an interaction with Src kinase, previously shown as a key upstream signaling molecule in adenovirus keratitis.37
Results
MyD88 is required in adenovirus keratitis
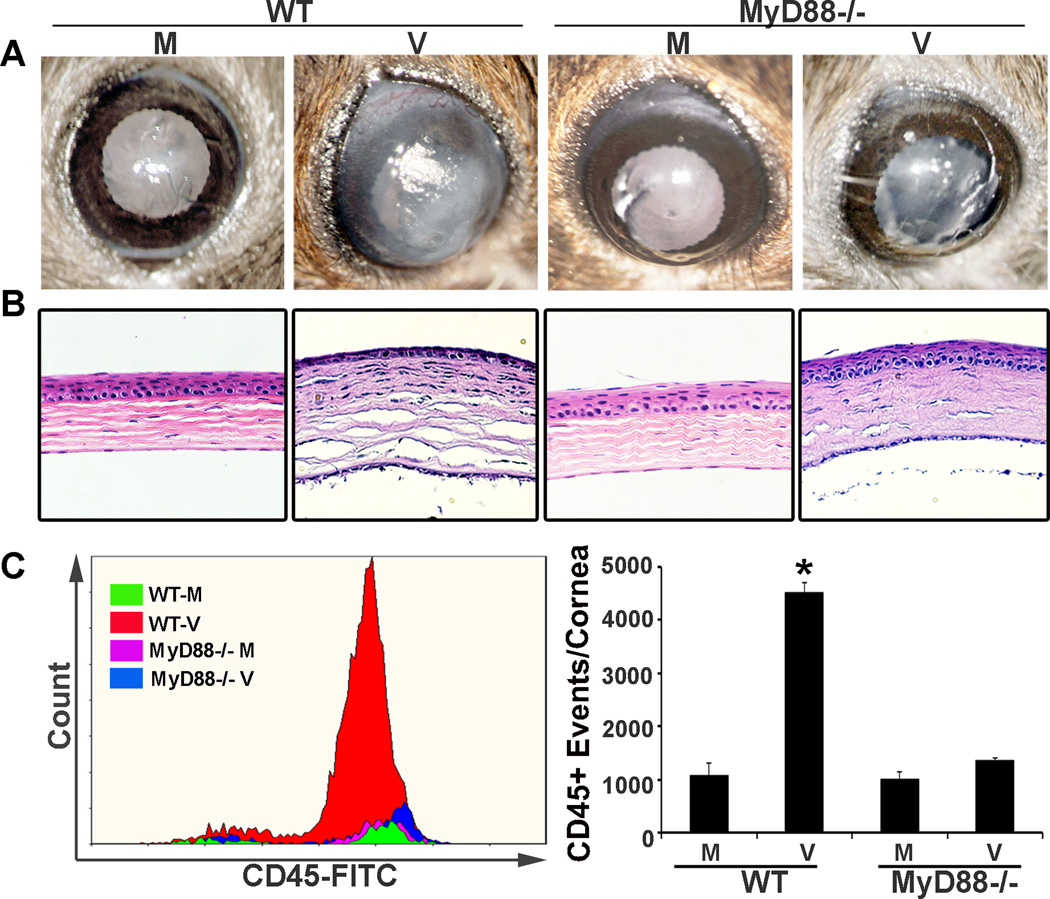
To determine a potential role for MyD88 in adenovirus keratitis, the corneas of wild type (WT) C57BL/6j and MyD88−/− mice on a C57BL/6j background were injected intrastromally with 1 µl of 105 TCID50 HAdV-D37 or virus-free dialysis buffer control, as previously described.17, 18 Clinical photographs and histology with H&E staining, both shown at 4 dpi, demonstrate widespread corneal opacity and stromal infiltration in infected WT corneas as compared to MyD88−/− corneas (Figs. 1A and 1B), as can be seen by loss of iris detail in the former. The latter developed only mild opacity clinically and minimal cell infiltration by histology. Previous studies have shown the infiltration of neutrophils at 1 dpi and activated monocytes at 4 dpi (both are CD45+), in the adenovirus mouse keratitis model using WT mice.17, 18 Flow cytometry performed 4 dpi for total CD45+ cell infiltration (Fig. 1C), demonstrated levels of CD45+ events in virus infected MyD88−/− corneas similar to that seen in mock infection (1363+/−54 vs. 997+/−154 CD45+ events, respectively), whereas CD45+ events in WT corneas were significantly increased by infection as compared to mock (4511+/−183 events vs. 1086 +/− 233; p<.05, ANOVA). As previously demonstrated for the adenovirus mouse keratitis model,17 virus replication was absent in both WT and MyD88−/− corneas over the first 4 dpi (data not shown).
FIGURE 1.
MyD88−/− keratitis after HAdV-D37 infection. The corneas of WT C57BL/6j and MyD88−/− mice on a C57BL/6j background were injected intrastromally with 1 µl of 105 TCID50 HAdV-D37 or virus-free dialysis buffer control. Representative clinical photographs (A) and histology with H&E staining (B), both at 4 dpi, show central corneal opacity and stromal infiltration, respectively, in infected WT corneas as compared to MyD88−/− corneas. Flow cytometry (C) performed 4 dpi for CD45+ cell infiltration shows significantly more CD45+ events in infected WT than in similarly infected MyD88−/− corneas (*p<0.05). Error bars represent SD of the mean (n=3 mice per group). No keratitis or cellular infiltration was observed in corneas injected with virus-free dialysis buffer (mock infection). Similar results were obtained in three independent experiments.
Absence of MyD88 reduces cytokine expression in adenovirus infection
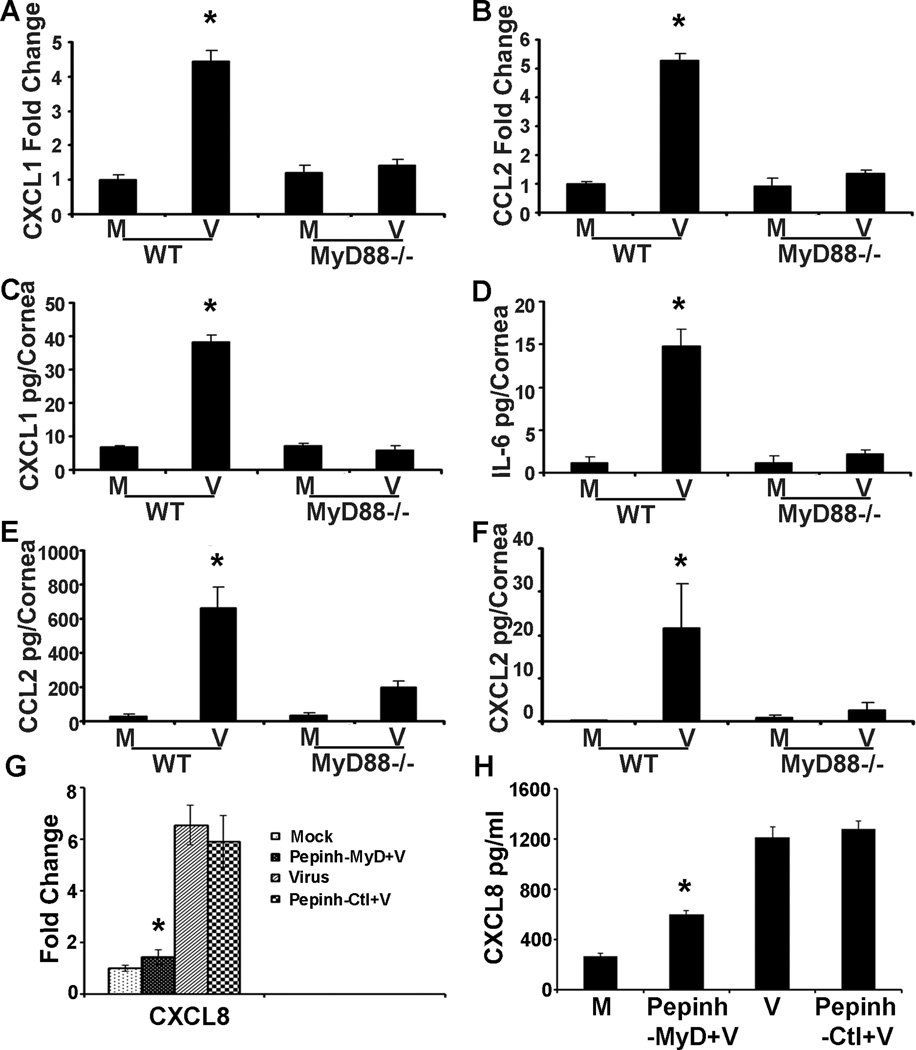
Adenovirus keratitis in the C57BL/6j mouse has been previously shown to involve expression of CXCL1 and 2, CCL2, and IL-6.17 Deficiency of CXCL1 and its receptor, CXCR2, reduces neutrophil infiltration in the acute keratitis.39 We compared cytokine expression in WT and MyD88−/− mice by qRT-PCR at 4 hpi, and by ELISA at 16 hpi, times previously identified to show early cytokine mRNA and protein expression, respectively.17 By real-time qRT-PCR, virus infection increased the expression of CXCL1 and CCL2 mRNA (Figs. 2A and B) in WT but not MyD88−/− corneas (CXCL1 fold change: 4.4+/−0.3 vs. 1.4+/−0.2, respectively as compared to mock infection; CCL2 fold change: 5.3+/−0.2 vs. 1.3+/−0.1, respectively; p<.05 for both comparisons). By ELISA, corneal protein expression of proinflammatory cytokines increased after infection in WT but not MyD88−/− mice (Figs. 2C–F; CXCL1 pg/cornea: 38.2+/− 2.0 vs. 5.7+/−1.5, respectively; IL-6: 14.8+/−2.0 vs. 2.1+/−0.5; CCL2: 667.8+/−115.2 vs. 199.7+/−37.7; CXCL2: 21.7+/−10.0 vs. 2.5+/−1.8; p<.05 for each comparison). In human corneal fibroblasts, the earliest proinflammatory cytokines expressed during in vitro infection by adenovirus include CXCL8, CCL2, and IL-6.37 We pretreated HCF with a MyD88 inhibitory peptide (InvivoGen) or a control peptide for 6 hr prior to infection and performed real-time qRT-PCR (Fig. 2G) and ELISA (Fig. 2H) for CXCL8 expression at 1 and 4 hpi, respectively. By both assays, MyD88 inhibitory peptide significantly reduced CXCL8 expression as compared to treatment with the control peptide (fold change: 1.4+/−0.3 vs. 5.9+/−1.3; pg/ml: 597.7+/−30.8 vs. 1280.1+/−59.3, respectively; p<.05 for both comparisons).
FIGURE 2.
MyD88−/− mouse corneal and human corneal cell cytokine expression after HAdV-D37 infection. Real-time qRT-PCR performed at 4 hpi shows increased mRNA expression for CXCL1 (A) and CCL2 (B) in WT but not MyD88−/− mouse corneas relative to mock infected corneas. By ELISA at 16 hpi, protein expression of CXCL1 (C), IL-6 (D), CCL2 (E), and CXCL2 (F) increases in WT but not MyD88−/− corneas as compared to controls (n=3 mice per group). HCF pretreated with MyD88 inhibitor peptide (Pepinh-MyD) or control inhibitor (Pepinh-Ctl) at 1 hpi show less CXCL8 gene expression by qRT-PCR (G) and at 4 hpi less CXCL8 protein by ELISA (H) in cells pretreated with the MyD88 inhibitory peptide. Error bars represent SD of the mean. All experiments were performed in triplicate with n=3 (*p<0.05).
Viral gene expression but not viral entry is reduced in MyD88−/− corneas
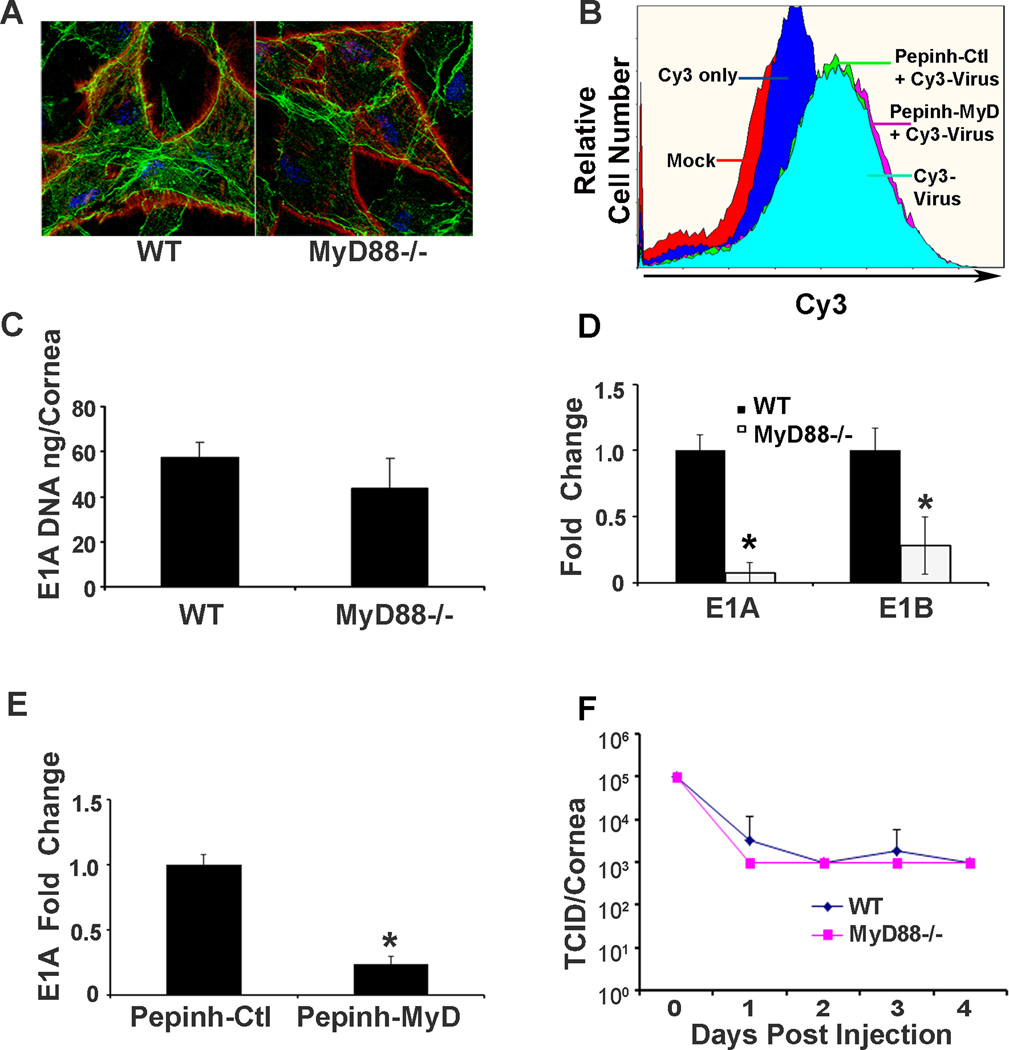
We showed previously that entry of adenovirus into HCF requires Src kinase activity.12 Other previous work suggested a relationship between Src kinase signaling and MyD88,40 so we sought to determine the effect of MyD88 on viral entry. Corneas of WT and MyD88−/− mice injected with Cy3-labeled HAdV-D37 were analyzed by confocal microscopy at 1 hpi. Confocal images suggested qualitatively equal binding to corneal cells of both mouse genotypes (Fig. 3A). We then applied flow cytometry to compare viral binding in HCF pretreated with the MyD88 inhibitory peptide or the control peptide prior to infection with Cy3 labeled virus, also at 1 hpi. The overlay plot indicated no difference in Cy3-labeled virus bound to cells pretreated with the inhibitor peptide as compared to the control peptide (Fig. 3B). qPCR performed at 1 dpi on mouse corneas also showed no significant difference in the amount of viral DNA in MyD88−/− corneas as compared to those of WT mice (Fig 3C, ng/cornea: 43.7+/−13.2 vs. 57.3+/−6.7, respectively; p=.21), consistent with equal viral entry. However, by real-time qRT-PCR, early viral gene expression was significantly reduced in MyD88−/− as compared to WT corneas (Fig. 3D; E1A and E1B: 14.3-fold and 3.6-fold, p=.00167 and p<.00001, respectively), suggesting that MyD88 is directly or indirectly important for early viral gene expression. These data were confirmed by qRT-PCR performed on infected HCF treated with the MyD88 inhibitory peptide (InvivoGen), in which MyD88 inhibition reduced E1A expression by 4.2-fold as compared to treatment with the control peptide (Fig. 3E; p=.001), suggesting a possible effect of MyD88 signaling on intracellular viral trafficking and/or nuclear entry by viral DNA, the latter requisite for viral gene expression. Notably, neither WT or MyD88−/− corneas supported viral replication over 4 dpi (Fig. 3F), consistent with prior studies.17, 18
FIGURE 3.
HAdV-D37 binding, entry, early gene expression, and DNA replication in the MyD88−/− corneas and human corneal fibroblasts treated with a MyD88 inhibitor peptide. Corneas of WT and MyD88−/− mice injected with Cy3-labeled HAdV-D37 (red) and visualized by confocal microscopy at 1 hpi (A) appear to show binding to corneal stromal cells of both mouse genotypes. Slides were co-stained with Topro-3 and phalloidin to visualize nuclei (blue) and cytosolic actin filaments (green). Flow cytometry performed at 1 hpi (B) shows no difference in viral binding to cultured HCF pretreated with the MyD88 inhibitory peptide or the control peptide (Pepinh-MyD, Pepinh-Ctl, respectively) prior to infection with Cy3 labeled virus. Real-time qPCR (C) performed at 1 dpi on mouse corneas shows no significant difference in the quantity of viral DNA in MyD88−/− corneas as compared to those of WT mice (p=.21). However, real-time qRT-PCR (D) for early viral gene expression shows a significant reduction in E1A and E1B expression in MyD88−/− as compared to WT corneas (*p=.00167 and *p<.00001, respectively). By qRT-PCR on infected HCF at 1 hpi (E), pretreatment with MyD88 inhibitory peptide is associated with reduced E1A expression compared to treatment with the control peptide (*p=.001). Viral titers performed over 4 dpi of WT or MyD88−/− corneas (F) show no increase in titer at any time point, indicating an absence of viral replication. Error bars represent SD of the mean. The values shown represent the mean of three separate experiments with n=3 mice per group for each experiment.
MyD88 and Src kinase interact in the adenovirus infected cornea
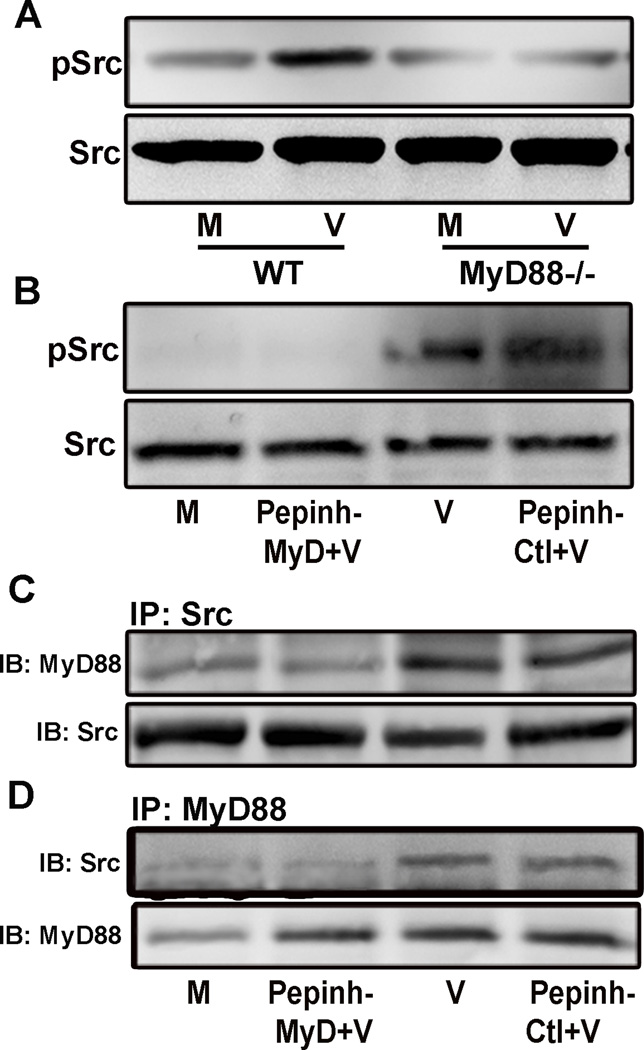
The MyD88 adapter protein is a major midstream mediator of TLR activation. In experimental adenovirus infection of HCF, it was previously shown that Src kinase activation is necessary for both viral entry and expression of proinflammatory mediators, including CXCL8 and CCL2.12, 41 Prior studies in other cell systems have linked Src kinase as a downstream effector of MyD88.40 We performed Western blot analysis of WT and MyD88−/− mouse corneal lysates at 1 hpi, and found Src phosphorylation was reduced in the absence of MyD88 (Fig. 4A). Subsequent experiments in infected HCF pretreated with the MyD88 inhibitory peptide showed reduced Src phosphorylation at 1 hpi in comparison to infected HCF pretreated with the MyD88 control peptide (Fig. 4B). Immunoprecipitates of infected HCF at 2 hpi using antibody against Src contained MyD88 protein by immunoblot (Fig. 4C), and immunoprecipitates using antibody against MyD88 contained Src protein (Fig. 4D). Pretreatment with the MyD88 inhibitory peptide reduced MyD88 in Src immunoprecipitates and Src in MyD88 immunoprecipitates. The MyD88 control peptide had no effect. These data are consistent with a direct or indirect physical interaction between MyD88 and Src kinase during infection.
FIGURE 4.
MyD88 and Src interaction in HAdV-D37 infection. Western blot analysis of WT and MyD88−/− mouse corneal lysates at 1 hpi (A) show increased phospho-Src in WT corneas infected with HAdV-D37, but not in MyD88−/− corneas. Infected HCF pretreated with the MyD88 inhibitory peptide at 1 hpi (B) show reduced phospho-Src in comparison to infected HCF pretreated with the MyD88 control peptide. Immunoprecipitates of infected HCF at 2 hpi using antibody against Src contain MyD88 protein by immunoblot (C), and immunoprecipitates using antibody against MyD88 contain Src protein (D). Pretreatment with the MyD88 inhibitory peptide is associated with reduced signal for MyD88 in Src immunoprecipitates and reduced Src in MyD88 immunoprecipitates, with no effect seen for the MyD88 control peptide. These experiments were performed twice with similar results.
Toll-like receptors 2 and 9 act synergistically in adenovirus keratitis
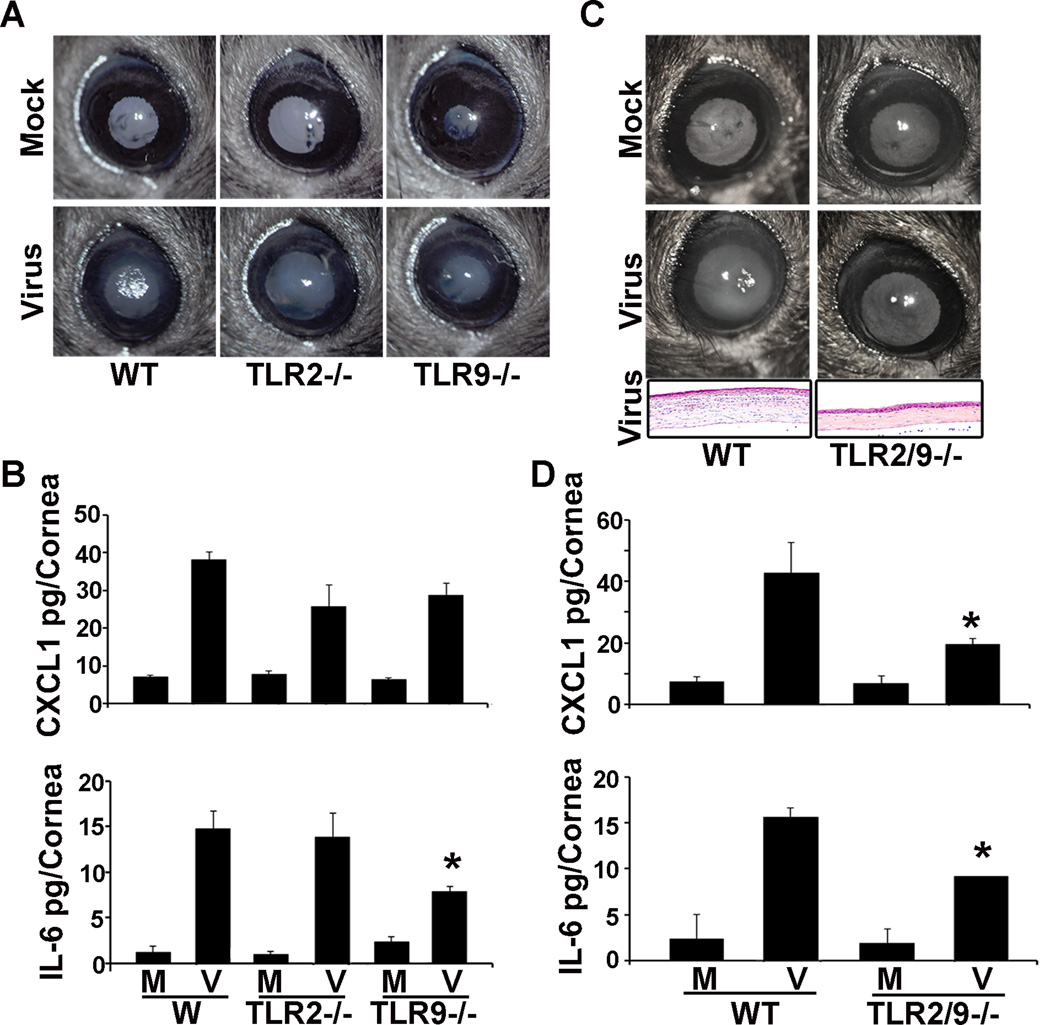
TLR9 has previously been shown as a PRR for human adenovirus DNA.42 TLR2, although known principally as a PRR for bacterial lipoteichoic acid,43 also binds virus coat proteins to induce proinflammatory signaling.44 In addition, TLR2 and 9 can signal cooperatively through a Src mediated pathway,45 and have been shown to synergize the innate immune response to adenoviral vectors.46 We began by comparing clinical keratitis in WT, TLR2−/−, and TLR9−/− mice. At 4 dpi, both wild type and TLR knockout mice demonstrated clinical keratitis, as can be seen as central whitening of the cornea (Fig. 5A). The expression of CXCL1 protein was not significantly reduced in either TLR2−/− or TLR9−/− mice, but IL-6 was reduced from 14.8+/−2.0 pg/cornea in WT mice to 7.8+/−0.6 in TLR9−/− mice (Fig. 5B, p<.05), similar to our previous report.18 In contrast, both clinical keratitis and corneal infiltration by histology were visibly diminished in TLR2/TLR9 double knockout mice as compared to WT (Fig. 5C), and CXCL1 and IL-6 protein levels were significantly decreased in TLR2/TLR9 double knockout mice corneas as compared to WT (Fig. 5D; CXCL1 pg/cornea: 19.6+/−1.9 vs. 42.7 +/−10.0; IL-6: 9.2+/−1.6 vs. 15.7+/−2.6, respectively; p<.05 for both comparisons).
FIGURE 5.
TLR2−/−, TLR9−/−, and TLR2/9−/− keratitis after HAdV-D37 infection. In representative photomicrographs (A), WT, TLR2−/−, and TLR9−/− mice corneas all demonstrate clinical keratitis at 4 dpi as compared to mock infected control mice. By ELISA at 16 hpi, expression of CXCL1 protein (B) is not statistically different in WT, TLR2−/−, and TLR9−/− mice corneas, whereas IL-6 is reduced (*p<.05). However, both clinical keratitis and corneal infiltration by histology (C) are diminished at 4 dpi in TLR2/TLR9 double knockout mice as compared to WT. By ELISA at 16 hpi (D), CXCL1 and IL-6 protein levels are significantly decreased in TLR2/TLR9 double knockout mice corneas as compared to WT (*p<.05 for both comparisons). Error bars represent SD of the mean. The data represent the means of three independent experiments with n=3 mice per group.
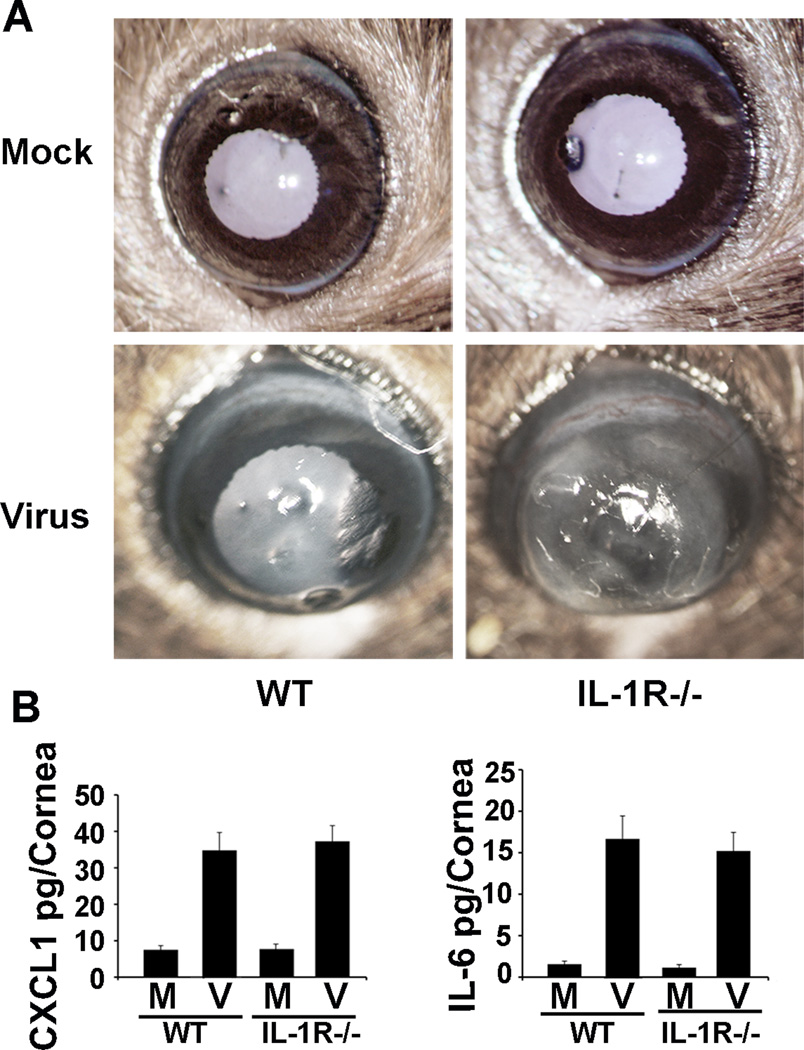
IL-1R is not required for adenovirus keratitis
IL-1R was reported to be required for the activation of hepatic macrophages by recombinant HAdV-C5.16 Murine corneas also contain macrophages.34 The IL-1 receptor (IL-1R) can signal through MyD88 to induce inflammatory mediators, bypassing the activation of TLRs. Previously, cytokine expression in response to adenoviral gene vectors administered intravenously was shown to be diminished in IL-1R−/− mice.47 Therefore, we compared virus infection in WT and IL-1R−/− corneas. On clinical examination at 4 dpi, IL-1R−/− corneal inflammation appeared possibly worse than in WT corneas (Fig. 6A). However, CXCL1 and IL-6 protein expression at 16 hpi were similar between WT corneas and IL-1R−/− corneas (Fig. 6B, CXCL1 pg/cornea: 34.8+/−1.0 vs. 37.3+/−4.2; IL-6: 16.7+/−2.8 vs. 15.2+/−2.2, respectively; p>.05 for both comparisons). These data indicate that IL-1R is not required for adenovirus keratitis in the mouse.
FIGURE 6.
Adenovirus keratitis after HAdV-D37 infection of IL-1R−/− mice. In representative photomicrographs (A) at 4 dpi, IL-1R−/− corneas show keratitis similar or possibly worse than in WT corneas. By ELISA at 16 hpi (B), CXCL1 and IL-6 protein expression are not significantly different in IL-1R−/− corneas as compared to WT (p>.05 for both comparisons). Error bars represent SD of the mean. The data represent the means of three independent experiments with n=3 mice per group.
Discussion
As demonstrated here in both cultured human corneal fibroblasts and a mouse keratitis model, MyD88 plays an essential role in the innate immune response to adenovirus infection of the cornea. MyD88 was previously reported as a significant mediator of innate immunity in corneal inflammation due to infection by Pseudomonas aeruginosa48, 49 and Fusarium species.50 It has also been previously shown that MyD88 integrates upstream TLR signaling in the living cornea, including that of TLR2 and 9.51 And it has long been known that TLR9 is a PRR for adenovirus unmethylated CpG DNA. However, our study points to several unanticipated features of MyD88 activation in adenovirus infection. One somewhat unexpected finding was a role for TLR2 in adenovirus keratitis. TLR2 is an important signaling component of lipid rafts.52 Ceramide, important in the formation of lipid rafts, was recently implicated as a target for adenoviral capsid protein VI, in which the interaction between pVI leads to membrane injury and increased viral entry.53 We recently showed a role for lipid rafts in adenoviral entry into human corneal fibroblasts.41 Select coat proteins of other viruses have been shown to bind TLR2.44 Although TLR2 was previously implicated as a possible PRR for adenovirus,46 a specific TLR2 ligand on the adenovirus has not been identified and deserves further study.
Rhee and coworkers demonstrated diminished antigen-specific CD8 T-lymphocyte responses in MyD88−/− mice immunized with recombinant adenoviral vectors, but saw no effect in mice missing any individual TLR,54 suggesting that TLR summation and/or synergy may be required for optimal signaling through MyD88. Synergy of TLRs on MyD88 activation has been previously shown.25 In particular, TLR2 and TLR9 were demonstrated to act synergistically in response to infection by Heliobacter pylori,45 and upon transfection of an adenovirus-based gene vector.46 However, TLR2/9 synergy has not been demonstrated during infection by a wild type adenovirus. Src kinase and MyD88 were previously shown jointly in a signaling complex with TLR2 and TRAF6 upon treatment with the TLR2 ligand lipoteichoic acid.55 Other studies have shown a central role for Src kinase in TLR2-mediated signaling,40, 56, 57 and Src has also been implicated in signaling through TLR9.58 Taken together, these data suggest a possible TLR/Src/MyD88 signalosome in corneal cells infected with adenovirus. However, the biochemical basis for TLR co-activation and synergy in MyD88 signaling is speculative and to what degree the observed interaction between Src and MyD88 is required for adenovirus keratitis remains to be explored. The failure of MyD88 inhibitory peptide to completely abrogate CXCL8 expression in infected HCF could be due to a redundancy in upstream activation.
Many studies of TLR signaling upon adenovirus infection have been carried out in a single cell type. One report showing co-activation of TLR2 and Src kinase utilized human synovial fibroblasts,57 likely more comparable to corneal fibroblasts than the bone marrow derived cells used in most studies of TLR and MyD88 signaling. Basner-Tschakarjan and colleagues demonstrated a role for TLR9 signaling in plasmacytoid dendritic cells infected with a recombinant replication-defective adenovirus but not in conventional dendritic cells.42 Fejer and coworkers showed that induction of type II interferons by adenovirus infection of myeloid dendritic cells was TLR independent, and also independent of known cytosolic PRRs.59 Specific differences in TLR activation between disparate cell types make interpretation of signaling in tissues, organs, and whole animals challenging, but may explain differences between studies performed in vitro and those done in vivo.
Adenoviral gene expression requires successful intracellular trafficking and delivery of viral DNA to the nucleus. In our study, MyD88−/− and WT mouse corneas showed equivalent degrees of viral binding and entry, and cultured human corneal fibroblasts similarly showed the same degree of viral binding and entry whether pretreated with the MyD88 inhibitory peptide or a control peptide. However, early viral gene expression was reduced in both mouse corneas and in human corneal fibroblasts when MyD88 was knocked down or inhibited, respectively. Therefore, in two distinct infectious models, inhibition of MyD88 appeared to have no effect on binding or internalization, and yet viral gene expression was reduced. Src kinase activation was previously shown to be important in viral gene expression,12 suggesting that the demonstrated interaction between Src and MyD88 upon infection may also impact the intracellular trafficking of adenovirus and delivery of viral DNA to the nucleus. Our results are in contrast to the findings of Suzuki and coworkers,60 who showed increased transgene expression in MyD88−/− mice transduced with a helper-dependent adenoviral vector. However, the β-galactosidase transgene used in their study was driven by a cytomegalovirus promoter. Therefore, it is possible that the effect of MyD88 on adenoviral gene expression was at the transcriptional level rather than on nuclear trafficking.
In summary, we have shown a role for TLR2, TLR9, MyD88, and Src kinase, but not IL-1R, in innate immune responses to adenovirus infection of the mouse cornea and cultured human corneal fibroblasts. MyD88 signaling appeared to play a role in viral gene expression, suggesting far-ranging effects on infection. Further studies are required to better understand the mechanism(s) of TLR2/9 synergy in adenovirus keratitis, and the role of MyD88/Src interaction in the innate immune response to corneal infection.
Methods
Cells, virus, and animals
Primary keratocytes (human corneal fibroblasts, HCF) were isolated from deceased human donor corneas as previously described,37 with approval by the Massachusetts Eye and Ear Infirmary Human Studies Committee, and adherence to the tenets of the Declaration of Helsinki. The human lung carcinoma cell line A549 (CCL-185) was obtained from the American Type Culture Collection (Manassas, VA). HAdV-D37 (GenBank accession number DQ900900) was also obtained from the American Type Culture Collection, grown in A549 cells, purified by cesium chloride gradient, and titered in triplicate on A549 cells by the 50% tissue culture infectious dose (TCID50) assay. HAdV-D37 was also conjugated with Cy3 dye (GE Healthcare, Piscataway, NJ) as previously reported.17 One milligram of Cy3 dye was reconstituted in 1 mL of 0.1 M sodium bicarbonate (pH 9.3). Labeling was performed by conjugating Cy3 dye to virus at a concentration approximately equal to 1012 AdV particles/mL, where reconstituted Cy3 dye was 20% of the final solution. The mixture was allowed to incubate for 30 min in the dark with gentle mixing every 10 min, followed by overnight dialysis to remove the excess dye. Cy3 labeling had no impact on viral titer or infectivity (data not shown).
Eight to 12-week-old wild type female C57BL/6j mice were purchased from Jackson Laboratories (Bar Harbor, ME). TLR9−/− mice bred on a C57BL/6j background were obtained as a kind gift from Drs. Paul Kincade (Oklahoma Medical research Foundation, Oklahoma City, Oklahoma) and Shizuo Akira (Osaka University, Osaka, Japan). TLR2−/− and Myd88−/−mice, both on the C57BL/6j background, were kind gifts of Dr. Michelle Callegan (Dean McGee Eye Institute, Oklahoma City, Oklahoma). TLR2 and TLR9 double knockout (TLR2/9−/−) mice were generated by cross-mating TLR2−/− and TLR9−/− mice past P10. The IL-1R−/− mice on a C57BL/6j background (B6.129S7-IL1r1tm1Imx/J) were obtained from Jackson Laboratories. All genotypes were confirmed by PCR prior to experimental use. Mice were maintained and used in accordance with the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals, and with approval from the Animal Care Committee at the Massachusetts Eye and Ear Infirmary.
Experimental Infections
Mice were anesthetized by intramuscular injection of ketamine and xylazine. One µl of virus (105 TCID50), or virus-free dialysis buffer control was injected in the central corneal stroma using a glass micropipette needle fitted with a gas-powered microinjection system (MDI, South Plainfield, NJ) under an ophthalmic surgical microscope (Carl Zeiss Meditec, Inc., Thornwood, NY) as previously described.17 At indicated time points post infection, corneas were photographed, the mice euthanized using CO2 inhalation, and the corneas excised for further analysis as below.
Monolayer HCF grown to 95% confluence in six-well plates were washed in DMEM with 2% FBS and infected with purified HAdV-D37 at a multiplicity of infection of 10 or mock-infected with virus-free dialysis buffer as a control. Virus was absorbed at 37 °C for 1 h and then incubated for an additional 1 h before RNA isolation. For protein analysis, cells grown to 95% confluence in six-well plates were serum-starved overnight before infection and then lysed 4 hpi. For MyD88 inhibition, cells were pretreated with 50 µM MyD88 inhibitory peptide (RQIKIWFQNRRMKWKK-RDVLPGTCVNS-NH2; InvivoGen, San Diego, CA) or the control peptide (RQIKIWFQNRRMKWKK-SLHGRGDPMEAFII-NH2) for 6 h, and then infected with HAdV-D37 or dialysis buffer as a control.
Flow Cytometry
Corneas were dissected from mouse eyes at 4 days post infection (dpi) and cut into small (1–2 mm diameter) fragments for subsequent digestion with 1 mg/ml collagenase type I and 0.5 mg/ml DNase (Sigma Chemical, St. Louis, MO)18. Single cell suspensions were washed twice (300 × g, 5 min/wash) in PBS and then incubated on ice for 15 min with 2 µl anti-mouse Fc block (BD Pharmingen, San Diego, CA) in a total volume of 100 µl PBS-1% BSA. Following incubation, cells were centrifuged (300 × g, 5 min) and resuspended in 5% normal rat serum (Jackson Immuno Research, West Grove, PA) for an additional 15 min on ice. Cells were then labeled with 4 µl anti-mouse FITC-conjugated anti-CD45 (clone 30-F11, BD Pharmingen), and incubated in the dark on ice for 30 min. Following incubation, the cells were washed 3 times with PBS-1% BSA (300 × g, 5 min/wash) and resuspended in PBS-1% BSA and flow cytometry performed using a Cytomics FC500 (Beckman Coulter, Brea, CA) for CD45+ events, representing the numbers of fluorescent events identified in infected and control corneas and roughly corresponding to the number of leukocytes per cornea.
Quantitative PCR
Real-time quantitative (q) PCR amplification of viral genomic DNA was performed using primers from the E1A gene (forward: 5’GGAGGTAGATGCCCATGATGA, reverse: 5’GTTGGCTATGTCAGCCTGAAGA), on an ABI Prism StepOnePlus (Life Technologies, Grand Island, NY) as previously described.41 Corneas were harvested at 1 dpi and the DNA obtained by phenol chloroform extraction. Results were normalized by standard curve. To quantify viral gene expression, RNA was isolated at 1 dpi by single step isolation with TRIzol (Life Technologies) according to manufacturer’s instructions. Following DNase treatment (Life Technologies), 2 µg of total RNA was used to synthesize cDNA using reverse transcriptase (Superscript II, Invitrogen). A total of 2 µL of cDNA obtained by reverse transcription was used for amplification in a final volume of 20 µL containing 10 µL of 2 × SYBR green master mixes (Applied Biosystems) and 250 nM of specific forward and reverse primers. Primers were designed using Primer Express Software (PE Applied Biosystems) and were as follows: E1A 10S (forward: 5’GGAGGTAGATGCCCATGATGA, reverse: 5’GTTGGCTATGTCAGCCTGAAGA); E1B: (forward: 5’ TGCTCTGGCCTGCTAGATTC, reverse: 5’CTGGCTCCATTTGTCAACCAG); GAPDH: (forward: 5’GACAATGAATACGGCTACAGCAACAGG, reverse: 5’GTTGGGATAGGGCCTCTCTTGCTCA). qPCR was performed as previously described,17 using an ABI Prism 7000 Sequence Detection System (PE Applied Biosystems), and according to the manufacturer’s instructions. RNA concentrations of samples were normalized using quantification of GAPDH mRNA as the internal control. Dissociation curves were analyzed for generation of a single product. Relative transcript levels were calculated according to the formula 1000 × 2−ΔCt, where ΔCt equals Ctgene of interest – Ctinternal control. Reaction mixtures lacking template and cDNA prepared without reverse transcriptase were used as controls in all experiments.
ELISA
Mouse corneas were harvested at indicated time points (n = 3/time point/group), flash frozen in liquid nitrogen, and homogenized in 400 µL of PBS with 1 mM PMSF, 1 µg/mL aprotinin, and 10 µg/mL leupeptin (Sigma-Aldrich, St. Louis, MO). Lysates were centrifuged at 10,000 × g for 10 min at 4°C, and the supernatants used for ELISA. Protein detection for mouse CXCL1, CXCL2, CCL2, and IL-6, and human CXCL8, was performed by sandwich ELISAs (R&D Systems, Minneapolis, MN), according to the manufacturer’s instructions. Each sample and standard was analyzed in duplicate. The plates were read on a microplate reader (Molecular Devices, Sunnyvale, CA) and analyzed using SOFTmax software SpectraMax M2 (Molecular Devices).
Confocal microscopy
Mouse corneas infected with Cy3 labeled HAdV-D37 were harvested at 1 hpi, fixed in 4 % paraformaldehyde for 30 min at room temperature, cut radially to flatten, stained with Alexa Fluor 488 phalloidin (Invitrogen) and TO-PRO-3 (Life Technologies, Grand Island, NY), and scanned with a Leica SP5 confocal microscope (Leica Microsystems, Buffalo Grove, IL) in the z-axis with step sizes of 1–2 µm.
Histology
Mouse corneas harvested at 4 dpi were washed in PBS, and fixed with 10% neutral buffered formalin for 24 h at room temperature. After paraffin embedding, whole eyes were cut into 5 µm-thick sections, mounted on positively charged slides and were air dried overnight. After deparaffinization and rehydration, slides were stained with hematoxylin and eosin. Slides were coverslipped using a synthetic resin, and photographed (Axiovert 135; Carl Zeiss Meditec, Jena, Germany), using a 40× objective.
Western blot and immunoprecipitation
Corneas were homogenized in cell lysis buffer (Cell Signaling, Beverly, MA). For immunoblotting, homogenized tissue or cell lysates were first boiled in 2× SDS sample buffer (Bio-Rad, Hercules, CA) and loaded on separating gels. Spectrophotometric bicinchoninic acid analysis was performed to normalize protein loading. After SDS-PAGE and transfer, nitrocellulose membranes were blocked overnight at 4°C in 5% bovine serum albumin. Incubation with antibody to phospho-Src (Cell Signaling) diluted in blocking buffer was performed for 2 h at room temperature, followed by wash thrice in Tris-buffered saline. Antibody reactivity was determined with enhanced chemiluminescent reagents (Amersham Biosciences, Piscataway, NJ) using horseradish peroxidase-coupled secondary antibodies (Amersham). Blots were then stripped in stripping buffer (BioRad) and reprobed with antibody against total Src (Millipore, Billerica, MA), and imaged with a Kodak Image Station 4000R (Rochester, NY).
For immunoprecipitation, cell lysates from infected HCF (300 µg) were precleared with protein A-Sepharose beads for 30 min. Precleared protein extracts were added to anti-MyD88 (Abcam, Cambridge, MA), anti-Src (Millipore) or isotype control antibodies in phosphate-buffered saline containing protease inhibitors (PMSF (5×10−5 M), leupeptin (1×10−2 mg/mL), aprotinin (5×10−3 mg/mL), and sodium vanadate (30 mM)) and 0.1% Tween 20 and rocked at 4 °C for 2 h before the addition of protein A-Sepharose (25 µL, 1:1 slurry) and further incubation at 4 °C for 12 h. Immunoprecipitates were washed five times with wash buffer (100 mM Tris-HCl, 500 mM NaCl, and 0.1% Tween 20 (pH 8)) containing protease inhibitors, and the proteins eluted by the addition of SDS–PAGE sample buffer and boiling for 5 min. Samples were run on 10% SDS–PAGE gels using standard protocols and transferred to nitrocellulose membranes (Bio-Rad). The membranes were probed with anti-MyD88 or anti-Src, and the bands were visualized with enhanced chemiluminescence (Amersham).
Statistical analysis
All qPCR, ELISA and flow cytometry experiments were performed at least three times. Arithmetic means from three or more experiments were compared by Students t test when two treatment groups, or by ANOVA with Scheffé multiple comparison test when more than two groups, using the statistical analysis software SAS (Cary, NC). Statistical significance was set at α ≤ 0.05.
Acknowledgments
We thank Shizuo Akira, Michelle Callegan, and Paul Kincade for TLR9−/−, TLR2−/−, and Myd88−/−mice, and Beth Benas for technical support.
This work was supported by NIH grants EY013124, EY021558, and P30EY014104, a Senior Scientific Investigator Award (to JC) from Research to Prevent Blindness, Inc., NY, NY, the Falk Foundation, and the Massachusetts Lions Eye Research Fund.
Footnotes
Conflict of Interest
The authors have no financial conflict of interest.
References
- 1.Wold WSM, Ison MG. Adenoviruses. In: Knipe DM, Howley P, editors. Fields Virology. Sixth. Vol. 2. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 1732–1767. [Google Scholar]
- 2.Matthes-Martin S, Feuchtinger T, Shaw PJ, Engelhard D, Hirsch HH, Cordonnier C, et al. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: summary of ECIL-4 (2011) Transpl Infect Dis. 2012;14:555–563. doi: 10.1111/tid.12022. [DOI] [PubMed] [Google Scholar]
- 3.Hoke CH, Jr, Snyder CE., Jr History of the restoration of adenovirus type 4 and type 7 vaccine, live oral (Adenovirus Vaccine) in the context of the Department of Defense acquisition system. Vaccine. 2013;31:1623–1632. doi: 10.1016/j.vaccine.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg HS, Moldawer LL, Sehgal PB, Redington M, Kilian PL, Chanock RM, et al. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci U S A. 1991;88:1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamanini A, Nicolis E, Bonizzato A, Bezzerri V, Melotti P, Assael BM, et al. Interaction of adenovirus type 5 fiber with the coxsackievirus and adenovirus receptor activates inflammatory response in human respiratory cells. J Virol. 2006;80:11241–11254. doi: 10.1128/JVI.00721-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 7.Li E, Brown SL, Stupack DG, Puente XS, Cheresh DA, Nemerow GR. Integrin alpha(v)beta1 is an adenovirus coreceptor. J Virol. 2001;75:5405–5409. doi: 10.1128/JVI.75.11.5405-5409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang K, Guan T, Cheresh DA, Nemerow GR. Regulation of adenovirus membrane penetration by the cytoplasmic tail of integrin beta5. J Virol. 2000;74:2731–2739. doi: 10.1128/jvi.74.6.2731-2739.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wickham TJ, Filardo EJ, Cheresh DA, Nemerow GR. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol. 1994;127:257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li E, Stupack D, Bokoch GM, Nemerow GR. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases. J Virol. 1998;72:8806–8812. doi: 10.1128/jvi.72.11.8806-8812.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li E, Stupack D, Klemke R, Cheresh DA, Nemerow GR. Adenovirus endocytosis via alpha(v) integrins requires phosphoinositide-3-OH kinase. J Virol. 1998;72:2055–2061. doi: 10.1128/jvi.72.3.2055-2061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajala MS, Rajala RV, Astley RA, Butt AL, Chodosh J. Corneal cell survival in adenovirus type 19 infection requires phosphoinositide 3-kinase/Akt activation. J Virol. 2005;79:12332–12341. doi: 10.1128/JVI.79.19.12332-12341.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Q, Muruve DA. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 2003;10:935–940. doi: 10.1038/sj.gt.3302036. [DOI] [PubMed] [Google Scholar]
- 14.Stilwell JL, McCarty DM, Negishi A, Superfine R, Samulski RJ. Development and characterization of novel empty adenovirus capsids and their impact on cellular gene expression. J Virol. 2003;77:12881–12885. doi: 10.1128/JVI.77.23.12881-12885.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higginbotham JN, Seth P, Blaese RM, Ramsey WJ. The release of inflammatory cytokines from human peripheral blood mononuclear cells in vitro following exposure to adenovirus variants and capsid. Hum Gene Ther. 2002;13:129–141. doi: 10.1089/10430340152712683. [DOI] [PubMed] [Google Scholar]
- 16.Di Paolo NC, Miao EA, Iwakura Y, Murali-Krishna K, Aderem A, Flavell RA, et al. Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity. 2009;31:110–121. doi: 10.1016/j.immuni.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chintakuntlawar AV, Astley R, Chodosh J. Adenovirus type 37 keratitis in the C57BL/6J mouse. Invest Ophthalmol Vis Sci. 2007;48:781–788. doi: 10.1167/iovs.06-1036. [DOI] [PubMed] [Google Scholar]
- 18.Chintakuntlawar AV, Zhou X, Rajaiya J, Chodosh J. Viral capsid is a pathogen-associated molecular pattern in adenovirus keratitis. PLoS Pathog. 2010;6:e1000841. doi: 10.1371/journal.ppat.1000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu J, Huang X, Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol. 2007;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nociari M, Ocheretina O, Schoggins JW, Falck-Pedersen E. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J Virol. 2007;81:4145–4157. doi: 10.1128/JVI.02685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 22.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 24.Lord KA, Hoffman-Liebermann B, Liebermann DA. Nucleotide sequence and expression of a cDNA encoding MyD88, a novel myeloid differentiation primary response gene induced by IL6. Oncogene. 1990;5:1095–1097. [PubMed] [Google Scholar]
- 25.Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, et al. MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. J Immunol. 2007;178:1164–1171. doi: 10.4049/jimmunol.178.2.1164. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 27.Kawai T, Takeuchi O, Fujita T, Inoue J, Muhlradt PF, Sato S, et al. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- 28.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki N, Suzuki S, Duncan GS, Millar DG, Wada T, Mirtsos C, et al. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature. 2002;416:750–756. doi: 10.1038/nature736. [DOI] [PubMed] [Google Scholar]
- 31.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 32.Muller LJ, Pels L, Vrensen GF. Novel aspects of the ultrastructural organization of human corneal keratocytes. Invest Ophthalmol Vis Sci. 1995;36:2557–2567. [PubMed] [Google Scholar]
- 33.Hamrah P, Liu Y, Zhang Q, Dana MR. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Vis Sci. 2003;44:581–589. doi: 10.1167/iovs.02-0838. [DOI] [PubMed] [Google Scholar]
- 34.Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci. 2002;43:2264–2271. [PMC free article] [PubMed] [Google Scholar]
- 35.Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, et al. The cellular basis of corneal transparency: evidence for 'corneal crystallins'. Journal of cell science. 1999;112:613–622. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- 36.Chodosh J, Astley RA, Butler MG, Kennedy RC. Adenovirus keratitis: a role for interleukin-8. Invest Ophthalmol Vis Sci. 2000;41:783–789. [PubMed] [Google Scholar]
- 37.Natarajan K, Rajala MS, Chodosh J. Corneal IL-8 expression following adenovirus infection is mediated by c-Src activation in human corneal fibroblasts. J Immunol. 2003;170:6234–6243. doi: 10.4049/jimmunol.170.12.6234. [DOI] [PubMed] [Google Scholar]
- 38.Muruve DA, Barnes MJ, Stillman IE, Libermann TA. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- 39.Chintakuntlawar AV, Chodosh J. Chemokine CXCL1/KC and its receptor CXCR2 are responsible for neutrophil chemotaxis in adenoviral keratitis. J Interferon Cytokine Res. 2009;29:657–666. doi: 10.1089/jir.2009.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Byeon SE, Yi YS, Oh J, Yoo BC, Hong S, Cho JY. The role of Src kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2012;2012:512926. doi: 10.1155/2012/512926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yousuf MA, Zhou X, Mukherjee S, Chintakuntlawar AV, Lee JY, Ramke M, et al. Caveolin-1 associated adenovirus entry into human corneal cells. PLoS One. 2013;8:e77462. doi: 10.1371/journal.pone.0077462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basner-Tschakarjan E, Gaffal E, O'Keeffe M, Tormo D, Limmer A, Wagner H, et al. Adenovirus efficiently transduces plasmacytoid dendritic cells resulting in TLR9-dependent maturation and IFN-alpha production. J Gene Med. 2006;8:1300–1306. doi: 10.1002/jgm.964. [DOI] [PubMed] [Google Scholar]
- 43.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 44.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 45.Chang YJ, Wu MS, Lin JT, Sheu BS, Muta T, Inoue H, et al. Induction of cyclooxygenase-2 overexpression in human gastric epithelial cells by Helicobacter pylori involves TLR2/TLR9 and c-Src-dependent nuclear factor-kappaB activation. Mol Pharmacol. 2004;66:1465–1477. doi: 10.1124/mol.104.005199. [DOI] [PubMed] [Google Scholar]
- 46.Appledorn DM, Patial S, McBride A, Godbehere S, Van Rooijen N, Parameswaran N, et al. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J Immunol. 2008;181:2134–2144. doi: 10.4049/jimmunol.181.3.2134. [DOI] [PubMed] [Google Scholar]
- 47.Shayakhmetov DM, Li ZY, Ni S, Lieber A. Interference with the IL-1-signaling pathway improves the toxicity profile of systemically applied adenovirus vectors. J Immunol. 2005;174:7310–7319. doi: 10.4049/jimmunol.174.11.7310. [DOI] [PubMed] [Google Scholar]
- 48.Sun Y, Karmakar M, Roy S, Ramadan RT, Williams SR, Howell S, et al. TLR4 and TLR5 on corneal macrophages regulate Pseudomonas aeruginosa keratitis by signaling through MyD88-dependent and -independent pathways. J Immunol. 2010;185:4272–4283. doi: 10.4049/jimmunol.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaidi TS, Zaidi T, Pier GB. Role of neutrophils, MyD88-mediated neutrophil recruitment, and complement in antibody-mediated defense against Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2010;51:2085–2093. doi: 10.1167/iovs.09-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tarabishy AB, Aldabagh B, Sun Y, Imamura Y, Mukherjee PK, Lass JH, et al. MyD88 regulation of Fusarium keratitis is dependent on TLR4 and IL-1R1 but not TLR2. J Immunol. 2008;181:593–600. doi: 10.4049/jimmunol.181.1.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson AC, Heinzel FP, Diaconu E, Sun Y, Hise AG, Golenbock D, et al. Activation of toll-like receptor (TLR)2, TLR4, and TLR9 in the mammalian cornea induces MyD88-dependent corneal inflammation. Invest Ophthalmol Vis Sci. 2005;46:589–595. doi: 10.1167/iovs.04-1077. [DOI] [PubMed] [Google Scholar]
- 52.Soong G, Reddy B, Sokol S, Adamo R, Prince A. TLR2 is mobilized into an apical lipid raft receptor complex to signal infection in airway epithelial cells. J Clin Invest. 2004;113:1482–1489. doi: 10.1172/JCI20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luisoni S, Suomalainen M, Boucke K, Tanner LB, Wenk MR, Guan XL, et al. Co-option of Membrane Wounding Enables Virus Penetration into Cells. Cell Host Microbe. 2015;18:75–85. doi: 10.1016/j.chom.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 54.Rhee EG, Blattman JN, Kasturi SP, Kelley RP, Kaufman DR, Lynch DM, et al. Multiple innate immune pathways contribute to the immunogenicity of recombinant adenovirus vaccine vectors. J Virol. 2011;85:315–323. doi: 10.1128/JVI.01597-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee IT, Wang SW, Lee CW, Chang CC, Lin CC, Luo SF, et al. Lipoteichoic acid induces HO-1 expression via the TLR2/MyD88/c-Src/NADPH oxidase pathway and Nrf2 in human tracheal smooth muscle cells. J Immunol. 2008;181(7):5098–5110. doi: 10.4049/jimmunol.181.7.5098. [DOI] [PubMed] [Google Scholar]
- 56.Chun J, Prince A. Ca2+ signaling in airway epithelial cells facilitates leukocyte recruitment and transepithelial migration. Journal of leukocyte biology. 2009;86:1135–1144. doi: 10.1189/jlb.0209072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang CH, Hsu CJ, Yang WH, Fong YC. Lipoteichoic acid enhances IL-6 production in human synovial fibroblasts via TLR2 receptor, PKCdelta and c-Src dependent pathways. Biochem Pharmacol. 2010;79:1648–1657. doi: 10.1016/j.bcp.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen TT, Johnsen IB, Knetter CF, Drablos F, Fitzgerald KA, Lien E, et al. Differential gene expression downstream of Toll-like receptors (TLRs): role of c-Src and activating transcription factor 3 (ATF3) J Biol Chem. 2010;285:17011–17019. doi: 10.1074/jbc.M109.068817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fejer G, Drechsel L, Liese J, Schleicher U, Ruzsics Z, Imelli N, et al. Key role of splenic myeloid DCs in the IFN-alphabeta response to adenoviruses in vivo. PLoS Pathog. 2008;4:e1000208. doi: 10.1371/journal.ppat.1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzuki M, Cerullo V, Bertin TK, Cela R, Clarke C, Guenther M, et al. MyD88-dependent silencing of transgene expression during the innate and adaptive immune response to helper-dependent adenovirus. Hum Gene Ther. 2010;21:325–336. doi: 10.1089/hum.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]