Abstract
Psychotropic medications are often prescribed to reduce challenging behavior in individuals with intellectual and developmental disabilities (IDD). Functional analyses (FAs) have demonstrated utility in assessing medication impact on behavior; however, the impact of adverse side effects (ASE) on challenging behavior is under-assessed. The purpose of this study was to develop a methodology, similar to FAs, to explore potential medication ASE impact on challenging behavior in seven individuals with IDD. Results revealed response rate differences in designed ASE conditions for most participants. Outcomes support further development and use of this methodology to assess the presence and impact of ASEs.
Keywords: adverse side effects, psychotropic medication, functional analysis methodology
Treatment of challenging behavior in individuals with intellectual and developmental disabilities (IDD) often consists of behavioral, pharmacological, and combined interventions (Heyvaert, Maes, & Onghena, 2010). Psychotropic medication use in this population is often prescribed off-label (i.e., not for the purposes approved by the FDA) to manage challenging behavior (Glover, Bernard, Branford, Holland, & Strydom, 2014; Sheehan et al., 2015). Often, multiple medications are prescribed to achieve optimal effect (Deb, Unwin, & Deb, 2015). Deb and colleagues (2015) reported that approximately 41% of their sample was prescribed two or more psychotropic medications off label. Kelly and Su (2015) found that the percentage of individuals with IDD prescribed with psychotropic medications increased to 44% from 21% after transitioning to community residential settings from institutions in the state of Georgia, and the average number of medications prescribed per individual raised to 1.84 from 0.68. These studies illustrate how conventional psychotropic medication use has become in treating challenging behavior among populations with IDD.
Despite its common practice, research has only recently begun to explore how polypharmacy, or simultaneous use of multiple psychotropic medications, impact challenging behavior (Valdovinos et al., 2016). Although these medications may produce reductions in challenging behavior frequency or rate, these medications also produce adverse side effects (ASEs). Limited work examining the impact of adverse side effects (ASEs) of psychotropic medication on challenging behavior exists. This is significant since evidence suggests individuals on combined psychotropic treatments are more likely to experience ASEs (Hess et al., 2010). Commonly reported ASEs of psychotropic medication include thirst, changes in appetite, headaches, and changes in movement (e.g., Givens, 2016). In a recent evaluation of psychotropic medication use, Scheifes and colleagues (2016) found that 84.4% of their sample had experienced at least one ASE as a result of psychotropic medication use, and 45.6% of those individuals experienced ASEs across different psychotropic medication classes.
At present, ASEs are typically assessed using indirect measures (e.g., Matson Evaluation of Drug Side-effects; Hess et al., 2010). However, these measures (e.g., Likert scales) may not measure accurately ASEs in those with IDD with communication impairments (Matson & Neal, 2009). Other instruments may be ineffectively time-consuming, unreliable, or invalid (Cleary et al., 2012). Furthermore, indirect measures rely on rater subjectivity rather than objective observations. This subjectivity may be problematic given that caregivers and staff may not have sufficient knowledge regarding medication ASEs (Fretwell & Felce, 2007).
It is conceptually feasible to assume that an ASE, like other biological events (e.g., O’Reilly, 1997), may function as a motivating operation (MO), thereby impacting the value of common reinforcers (Valdovinos & Kennedy, 2004). For example, the ASE dry mouth may establish liquids as more reinforcing. This may increase the likelihood that challenging behavior (e.g., aggression), or other liquid seeking behavior (e.g., elopement, wandering), will occur, particularly within environments in which liquids might be obtained. Psychotropic medications introduce changes in physiology (i.e., intended effects and ASEs), which could theoretically alter the relative value of stimuli. Without assessing ASEs, any changes in challenging behavior could be misattributed to medication ineffectiveness, potentially resulting in medication dosage change or adjunctive treatment (i.e., additional medication).
A behavioral analog for assessing ASE presence and potential relation to challenging behavior is needed. An objective, direct method commonly employed to assess challenging behavior is functional analysis (FA; Iwata, Dorsey, Slifer, Bauman, & Richman, 1994). Further, FAs have also been used to evaluate the impact psychotropic medications have on challenging behavior (e.g., Zarcone et al., 2004); however, FAs have not yet been used in the context of monitoring ASEs. The purpose of the current study was to develop test conditions within the context of an FA to evaluate the impact of commonly reported ASEs on challenging behavior.
Method
Participants
The seven participants in this study were a subset of a two-year project evaluating the impact of psychotropic medication changes on challenging behavior within FAs (see Authors et al., year). All participants had a diagnosis of IDD, and five participants also had a diagnosis of autism spectrum disorder (ASD); six or fewer psychotropic medications prescribed; and lived in institutional or community residential settings (see Table 1 for participant characteristics).
Table 1.
Participant Characteristics
| Participant | Sex | Age | Diagnoses | Challenging Behavior(s) | Medication Starting Regimen | Medication Changes | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Greg | M | 42 | ASD, Generalized anxiety disorder, Insomnia, Severe ID | SIB, problem vocalizations | Depakote 2500mg | 1. | D/C Thorazine |
| Thorazine 25mg | |||||||
| Ativan 1.5mg | |||||||
|
| |||||||
| Shelia | F | 32 | ASD, Moderate ID | SIB, problem vocalizations | Depakote ER 250mg | 1. | ↑Depakote ER 500mg |
| Vistaril 50mg | T Zyprexa 10mg | ||||||
| Ambien 10mg | 2. | ↑Depakote 1000m | |||||
| Zyprexa 10mg | D/C Risperdal | ||||||
| Klonopin 4.5mg | 3. | ↑Depakote 1500mg | |||||
| Risperdal 1mg | 4. | + Lamcital 50mg | |||||
| Ativan 0.5mg PRN | ↓Klonopin 4mg | ||||||
| 5. | ↑ Klonopin 4.5mg | ||||||
| 6. | D/C Ambien | ||||||
| 7. | ↓Klonopin 1.5mg | ||||||
| D/C Vistaril | |||||||
| 8. | ↑Zyprexa 15mg | ||||||
| 9. | ↑ Lamcital 75mg | ||||||
| ↑ Klonopin 2.5mg | |||||||
| 10. | ↑ Lamcital 100mg | ||||||
|
| |||||||
| Ronald | M | 28 | ASD, Bipolar I Disorder, Severe ID | SIB, problem vocalizations | Depakote 1000mg | 1. | + Latuda 80mg |
| Mellarill 200mg | D/C Mellarill | ||||||
| Lexapro 5mg | D/C Lexapro | ||||||
| 2. | ↑ Latuda 160mg | ||||||
|
| |||||||
| Blake | M | 45 | ASD, Profound ID, Polydipsia, Binge Eating Disorder, Impulse Control Disorder (NOS) | SIB, problem vocalizations | Zoloft 200mg | 1. | ↓ Risperdal 1.5mg |
| Risperdal 2mg | 2. | ↑Topamax 200mg | |||||
| Topamax 150mg | 3. | ↓Risperdal 1.25mg | |||||
|
| |||||||
| Theodore | M | 38 | ASD, Profound ID | SIB | Vistaril 50mg | 1. | ↑Vistaril75mg |
| Risperdal 3mg | 2. | +Oleptro 75mg | |||||
| 3. | ↑Risperdal 4mg | ||||||
| ↓Vistaril 50mg | |||||||
| 4. | ↑Risperdal 4.25mg | ||||||
|
| |||||||
| Matthew | M | 37 | ASD, Profound ID | SIB, problem vocalization, aggression | Tegratol 800mg | 1. | ↓Oleptro 250mg |
| Oleptro 300mg | 2. | ↓Oleptro 200mg | |||||
| Vistaril 50mg | 3. | T Risperdal 4mg | |||||
| Risperdal 4mg | 4. | ↓ Risperdal 3.5mg | |||||
|
| |||||||
| Ryder | M | 49 | Profound ID, Bipolar Disorder (NOS) | SIB, aggression | Lithobid 1650mg | 1. | Ativan protocol (x4) |
| Seroquel 400mg | 2. | Ativan protocol | |||||
| Fanapt 6mg | ↑Seroquel 500mg | ||||||
| + Ativan 0.5mg | |||||||
Note: (↑) increase in medication dose; (↓) decrease in medication dose; (+) medication added; (D/C) discontinued medication; (T) time of administration change. Italics and bold denote different changes in medication regimen.
Study Design
In the larger study, medication decisions made by participants’ treatment teams were not informed by FA outcomes, thus an ABC, AA′A″, or combination, parametric design was used to make comparisons across medication regimens. Use of this design to evaluate medication impact on behavior is novel and perhaps unconventional, but it allowed for comparisons of a given ASE condition across medication regimens (Cox & Virues-Ortega, 2016). Thus, results from one condition were compared with the those from the same condition across different medication regimens. In this design, “A” represents starting dose/medication regimen and “ABC” indicates changes in medication kind. Whereas “AA′A″” indicates changes in doses, an “ABB′” indicates changes in both medication kind and dose. This design was applied across all participants with one exception. Ryder experienced the same medication change repeatedly. He was prescribed a medication protocol (five times) at the onset of a “manic phase”. This protocol involved the initiation of Ativan (an anxiolytic) at a dose of 1mg for one week, then a decrease to 0.5mg of Ativan for a week, and then it was discontinued. Participants’ caregivers reported medication changes and Medication Administration Records confirmed these alterations.
Response Measurement and Interobserver Agreement
Data were collected on ASE-related responses and challenging behavior for 5 min sessions. Responses were: drink/food requests (any verbal or gestural request for drink/food), headache-related behavior (request to reduce radio volume or saying “headache”), tardive dyskinesia (TD) responses (tongue produced a bulge or moved in and out of mouth), range of motion deficits (ROMD; inability to extend arms beyond 135 degrees or perpendicular to the body or extend legs beyond 90 degrees in response to a demand), aggression (pinching, grabbing, biting, kicking, hitting, pushing; n=2), self-injurious behavior (SIB; skin picking, head hitting, head banging, self-hitting; n=8), and problem vocalizations (grunting, whining, growling, screaming; n=5). ASE-related responses were only measured in the relevant ASE condition.
Interobserver agreement (IOA) was calculated between two independent observers for 100% of the ASE conditions using Cohen’s Kappa frequency/sequence comparisons with a 5s tolerance window. Across all participants, IOA averaged 91.48% (range, 25% to 100%) for ASEs and 92.2% (range, 74.4% to 100%) for challenging behavior.
ASE Conditions
A functional analysis (FA) was conducted for each participant at the onset of the larger study. Prior to the FA, individualized ASE conditions were tested in a fixed order. Participants were only exposed to ASE conditions relevant to potential ASEs associated with their medication regimens. All conditions were 5 min each with the exception of “Movement,” which is described below. All conditions were conducted once per medication regimen. Two weeks following a medication change, another FA with ASE conditions was conducted.
The ASE conditions were designed to identify and measure the impact of potential ASEs on challenging behavior. All participants were exposed to the thirst, hunger, and ROMD conditions. Additional conditions (i.e., TD, headache and movement) were conducted if the relevant ASE was common for one or more of the individuals’ prescribed medication. For example, dizziness is associated with Depakote use and thus an ROMD condition was conducted (FDA, 2016), and the movement condition was conducted for Ryder because caregivers reported that he might have akathisia, a medication-induced movement disorder in which individuals have difficulty staying motionless. Although the ASE conditions were designed to assess presence of common ASEs, programmed consequences (i.e., the removal or provision of stimuli) were delivered contingent upon occurrences of challenging behavior and ASE-related behavior had no programmed consequence. Some of the ASE-related behavior (e.g., hand tremors) occurred across conditions; however, only data on ASEs associated with the test condition are presented.
Thirst and hunger
For each condition, the participant and experimenter were seated at a table with a preferred drink or food, as identified by caregivers. Prior to the session, the participant was given a sip (~0.25oz) of the drink, or single piece of food. During session, access to these items were withheld and provided contingent upon challenging behavior with requests for drink or food ignored. The next trial began once the edible or drink was consumed.
TD and ROMD
In TD, fine motor demands required small hand movements and manipulation or finger movements (e.g., cutting paper, folding paper, stuffing envelopes, stringing beads, working a screw and bolt peg board). In ROMD, gross motor demands using large range movements involving the extremities (i.e., arms and legs; e.g., walking, lifting arms and legs). During sessions, the experimenter presented the participant with demands presented using three-step guided compliance (verbal, gestural, and physical prompt; Iwata et al., 1994) with 5 s between prompts. Brief praise was delivered upon completion of tasks following verbal and gestural prompts and a subsequent demand immediately followed. Tasks were removed for 10 s following challenging behavior.
Headache
A radio was set at its highest volume and turned off for 10 s if challenging behavior occurred. Requests to lower volume or complaints of headache were ignored.
Movement
Ryder was given the initial demand to sit down. The session was terminated if he stood or engaged in challenging behavior, or once 5 min passed.
Results
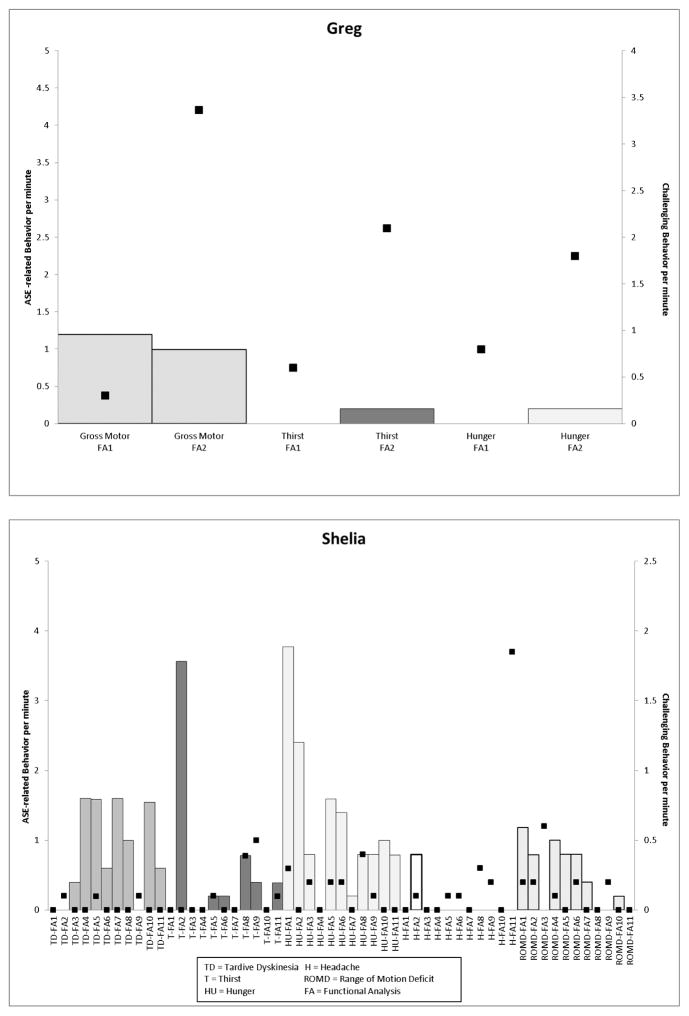
The top panel of Figure 1 depicts ASE-related behavior experienced by Greg and the rate of challenging behavior across conditions tested. Once Thorazine (a typical antipsychotic) was discontinued, higher rates of challenging behavior were observed across all conditions in addition to an increase in the number of drink (0.2 rpm) and food requests (0.2 rpm) made in the thirst and hunger conditions, respectively, and a decrease in ROMD (from 1.2 rpm to 0.9 rpm). Shelia’s data are presented in the bottom panel of Figure 1. Her medication changes resulted in different effects for each ASE-related behavior across medication regimens. For example, in the TD sessions, the presence of dyskinetic movements occurred when Risperdal (an atypical antipsychotic) was discontinued and these movements persisted at varying rates with the exception of when Zyprexa (an atypical antipsychotic) was increased (this pattern is not uncommon as antipsychotics are associated with movement-related side effects). Whereas rates of food requests seemed to decrease across medication changes with the last four medication changes producing stable rates in food requests and challenging behavior. Contrast both of these with the ASE-related behavior noted in the headache condition. Although ASE-related behavior did not occur outside of the second medication regimen (Depakote, an anticonvulsant, was increased and the time of day administration of Zyprexa was changed), the rates of challenging behavior in this condition increased in subsequent medication changes (0 to 1.8rpm).
Figure 1.
Graphs depicting ASE-related behavior (left y-axis) and challenging behavior (right y-axis) per minute (Greg, top panel; Shelia, bottom panel) across medication regimens.
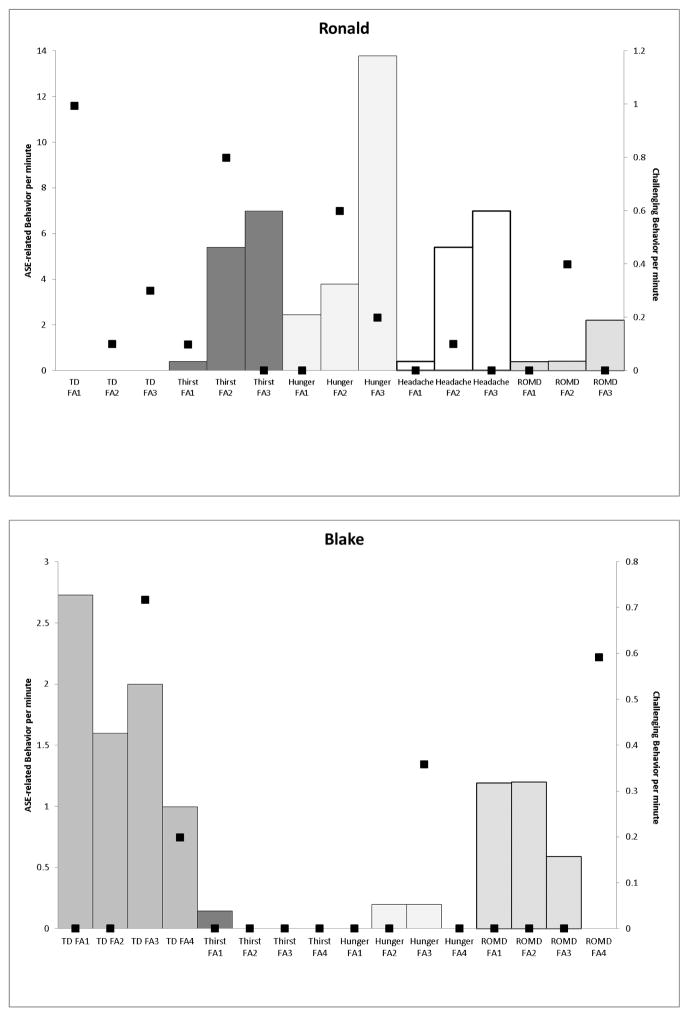
In Figure 2, the top panel presents data for Ronald. With the addition of Latuda (atypical antipsychotic) and discontinuation of Mellaril (typical antipsychotic) and Lexapro (an antidepressant) increases in ASE-related behavior and challenging behavior rates occurred in the thirst (5.4 rpm and 0.4 rpm, respectively), hunger (3.8 rpm, 0.6 rpm, respectively), and headache (5.4 rpm and 0.8 rpm, respectively) conditions. An increase in the dose of Latuda further increased rates of ASE-related behavior but challenging behavior rates in these conditions decreased. The bottom panel of Figure 2 depicts Blake’s data. Challenging behavior seldom occurred in these conditions. Dyskinetic movements decreased as Risperdal was decreased (decrease in hand tremor rate; 2.7 rpm to 1.6 rpm); however when Topamax (an anticonvulsant) was increased, an increase in the rate of challenging behavior (0.7 rpm) and ASE-related behavior (2.0 rpm) occurred and a reduction in Risperdal in the last medication regimen produced decreases in both. With respect to responding in the ROMD condition, ASE-related behavior remained constant until Topamax was increased and the rate of ASE-related behavior decreased to 0.6 rpm and then ceased occurring when Risperdal decreased further although challenging behavior rates increased to 0.6rpm.
Figure 2.
Graphs depicting ASE-related behavior (left y-axis) and challenging behavior (right y-axis) per minute (Ronald, top panel; Blake, bottom panel) across medication regimens.
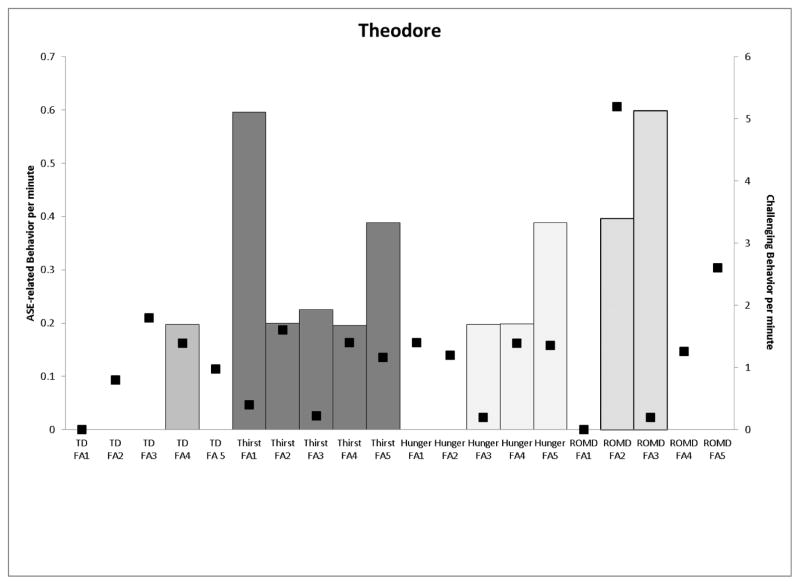
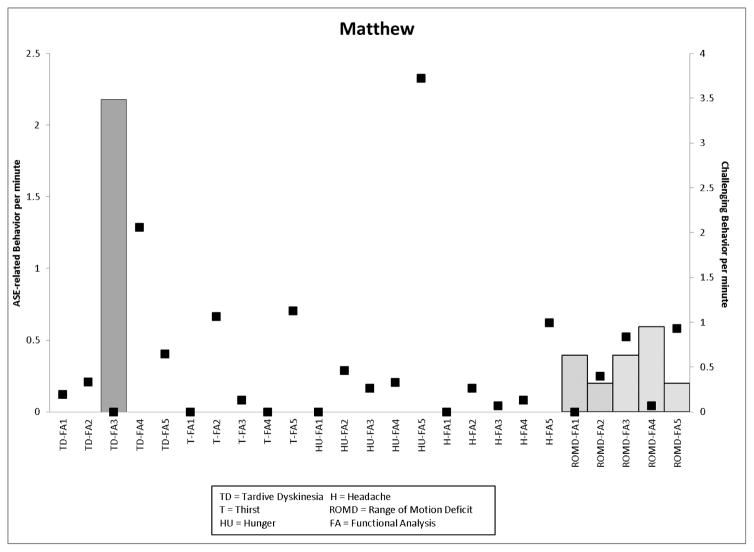
The top panel of Figure 3 presents data for Theodore. In the TD condition, ASE-related behavior did not occur until Risperdal was increased and Vistaril was decreased (from 0 to 0.2rpm). The rate of drink requesting was highest in the initial thirst condition and the final thirst condition when Risperdal was increased (food requests were also highest on this medication regimen). In the ROMD conditions, ASE-related behavior were highest when Vistaril was increased and Oleptro (an antidepressant) was added (0.4 rpm and 0.6 rpm, respectively). The bottom panel presents the results of Matthew’s assessment. Decreases in Oleptro dosages were associated with a large increase in the rate of movement-related ASEs (hand tremor, 2.2 rpm and ROMD, 0.4 rpm). Alterations in the timing of Risperdal administration increased the rate of ROMD observed (0.6 rpm). A final decrease in Risperdal was associated with higher rates of challenging behavior across all conditions and a reduction in the rate of ROMD (0.2 rpm).
Figure 3.
Graphs depicting ASE-related behavior (left y-axis) and challenging behavior (right y-axis) per minute (Theodore, top panel; Matthew, bottom panel) across medication regimens.
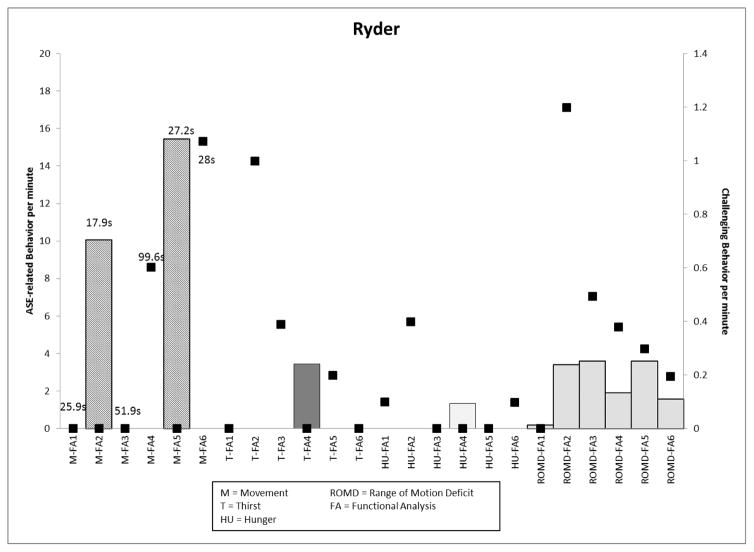
Ryder’s data are presented in Figure 4. The main medication change, Ativan (an anxiolytic) protocol, was experienced four times with different effects observed in his standing latency, TD symptoms (in “movement” and “ROMD” conditions), and rates of challenging behavior. In the final medication phase, when the Ativan protocol was combined with an increase in Seroquel (an atypical antipsychotic) and the permanent addition of Ativan, rates of challenging behavior in the movement and hunger conditions increased (1.1 rpm, 0.1 rpm respectively) and decreased in the thirst and ROMD conditions (0.2 rpm).
Figure 4.
Graph depicting Ryder’s ASE-related behavior (left y-axis; latency to movement provided in seconds) and challenging behavior (right y-axis) per minute across medication regimens.
Discussion
Differences in rates of ASE-related and challenging behavior were observed across ASE conditions and varied by participant and medication regimen. There were occasions when rates of challenging behavior increased as rates of ASE-related behavior increased. Conversely, there were occasions when challenging behavior did not occur but ASE-related behavior occurred. Finally, there were conditions in which challenging behavior occurred but ASE-related behavior did not. Thus, the presence of targeted ASE-related behavior did not consistently co-vary with the rate of challenging behavior. In other words, ASE-related behavior rate was greater (or lower) in a given ASE test condition on a particular medication regimen, but challenging behavior did not necessarily occur at a higher (or lower) corresponding rate. It did appear that the type of medication change impacted the presentation of ASE-related behavior. For example, when antipsychotic medications were reduced, movement-related ASEs were more likely to occur (Greg, Shelia, Ronald, and Matthew) which could suggest antipsychotic withdrawal related dyskinesia. Decreases in antidepressants were associated with increases in challenging behavior and increases in ASE-related behavior (Ronald and Matthew). The addition of an antidepressant was also found to be associated with an increase in ASE-related behavior (Theodore). The addition or increase in anticonvulsant and anxiolytic medication produced mixed results with both increases and decreases in rates challenging behavior and often increases in the ASE-related behavior observed, specifically hand tremors and food requests (Shelia and Blake).
This study introduces a preliminary method for evaluating potential MO function of psychotropic medication ASEs, assuming that ASEs of psychotropic medication do alter the effectiveness of stimuli thereby potentially evoking and/or increasing the occurrence of behavior historically reinforced by these stimuli. The impact of ASEs on challenging behavior is not intuitive, nor is it predictable, as different patterns may be observed. The participant patterns observed did not consistently demonstrate correspondence between challenging behavior and ASE-related behavior. Although FAs provide reinforcement for target responses (i.e., challenging behavior), participants in this study were presented with stimuli to evoke responding while consequences were kept constant (Carr & Durand, 1985). Therefore, there is the possibility that challenging behavior increased during FA sessions for some participants and ASE-related behavior did not, given the differing reinforcement schedules within each session. However, the means with which we objectively evaluated ASEs may still provide valuable clinical information for the treatment of individuals with IDD. Objective evaluation is valuable because research has demonstrated that current tools relying on third-party subjective/anecdotal reports over- and/or underestimate the presence of ASEs (Cleary et al., 2012). Using direct and objective measures of ASE presence may facilitate better pharmacological treatments but also inform behavioral programming. For example, Ronald had an increasing number of food requests across medication regimens. Conceptualization of food requests as an indication of hunger suggests that food may serve as a stronger motivator and may be used to reinforce adaptive behavior or the absence of challenging behavior. It would also be important to monitor his eating habits and weight to protect against other potential health conditions associated with psychotropic medication use (i.e., weight gain, metabolic disorders; Teluckdharry et al., 2013).
This study is not without limitations. First, a control condition for the ASE conditions was not run. Developing a condition in which there was free access to liquids and food, a quiet environment, and zero requests for motor engagement may have provided a means by which to assess if the manipulations in the conditions were indeed accounting for the responses observed within each condition. Additionally, the ASE conditions were only tested once prior to an FA and not repeatedly throughout the FA for each medication regimen. Multiple presentations of these conditions, and subsequent assessment of challenging behavior within them, may have provided additional information regarding the impact of ASEs. For example, it is possible challenging behavior occurred in the thirst condition because of limited access to fluids prior to the assessment but once access to liquids had been provided, thirst was quenched. Thus, thirst may have been a temporary state not a chronic psychotropic medication ASE. Finally, tests for ASEs could have also been conducted between medication changes to monitor the continued presence of ASEs as some are known to be transient in nature.
Using an experimental approach to monitor the impact of ASEs on challenging behavior has the potential to better inform prescriptive practices and behavioral interventions to best serve adults with IDD. The use of direct measures, such as the ASE conditions presented, may reduce subjectivity that potentially contributes to misaligned use of psychotropic medication and provide behavioral basis for prescriptive practices. Future research should extend the findings of this study by addressing limitations identified, specifically the lack of experimental control and repeated measures, to systematically evaluate if the presence of ASEs have a more direct impact on challenging behavior. Additionally, research could target appropriate requests during this paradigm to determine if adaptive behavior is impacted by psychotropic medication use.
Acknowledgments
Research funded by NICHD grant 1R15HD072497-01.
Funding: This study was funded by NICHD grant 1R15HD072497-01.
Footnotes
Compliance with Ethical Standards
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Author A declares that he/she has no conflict of interest. Author B declares that he/she has no conflict of interest. Author C declares that he/she has no conflict of interest. Author D declares that he/she has no conflict of interest. Author E declares that he/she has no conflict of interest. Author F declares that he/she has no conflict of interest.
References
- Carr EG, Durand VM. Reducing behavior problems through functional communication training. Journal of Applied Behavior Analysis. 1985;18:111–126. doi: 10.1901/jaba.1985.18-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary A, Walsh F, Connolly H, Hays V, Oluwole B, Macken E, Dowling M. Monitoring and documentaion of side effects from depot antipsychotic medication: An interdisciplinary audit of practice in a regional mental health service. Journal of Psychiatric and Mental Health Nursing. 2012;19:395–401. doi: 10.1111/j.1365-2850.2011.01807.x. [DOI] [PubMed] [Google Scholar]
- Cox AD, Virues-Ortega J. Interactions between behavior function and psychotropic medication. Journal of Applied Behavior Analysis. 2016;49:85–104. doi: 10.1002/jaba.247. [DOI] [PubMed] [Google Scholar]
- Deb S, Unwin G, Deb T. Characteristics and the trajectory of psychotropic medication use in general and antipsychotics in particular among adults with an intellectual disability who exhibit aggressive behaviour. Journal of Intellectual Disability Research. 2015;59(1):11–25. doi: 10.1111/jir.12119. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (FDA) Depakene (valproic acid) capsules and oral solution, depakote (divalproex sodium) delayed release and depakote er (extended release) tablets, depakote sprinkle capsules (divalproex sodium coated particles in capsules), depacon (valproate sodium) injection. 2016 Retrieved from http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/018081s064,018082s047lbl.pdf.
- Fretwell C, Felce D. Staff knowledge of the side effects of anti-psychotic medication. Journal of Applied Research in Intellectual Disabilities. 2007;6:580–85. [Google Scholar]
- Givens CJ. Adverse drug reactions associated with antipsychotics, antidepressants, mood stabilizers, and stimulants. The Nursing Clinics of North America. 2016;51:309–321. doi: 10.1016/j.cnur.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Glover G, Bernard S, Branford D, Holland A, Strydom A. Use of medication for challenging behaviour in people with intellectual disability. The British Journal of Psychiatry. 2014;205:6–7. doi: 10.1192/bjp.bp.113.141267. [DOI] [PubMed] [Google Scholar]
- Hess J, Matson J, Neal D, Mahan S, Fostad J, Bamburg J, Holloway J. A comparison of psychotropic drug side effect profiles in adults diagnosed with intellectual disabilities and autism spectrum disorders. Journal of Mental Health Research in Intellectual Disabilities. 2010;3:85–96. [Google Scholar]
- Heyvaert M, Maes B, Onghena P. A meta-analysis of intervention effects on challenging behaviour among persons with intellectual disabilities. Journal of Intellectual Disability Research. 2010;54:643–49. doi: 10.1111/j.1365-2788.2010.01291.x. [DOI] [PubMed] [Google Scholar]
- Iwata BA, Dorsey MF, Slifer KJ, Bauman KE, Richman GS. Toward a functional analysis of self-injury. Journal of Applied Behavior Analysis. 1994;27:197–209. doi: 10.1901/jaba.1994.27-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S, Su Y. Psychotropic and anticonvulsant medication: Individuals with intellectual and developmental disabilities who transitioned to the community from an institution. Intellectual and Developmental Disabilities. 2015;53:289–300. doi: 10.1352/1934-9556-53.4.289. [DOI] [PubMed] [Google Scholar]
- Matson JL, Neal D. Psychotropic medication use for challenging behaviors in persons with intellectual disabilities: An overview. Research in Developmental Disabilities. 2009;30:572–86. doi: 10.1016/j.ridd.2008.08.007. [DOI] [PubMed] [Google Scholar]
- O’Reilly MF. Functional analysis of episodic self-injury correlated with recurrent otitis media. Journal of Applied Behavior Analysis. 1997;30:165–167. doi: 10.1901/jaba.1997.30-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheifes A, Walraven S, Stolker JJ, Nijman HL, Egberts TC, Heerdink ER. Adverse events and the relation with quality of life in adults with intellectual disability and challenging behaviour using psychotropic drugs. Research in Developmental Disabilities. 2016;49–50:13–21. doi: 10.1016/j.ridd.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Sheehan R, Hassiotis A, Walters K, Osborn D, Strydom A, Horsfall L. Mental illness, challenging behaviour, and psychotropic drug prescribing in people with intellectual disability: UK population based cohort study. BMJ. 2015;351:h4326. doi: 10.1136/bmj.h4326. doi: http://dx.doi.org/10.1136/bmj.h4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teluckdharry S, Sharma S, O’Rourke E, Tharian P, Gondalekar A, Nainar F, Roy M. Monitoring metabolic side effects of atypical antipsychotics in people with an intellectual disability. Journal of Intellectual Disabilities. 2013;17:223–235. doi: 10.1177/1744629513495261. [DOI] [PubMed] [Google Scholar]
- Valdovinos MG, Henninger-McMahon M, Schieber E, Beard L, Conley B, Haas A. Assessing the impact of psychotropic medication changes on challenging behavior of individuals with intellectual disabilities. International Journal of Developmental Disabilities. 2016;62:200–211. doi: 10.1080/20473869.2016.1177301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdovinos MG, Kennedy CH. A behavior-analytic conceptualization of psychotropic medication side effects. The Behavior Analyst. 2004;27:231–38. doi: 10.1007/BF03393182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarcone JR, Lindauer SL, Morse PS, Crosland KA, Valdovinos MG, McKerchar TL, Reese RM, Hellings JA, Schroeder SR. Effects of risperidone on destructive behavior of persons with developmental disabilities: III. Functional analysis. American Journal on Mental Retardation. 2004;109:310–321. doi: 10.1352/0895-8017(2004)109<310:EORODB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]