Abstract
Purpose of review
The outcome of allogeneic stem cell transplantation (allo-HCT) is still compromised by relapse and complications. NK cells and γδT cells, effectors which both function through MHC-unrestricted mechanisms, can target transformed and infected cells without inducing Graft-versus-Host Disease (GVHD). Allo-HCT platforms based on CD34+ selection or αβ-TCR depletion result in low grades of GVHD, early immune reconstitution (IR) of NK and γδT cells and minimal usage of GVHD prophylaxis. In this review we will discuss strategies to retain and expand the quantity, diversity and functionality of these reconstituting innate cell types.
Recent findings
Bisphosphonates, IL-15 cytokine administration, specific antibodies, checkpoint inhibitors and (CMV based) vaccination are currently being evaluated to enhance IR. All these approaches have shown to potentially enhance both NK and γδT cell immuno-repertoires.
Summary
Rapidly accumulating data linking innate biology to proposed clinical immune interventions, will give unique opportunities to unravel shared pathways which determine the Graft-versus-Tumor effects of NK and γδT cells.
Keywords: NK cells, γδT cells, allogeneic stem cell transplantation, immune reconstitution
1.1 Introduction
Allogeneic stem cell transplantation (allo-HCT) is the only accepted curative option for many patients with advanced hematological malignancies. The chief long-term goal of allo-HCT is to develop low-toxicity platforms that will not only reduce morbidity and mortality but also allow disease-specific interventions to be applied after transplantation. Graft manipulation using profound T cell depletion with antibodies like Campath reduces acute and chronic Graft-versus-Host Disease (GVHD), but with an increase in relapse and infectious complications, most likely attributable to delayed immune reconstitution (IR) [1, 2]. More recently, there has been a surge of interest in the usage of post-transplantation high dose cyclophosphamide (PTCy) as short-term GVHD-prophylaxis. Incidence of severe aGVHD and cGVHD is low [3], but it appears that as transplant-related mortality declines, relapse is unacceptably high. Clearly, further modifications are needed to reach the goal of long-term disease free survival●[4]. To avoid long-term immune deficiency mediated by circulating Campath after transplantation or PTCy acting early after transplantation on all engrafted immune cells, graft manipulation by CD34+ selection[5] or αβ TCR depletion[6–8] has been explored. Such strategies have been associated with lower incidences of aGVHD and cGHVD, in some cases without the need for post-transplant immune suppression and without an increase in relapse [5].
Individual variations in IR remain a challenge. Even in unmanipulated grafts, αβ T cell-based immunity is severely impaired the first months post allo-HCT[9], with a substantial variability between individuals. In response, patient-tailored grafts and individualized dosing of conditioning regimens have been proposed to equalize immune reconstruction [10]. Innate immune subsets including NK cells and γδT cells present an additional opportunity to improve tumor control shortly after transplant. These cells reconstitute swiftly post allo-HCT [11, 9] and are not associated with GVHD[12]. NK cells recover faster in numbers after αβ TCR depletion as compared to CD34+ selection, presumably due the co-transferred NK cells within the graft [13, 14]. Moreover, γδT cells can be detected at physiological numbers in the first month post allo-HCT after αβ TCR depletion [7]. The major challenge for both subpopulations is to retain functionality and / or diversity even in the absence of early immune suppression. This challenge is very well illustrated in recipients of ex vivo expanded NK and γδT cells. Although some successes are reported, clinical efficacy is often disappointing, most likely due to loss of function of the transferred innate cells [15, 16]. Interventions aiming to improve early outcome after allo-HCT should result in profound enrichment and improved functionality of both NK and γδT cells [17]. Administration of bisphosphonates, cytokines and bispecific or trispecific immune engagers, checkpoint inhibitors, and “controlled infection” or vaccination (Figure 1) are all examples of such interventions and will therefore be the focus of this review.
Figure 1.
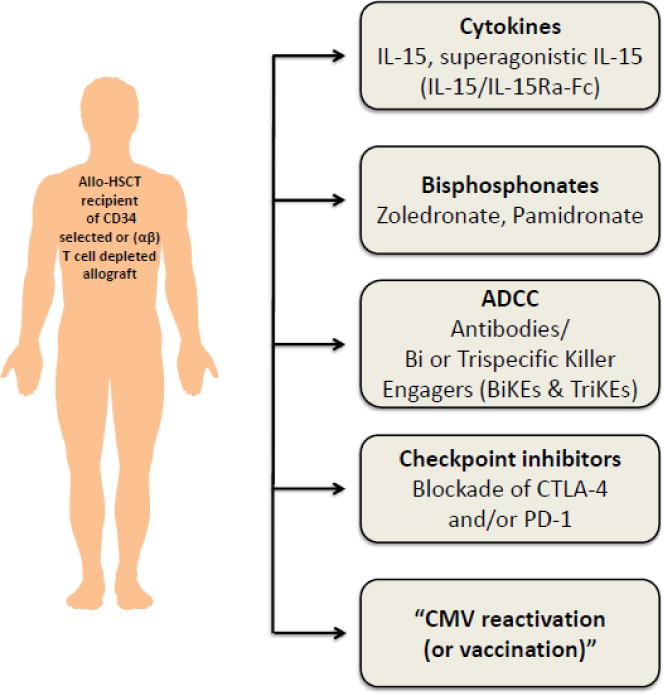
Potential strategies to induce NK and γδ T cell based anti-tumor responses after allo-HCT. ADCC (antibody-dependent cytotoxicity); Allo-HCT (allogeneic hematopoietic cell transplantation); CMV (cytomegalo virus); CTLA-4 (cytotoxic T-lymphocyte–associated antigen); IL-15 (interleukin 15); PD-1 (programmed death-1).
1.2 Basic biology of NK and γδT cells
1.2.1 NK cells
NK cells and γδT cells are major factors in cancer immune surveillance [18, 19]. NK cells are large granular lymphocytes with the ability to lyse virally-infected cells and tumor targets without MHC-restriction or prior sensitization. Human NK cells comprise around 10–15% of the lymphocyte pool. NK cells can kill infected or transformed cells both by direct cytotoxicity or by the production of cytokines [20]. NK cell reactivity is tightly regulated via activating and inhibiting receptors, the balance of which will dictate the fate of an NK cell [20]. In order to react with a target cell, NK cells require engagement of an activating receptor and functional competence through inhibitory receptor signaling. This process, referred to as NK cell education or licensing, prevents auto reactivity of NK cells not expressing inhibitory receptors recognizing self-MHC [21]. By contrast, chronic triggering of activating receptors can lead to loss of function of an NK cell [21]. Thus, NK cell function is adaptable or fluid.
Activating receptors detect ligands representing a cell in ‘distress’. These receptors include natural cytotoxicity receptors (NCRs), c-type lectins (including NKG2D which can engage with MICA/MICB or ULBPs and NKG2C/CD94 which can engage with HLA-E), DNAM-1 (which is specific for poliovirus receptor (PVR) and Nectin-2) [22], and the low affinity Fc receptor CD16 [23]. Inhibitory receptors include the killer immunoglobulin-like receptors (KIRs) and the C-type lectin NKG2A/CD94, which also recognizes HLA-E. KIRs are polymorphic molecules that are not only inhibitory, but can also be activating. The ligand for inhibitory KIRs are HLA class I molecules [24]. Four types of KIRs can provide inhibitory signals through engagement with defined HLA molecules. When inhibitory molecules engage with HLA molecules, the target cells will be considered as ‘self’. In allo-HCT (especially haplo-HCT), donor-recipient KIR mismatches (‘missing self’) are reported to contribute to the Graft-versus-Leukemia (GVL) effect [25].
NK cells are divided in CD56dim and CD56bright subsets (Table 1). In a steady state situation, 90% of the NK cells in peripheral blood (PB) are CD56dimCD16+. CD56dim NK cells highly express granzyme/perforin and can mediate direct cytotoxicity. CD56bright cells lack perforin, but are strong producers of cytokines such as IFNγ upon stimulation (e.g. with IL-15, IL-12 and IL-18) [26].
Table 1.
Potential strategies to induce NK and γδ T cell-based anti-tumor responses after allo-HCT
| Name | Brief description | Ref. |
|---|---|---|
| CD56 bright NK cells | 10% NK cells in PB with as most important function cytokine production | [26] |
| CD56dim NK cells | 90% NK cells in PB with as most important function cytotoxicity | [26] |
| Adaptive NK cells | NKG2C+CD56+CD57 NK cells which are induced by CMV infection. Adaptive NK cells persist long time and can expand upon reactivation of CMV. | [83] |
| Unconventional T cells | CD3+ lymphocytes (including γδ T cells and NKT cells) with unconventional TCR recognizing peptides, lipids or other molecules representing an altered metabolic state of a target cell. | [30] |
| Vδ2+ T cells | γδ T cells with the vγ9δ2 TCR. Normally up to 95% of γδ T cells in PB. Vδ2+ T cells recognize derivatives of an altered mevalonate pathway. | [37, 36] |
| Vδ2- T cells | γδ T cells not expressing a vδ2 delta chain of the γδ TCR. The majority of vδ2- γδ T cells are vδ1+. Vδ2- γδ T cells usually comprise the minority of circulating γδ T cells in PB, but up to 50% of tissue residing γδ T cells. CMV infection can result in proliferation of vδ1+ γδ T cells. | [89, 88] |
1.2.2 γδT cells
γδT cells are CD3+ lymphocytes and comprise 1–5% of the PB lymphocyte pool. γδT cells can also be found in secondary lymphoid organs and peripheral tissue like skin, lung and intestine, where they can represent up to 50% of the T cell population [27]. γδT cells express a range of activating and inhibitory molecules, many of which are shared with NK cells [28, 29]. Like NK cells, activation of γδT cells depends on the net balance of activating and inhibitory signals. In cases of ‘distress’, γδT cells are activated rapidly and contribute to the ‘lymphoid stress-surveillance’ response. This immune response includes direct cytotoxicity and production of cytokines as well as more adaptive features like antigen presentation [29].
γδT cells are sometimes also referred to as ‘unconventional T cells’ (Table 1). Unlike conventional (αβ)T cells, the TCR of unconventional T cells does not bind with antigens presented on MHC molecules, but instead with alternative ligands on target cells[30]. The most extensively characterized γδTCR is the vγ9δ2 TCR [27], which senses elevated levels of isopentenyl pyrophosphate (IPP) of a dysregulated mevalonate pathway[31]. This concept will be expanded in section 1.3.1. Other ligands for γδTCRs include MICA/B, CD1 and endothelial protein C receptor (EPCR)[28].
γδT cells are commonly divided into vδ2+ and vδ2- γδT cells (Table 1). Most vδ2+ T cells express the vγ9δ2. They represent the up to 95% of the γδT cells in PB. The majority of γδT cells in lymphoid organs and peripheral tissue like skin, lung and intestine express either the vδ1 or vδ3 TCR chain, and not vδ2. They are often collectively referred to as vδ2- γδT cells. γδT cells can recognize a range of hematological and solid malignancies [18]. Recently, analysis of tumor-infiltrating lymphocyte revealed that γδT cells have the strongest association with a beneficial outcome in a large series of diverse cancers, suggesting an important role in tumor immune surveillance for these cells●●[32].
1.3 Potential strategies to simultaneously enhance NK and γδT cell immunity post allo-HCT
1.3.1 Bisphosphonates
Bisphosphonates (BPs) like pamidronate (PAM) or zoledronate (ZOL) are routinely administered to prevent osteoporosis and prevent bone loss in cancer patients. In multiple myeloma (MM) patients the development of an acute phase reaction after administration of BPs was associated with expansion of vγ9δ2 γδT cells [33]. Vγ9δ2 γδT cells target tumor cells by sensing elevated levels of isopentenyl pyrophosphate (IPP) from the dysregulated mevalonate pathway (MVA) [34]. The MVA pathway, an essential metabolic pathway for many tumor types, uses acetyl-CoA to produce sterols (including cholesterol) and isoprenoid metabolites[35]. BPs disrupt the MVA pathway, resulting not only in impaired protein prenylation in the osteoclast, but also in accumulation of IPPs in a wide range of cell types. Vγ9δ2 γδT cells recognize a conformational change of the ubiquitously expressed surface protein butyrophilin A1 (BTN3A1). This conformational change is caused by accumulation of IPPs and depends on an altered cellular localization of the small GTP-ase Rho-B[36, 37]. An altered localization of Rho-B can be found in many tumors, but only in a few non-transformed cells (e.g. APCs)●[36]. Administration of BPs has contributed to γδT cell-mediated immune responses in a number of cancer patients[38]. Accumulating knowledge that BPs can induce γδT cell-mediated tumor reactivity [39, 40], and in particular reduce the frequency of EBV-induced lymphoma in mouse models [39], as well as awareness that bone loss and osteoporosis is a common complication post allo-HCT [41], suggest that BPs are logical agents to stimulate γδT cell immunity post allo-HCT. Clinical trials have shown that administration of BPs after allo-HCT is safe and results in increased bone densities[42]. In addition, administration of ZOL shortly after αβ TCR / CD19-depleted haploidentical allo-HCT results in activation of vδ2+ γδT cells, associating with improved clinical outcomes ● [43]. Interestingly, administration of ZOL also resulted in an increase of vδ1+ γδT cells with increased cytotoxicity [43].
BPs may also impact alternative immune mediated anti-tumor responses. Pre-clinical studies have shown that ZOL can activate NK cells[44, 45]. An indirect mechanism is hypothesized in which ZOL stimulates DC-like cells and γδT cells, subsequently enhancing NK cell cytotoxicity [45]. Osteoclasts have also been shown to contribute to an immunosuppressive microenvironment in cancer patients. Part of the immunosuppressive nature of osteoclasts is mediated by expression of checkpoint inhibitors like PD-L1, expression of T-cell metabolism regulators like indoleamine 2, 3-dioxygenase (IDO) [46] and stimulation of regulatory T cells [47], mechanisms likely to also negatively impact NK and γδT cells. Inhibition of the osteoclastogenesis is therefore proposed to create a more pro-inflammatory microenvironment in the bone marrow niche ●[46].
1.3.2 Cytokines (IL-2 and IL-15)
Cytokine therapy can promote NK cell antitumor reactivity [16]. NK cells respond to both IL-2 and IL-15, which signal via the common γ chain receptors. High dose IL-2 was the first cytokine to show, in metastatic melanoma patients, that it can be used to prime the immune system to destroy tumor cells[48]. It is currently the only lymphocyte cytokine approved by the FDA [49]. Clinical translation of this observation to a broadly applicable immune intervention is challenged by the considerable toxicity of high dose IL-2 and the induction of regulatory T cells (even with low dose IL-2). When IL-2 was used to promote γδT cells, clinical trials showed only modest efficacy and significant toxicity [38].
In contrast to IL-2, IL-15 does not induce the proliferation of regulatory T cells [50, 51]. IL-15 induces the association of IL-15Rα, IL-2Rβ and γc, which promotes NK cell development, expansion and homeostasis [52]. In contrast to other γc cytokines, IL-15 requires trans presentation of IL-15Rα to activate NK cells [53]. The cleaved IL-15/IL-15Rα heterodimer is bioactive, whereas the single-chain IL-15 is poorly secreted and unstable. Therefore, serum IL-15 is present in heterodimeric forms [54]. A recently identified mechanism on how IL-15 promotes immune responses is via activation of the mTOR pathway ●[55], which results in subsequent phosphorylation STAT-3 and STAT-5 [52]. In that light, the use of the mTOR inhibitor rapamycin is likely not the optimal immunosuppressive agent for restoration of NK cell function post allo-HCT.
IL-15 also plays an important role in γδT cell function. Loss of IL-15 or any component of its receptor complex in mice not only results in a severe reduction of NK cells, but also of γδT cells, NKT cells and a population of memory CD8 T cells [56]. IL-15 promotes the maturation of a naïve γδT cell repertoire both in cord blood[57] and in thymocytes [58] towards a more cytotoxic repertoire. It also enhances the function of vδ2+ γδT cells in the presence of bisphosphonates [59]. In addition, IL-15 deficiency has been reported to deplete tumor-infiltrating γδT cells, resulting in loss of tumor control ●●[60]. Not surprisingly and in line with ex vivo NK cell expansion [16], IL-15 has recently been described as the key cytokine to generate GMP grade γδT cells active against many hematological malignancies [61, 62]. Like NK cells, IL-15 also signals via the JAK/STAT pathway in γδT cells [59, 58], suggesting a common pathway for IL-15 signaling in both innate immune subsets.
In the first clinical phase I trial using recombinant IL-15 (rh-IL15) in cancer patients, 0.3ug of rh-IL15 daily was determined as the maximum tolerated dose (MTD) ●●[63]. NK cells and γδT cells showed the most marked expansion, followed by a contraction in subsequent weeks. Objective clinical responses were observed in a small fraction of patients. Current efforts therefore focus on reducing toxicity and increasing efficacy of rh-IL15 administration. In line with the increased biological activity of trans-presented heterodimeric IL-15, superagonistic formulas of synthetic IL-15 coupled to the IL-15Rα significantly promoted NK cell development and differentiation in vivo [64]. These include soluble IL-15Rα coupled to the human portion of IgG1 associated with recombinant rh-IL15[65] or a mutant form of rhIL-15 (ALT-803) [66], as well as rhIL-15 directly linked to the high affinity IL-15Rα ‘sushi+ domain’ (CYP0150) [67]. Pre-clinical data showed superior NK activation of these superagonists as compared to conventional rhIL-15. Clinical trials are in progress to determine safety and efficacy. In the context of allo-HCT, (superagonistic) rh-IL15 appears to be a logical intervention to promote early IR of NK and γδT cells, especially after αβ TCR depletion, where the reconstituting immune repertoire primarily consists of NK and γδT cells and the duration of immunosuppression is anticipated to be short.
1.3.3 Bi-and trispecific antibodies
An effective adaptive immune response is characterized by a clonal expansion directed towards a few high- affinity antigens, a concept which is being implemented in all tumor vaccination programs. As NK cells do not perform antigen-specific clonal expansion, alternative approaches will need to be explored to induce NK-mediated immune responses with a magnitude and specificity comparable to adaptive immune responses. A unique feature of NK cells is the high expression of CD16 (FcγRIII), which allows NK cells to perform antibody-dependent cytotoxicity (ADCC). Even though CD16 is a low affinity binding receptor to natural Fc, NK cells are key contributors to the anti-tumor effect seen with the first FDA-approved monoclonal antibody rituximab [68].
To further optimize NK-cell mediated ADCC, bi-and trispecific killer engagers (BiKEs and TriKEs) have been designed which bind CD16 at higher affinity than a natural Fc binder to create a specific immune synapse between NK cells and tumor cells[69]. BiKEs bind to NK cells through a single chain variable fragment (scFv) spaced with an inert linker and combined in a single sequence to a scFv directed against one or more desired tumor antigens (e.g. CD19/CD22/CD33). Upon BiKE administration, NK cells are activated through CD16 signaling and gain specificity via the scFv fragment towards for instance CD33 [70]. Importantly, levels of CD16 expression are not a limiting factor as patient-derived NK cells with lower CD16 expression also respond upon administration of a BiKE [71]. However, even high affinity binding of CD16 with a BiKE does not deliver a proliferation signal to NK cells., We hypothesized that addition of an an IL-15 linker between the scFV against antigen and CD16 would result in promotion of expansion, activation and enhance the survival of NK cells and therefor developeda 161533 TriKE. Compared to the 1633 BiKE, the 161533 TriKE showed superior NK cell function both in patient-derived NK cells as well as in murine models ●[72]. Based on this data, TriKEs are targeted for clinical development.
Although the capacity of NK cells to mediate ADCC via the abundantly expressed FcγRIII CD16 is well-recognized [73], γδT cell-mediated responses upon antibody based immunotherapy are generally not reported. This is somewhat remarkable since it has been long-known that γδT cells express CD16 [74], albeit at much lower and heterogeneous levels as compared to NK cells. In addition, γδT cells have been shown to be able to induce ADCC upon incubation with both monomeric antibodies, as well as CD19 and CD33-based BiKEs and TriKEs [75, 76].
Based upon these promising pre-clinical data, clinical trials planned in the near future may elucidate how TriKEs can improve NK cell and perhaps γδT cell-mediated immune responses in cancer patients. Pending results, FDA- approved antibodies such as rituximab or daratumumab may have potential to induce NK and γδT cell-mediated tumor-specific immune responses shortly post allo-HCT indirectly via CD16 (FcγRIII).
1.3.4 Keeping innate cells functionally active
To prevent uncontrolled immune activation, lymphocytes express a range of inhibitory checkpoint molecules. Blockade of ‘programmed death-1’ (PD-1) or ‘cytotoxic T-lymphocyte–associated antigen’ (CTLA-4) mediated signaling can restore αβ-T cell mediated tumor responses. After decades of translational research, administration of ‘checkpoint inhibitors’ is implemented in daily clinical practice for many types of malignancies[77]. As NK cells[78] and also γδT cells[79] express checkpoint proteins, administration of checkpoint inhibitors is expected to increase NK and γδT cell responses. For example, blocking CTLA-4 in melanoma patients showed an association between tumor control and reconstitution of γ9δ2T cell repertoires [80].
After allo-HCT, checkpoint inhibitors have thus far been administered to only a small series of allo-HCT patients, given the potential risk of inducing severe GVHD, [81, 82]. Larger clinical trials are necessary to establish the safety and efficacy of checkpoint blockade after allo-HCT. If the risk of detrimental side effects of immune checkpoint inhibitors is predominantly determined by αβ T cells, T cell depletion platforms may be a more suitable platform for implementation as compared to T cell replete allo-HCT.
1.3.5 CMV exposure exerts a profound effect on the innate immune repertoire
CMV is a herpes virus which establishes a lifelong latent infection following primary infection. CMV infection is common, with seroprevalence rates ranging from 45–100% [83]. Post allo-HCT, CMV reactivation is observed in a 30–60% of recipients, with GVHD and T cell depletion as risk factors [84].
During a primary CMV infection, NKG2C+ NK cells expand and become strong producers of IFNγ. Importantly, increased frequencies of CD56dimCD57+NKG2C+ NK cells persist, with an ability to expand and become functionally active upon a CMV reactivation [85]. These CMV-induced ‘adaptive NK cells’ share DNA methylation patterns similar to cytotoxic effector T cells but different from conventional NK cells ●[86]. Consistent with other studies [87, 88], we have recently shown that allo-HCT recipients with reactivated CMV had lower leukemia relapse, associating with both higher frequencies and greater absolute numbers of these adaptive NK cells ●[89]. Collectively, these data reinforce the concept that CMV can result in NK cells with properties of immune memory. These properties include priming after secondary target exposure, which may potentially result in lasting malignant disease relapse protection [89].
Vδ1+ T cells are the primary γδT cell subset proliferating upon CMV infection/reactivation [90]. After allo-HCT, CMV induced vδ1+ T cells are capable of recognizing both CMV-infected cells and primary leukemic blasts ●[8, 91]. Next generation sequencing of the γδTCRs revealed that CMV reactivation post allo-HCT induced a clonal proliferation of distinct γδT cell clones, suggesting a γδTCR-mediated response of γδT cells upon CMV infection ●●[92]. Longitudinal analysis of γδT cells in series of allo-HCT recipients demonstrated that γδT cell numbers are significantly higher in recipients with CMV viraemia as compared to recipients without CMV reactivation [93–95, 91]. Memory-like γδT cell responses are increasingly recognized, but many questions remain, including the driving ligands and other signal-driving γδT cell responses [96].
Harnessing the beneficial impact of CMV infection/reactivation on the innate immune repertoire remains challenging. Theoretically, development of a CMV-based vaccine seems the most straightforward approach. Currently there is no FDA-approved CMV vaccine, but over 25 phase 1–3 clinical trials are currently evaluating CMV vaccination [97]. Amongst candidate CMV vaccines are those expressing T cell epitope(s) and/or the CMV glycoprotein B (gB). The CMVPepVax (chimeric peptide composed of a the HLA A*0201 restricted pp65 CD8 T-cell epitope and a tetanus T-helper epitope) and the TransVax vaccine (a CMV DNA vaccine encoding the pp65 epitope and gB) have an attractive safety profile and lower CMV reactivation post allo-HCT in patients still on immunosuppressive medication[98, 99]. Interestingly, a higher relapse- free survival was observed in recipients of CMVPepVax, warranting confirmation in a phase 2 trial ●●[99]. Open questions concern the effectiveness of peptide-based vaccines aiming for a CD8 T cell-based immune response in situations where the αβ T cell repertoire is more strongly impaired and what alternatives are suitable for patients who do not have the desired HLA haplotype.
Though γδT cell responses have also been observed after vaccination with CMV vaccines developed for inducing αβ T cell repertoires[100], those inducing strong (adaptive) NK and γδT cells responses probably have alternative requirements. Two approaches for enhancing NK and γδT cells responses are conceivable. The first involves identifying CMV vaccines with a potent effect on NK and γδT cells. In particular, analysis of the NK and γδT cell repertoire will be of special interest following use of attenuated or replication-incompetent CMV vaccines currently under clinical evaluation [97]. A second approach to harness NK and γδT cell immune responses entails indirect stimulation of already existing (donor derived) adaptive NK and γδT cell repertoires. For example, vaccination with a seasonal influenza vaccine in healthy individuals has shown long-lasting NK cell responses, in which the CMV serostatus impacts the outcome [101]. This finding suggests that the recommended influenza vaccination for allo-HCT recipients[102] might result in adaptive innate responses that are potentially cross-reactive towards other infections or malignant cells. Additionally, within EBV infected individuals, NK and γδT cell repertoires show differential, both redundant and non-redundant, activation patterns [103], which might also apply to CMV infection. Therefore, a next - generation vaccine is required to fulfill the needs synergistic activation of both subsets.
1.4 Concluding remarks
For more than 6 decades, allo-HCT has been the principle mode of cellular immunotherapy. Traditionally, focus has been upon the anti-tumor effect αβ T cells, in which separating GVL from GVHD remains challenging [104]. Adoptive transfer of tumor specific αβ-T cells has been proposed as a way to overcome these challenges. Both ex vivo expanded tumor-infiltrating lymphocytes and genetically engineered αβ-CAR-T cells have shown the impressive capacity of tumor-specific αβ T cells to induce sustained responses [105, 106]. However, widespread clinical implementation is challenged by a limited number of the target antigens suitable for CAR-T cells and by logistic and financial hurdles associated with CAR-T cells manufacturing [107].
Allo-HCT is currently providing hundreds of patients with an array of allogeneic NK and γδT cells [108]. Such allogeneic immune cells can recognize malignant cells, but do not cause GVHD. NK and γδT cells share many innate features, yet they also have been shown to be pleiotropic and diverse [17, 20]. In particular, the interaction between NK and γδT cells in the context of immunological challenge has only recently undergone appraisal [103]. Reconstituting innate repertoires will provide unique possibilities to further elucidate these interactions. A factor to consider is that the composition and function of the tumor-infiltrating lymphocyte repertoire is different as compared to that of circulating lymphocytes [109]. Recently developed high-resolution methods for cell analysis like Cytometry by time of Flight (CyTOF) ●[110], next-generation sequencing of γδ TCRs[92] as well as single-cell RNA-seq [109] have all contributed to new insights in complex (innate) immunological networks.
Substantial progress has been made to develop allo-HCT platforms facilitating early NK and γδ T cell reconstitution. The challenge for immediate future is to build on these efforts by implementing early ‘off the shelf ’ therapies which specifically stimulate reconstituting innate subsets in the timeframe in which αβ T cell IR is still incomplete. This will fulfill an urgent need, as mortality rates of HCT-recipients are highest in the first months after transplantation, due largely to impaired control of infections and relapse.
Acknowledgments
Moniek A. de Witte received the following funding: KWF UU 2015-7553. Jeffrey S. Miller received the following funding: NIH/NCI P01 CA65493, NIH/NCI P01 111412, NIH/NCI R35 CA197292. Funding for this study was provided by ZonMW 43400003 and VIDI-ZonMW 917.11.337, KWF UU 2010-4669, UU 2013-6426, UU 2014-6790 and UU 2015-7601, Vrienden van het UMCU, AICR 10-0736 & 15-0049 to J.K.
Jürgen Kuball is scientific co-founder and CSO of Gadeta BV (www.gadeta.nl) and is a shareholder. He is also an inventor on different patents given to Gadeta via the University Medical Centre Utrecht that deal with γδTCR, processing strategies, and their ligands. In addition, he has received grants from Novartis and Miltenyi Biotech.
Jeffrey S. Miller is a consultant and scientific advisory for Fate Therapeutics and Oxis Biotech. He also is on the scientific advisory board for Celgene. In addition, he has pending patents via the University of Minnesota for Fate Therapeutics and Oxis Biotech.
Footnotes
Compliance with Ethical Standards
Conflict of Interest
Moniek A. de Witte has no conflict of interest.
Human and Animal Rights and Informed Consent
This article refers to published studies with both human and animal subjects performed by the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Baron F, Labopin M, Blaise D, Lopez-Corral L, Vigouroux S, Craddock C, et al. Impact of in vivo T-cell depletion on outcome of AML patients in first CR given peripheral blood stem cells and reduced-intensity conditioning allo-SCT from a HLA-identical sibling donor: a report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone marrow transplantation. 2014;49(3):389–96. doi: 10.1038/bmt.2013.204. [DOI] [PubMed] [Google Scholar]
- 2.Zaia J, Baden L, Boeckh MJ, Chakrabarti S, Einsele H, Ljungman P, et al. Viral disease prevention after hematopoietic cell transplantation. Bone marrow transplantation. 2009;44(8):471–82. doi: 10.1038/bmt.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Luznik L, Bolanos-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115(16):3224–30. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs EJ. HLA-haploidentical blood or marrow transplantation with high-dose, post-transplantation cyclophosphamide. Bone marrow transplantation. 2015;50(Suppl 2):S31–6. doi: 10.1038/bmt.2015.92. Large clinical cohorts who received post transplant cyclophosphamide. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasquini MC, Devine S, Mendizabal A, Baden LR, Wingard JR, Lazarus HM, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(26):3194–201. doi: 10.1200/JCO.2012.41.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maschan M, Shelikhova L, Ilushina M, Kurnikova E, Boyakova E, Balashov D, et al. TCR-alpha/beta and CD19 depletion and treosulfan-based conditioning regimen in unrelated and haploidentical transplantation in children with acute myeloid leukemia. Bone marrow transplantation. 2016;51(5):668–74. doi: 10.1038/bmt.2015.343. [DOI] [PubMed] [Google Scholar]
- 7.Bertaina A, Merli P, Rutella S, Pagliara D, Bernardo ME, Masetti R, et al. HLA-haploidentical stem cell transplantation after removal of alphabeta+ T and B cells in children with nonmalignant disorders. Blood. 2014;124(5):822–6. doi: 10.1182/blood-2014-03-563817. [DOI] [PubMed] [Google Scholar]
- 8.Airoldi I, Bertaina A, Prigione I, Zorzoli A, Pagliara D, Cocco C, et al. gammadelta T-cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-alphabeta+/CD19+ lymphocytes. Blood. 2015;125(15):2349–58. doi: 10.1182/blood-2014-09-599423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosch M, Khan FM, Storek J. Immune reconstitution after hematopoietic cell transplantation. Current opinion in hematology. 2012;19(4):324–35. doi: 10.1097/MOH.0b013e328353bc7d. [DOI] [PubMed] [Google Scholar]
- 10.Admiraal R, Nierkens S, de Witte MA, Petersen EJ, Fleurke GJ, Verrest L, et al. Association between anti-thymocyte globulin exposure and survival outcomes in adult unrelated haemopoietic cell transplantation: a multicentre, retrospective, pharmacodynamic cohort analysis. Lancet Haematol. 2017;4(4):e183–e91. doi: 10.1016/S2352-3026(17)30029-7. [DOI] [PubMed] [Google Scholar]
- 11.Hirokawa M, Horiuchi T, Kawabata Y, Kitabayashi A, Miura AB. Reconstitution of gammadelta T cell repertoire diversity after human allogeneic hematopoietic cell transplantation and the role of peripheral expansion of mature T cell population in the graft. Bone marrow transplantation. 2000;26(2):177–85. doi: 10.1038/sj.bmt.1702478. [DOI] [PubMed] [Google Scholar]
- 12.Scheper W, Grunder C, Straetemans T, Sebestyen Z, Kuball J. Hunting for clinical translation with innate-like immune cells and their receptors. Leukemia. 2014;28(6):1181–90. doi: 10.1038/leu.2013.378. [DOI] [PubMed] [Google Scholar]
- 13.Handgretinger R. Negative depletion of CD3(+) and TcRalphabeta(+) T cells. Current opinion in hematology. 2012;19(6):434–9. doi: 10.1097/MOH.0b013e3283582340. [DOI] [PubMed] [Google Scholar]
- 14.Lang P, Feuchtinger T, Teltschik HM, Schwinger W, Schlegel P, Pfeiffer M, et al. Improved immune recovery after transplantation of TCRalphabeta/CD19-depleted allografts from haploidentical donors in pediatric patients. Bone marrow transplantation. 2015;50(Suppl 2):S6–10. doi: 10.1038/bmt.2015.87. [DOI] [PubMed] [Google Scholar]
- 15.Deniger DC, Moyes JS, Cooper LJ. Clinical applications of gamma delta T cells with multivalent immunity. Frontiers in immunology. 2014;5:636. doi: 10.3389/fimmu.2014.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Childs RW, Carlsten M. Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: the force awakens. Nat Rev Drug Discov. 2015;14(7):487–98. doi: 10.1038/nrd4506. [DOI] [PubMed] [Google Scholar]
- 17.Scheper W, Sebestyen Z, Kuball J. Cancer Immunotherapy Using gammadeltaT Cells: Dealing with Diversity. Frontiers in immunology. 2014;5:601. doi: 10.3389/fimmu.2014.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva-Santos B, Serre K, Norell H. gammadelta T cells in cancer. Nature reviews Immunology. 2015;15(11):683–91. doi: 10.1038/nri3904. [DOI] [PubMed] [Google Scholar]
- 19.Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nature reviews Cancer. 2016;16(1):7–19. doi: 10.1038/nrc.2015.5. [DOI] [PubMed] [Google Scholar]
- 20.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nature immunology. 2008;9(5):503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 21.Orr MT, Lanier LL. Natural killer cell education and tolerance. Cell. 2010;142(6):847–56. doi: 10.1016/j.cell.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198(4):557–67. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nature immunology. 2008;9(5):495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thielens A, Vivier E, Romagne F. NK cell MHC class I specific receptors (KIR): from biology to clinical intervention. Current opinion in immunology. 2012;24(2):239–45. doi: 10.1016/j.coi.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Ruggeri L, Mancusi A, Capanni M, Martelli MF, Velardi A. Exploitation of alloreactive NK cells in adoptive immunotherapy of cancer. Current opinion in immunology. 2005;17(2):211–7. doi: 10.1016/j.coi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Bjorkstrom NK, Ljunggren HG, Sandberg JK. CD56 negative NK cells: origin, function, and role in chronic viral disease. Trends in immunology. 2010;31(11):401–6. doi: 10.1016/j.it.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nature reviews Immunology. 2013;13(2):88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Correia DV, Lopes A, Silva-Santos B. Tumor cell recognition by gammadelta T lymphocytes: T-cell receptor vs. NK-cell receptors. Oncoimmunology. 2013;2(1):e22892. doi: 10.4161/onci.22892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31(2):184–96. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nature immunology. 2015;16(11):1114–23. doi: 10.1038/ni.3298. [DOI] [PubMed] [Google Scholar]
- 31.Sebestyen Z, Scheper W, Vyborova A, Gu S, Rychnavska Z, Schiffler M, et al. RhoB Mediates Phosphoantigen Recognition by Vgamma9Vdelta2 T Cell Receptor. Cell Rep. 2016 doi: 10.1016/j.celrep.2016.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32••.Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nature medicine. 2015;21(8):938–45. doi: 10.1038/nm.3909. Comprehensive analysis of tumor infitrating leucocytes, showing that γδT cells present at the tumor site are strongly associated with clinical benificial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunzmann V, Bauer E, Wilhelm M. Gamma/delta T-cell stimulation by pamidronate. The New England journal of medicine. 1999;340(9):737–8. doi: 10.1056/NEJM199903043400914. [DOI] [PubMed] [Google Scholar]
- 34.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197(2):163–8. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mullen PJ, Yu R, Longo J, Archer MC, Penn LZ. The interplay between cell signalling and the mevalonate pathway in cancer. Nature reviews Cancer. 2016;16(11):718–31. doi: 10.1038/nrc.2016.76. [DOI] [PubMed] [Google Scholar]
- 36•.Sebestyen Z, Scheper W, Vyborova A, Gu S, Rychnavska Z, Schiffler M, et al. RhoB Mediates Phosphoantigen Recognition by Vgamma9Vdelta2 T Cell Receptor. Cell Rep. 2016;15(9):1973–85. doi: 10.1016/j.celrep.2016.04.081. Authors provide a mechanism on how vγ9δ2+ T cells can specically target cancer cells. Altered RhoB activity and distribution in tumor cells induces membrane immobility of BTN3A1, the ligand for the vγ9δ2 TCR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harly C, Guillaume Y, Nedellec S, Peigne CM, Monkkonen H, Monkkonen J, et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human gammadelta T-cell subset. Blood. 2012;120(11):2269–79. doi: 10.1182/blood-2012-05-430470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisher JP, Heuijerjans J, Yan M, Gustafsson K, Anderson J. gammadelta T cells for cancer immunotherapy: A systematic review of clinical trials. Oncoimmunology. 2014;3(1):e27572. doi: 10.4161/onci.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiang Z, Liu Y, Zheng J, Liu M, Lv A, Gao Y, et al. Targeted activation of human Vgamma9Vdelta2-T cells controls epstein-barr virus-induced B cell lymphoproliferative disease. Cancer cell. 2014;26(4):565–76. doi: 10.1016/j.ccr.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 40.Dharnidharka VR, Mohanakumar T. New approaches to treating B-cell cancers induced by Epstein-Barr virus. The New England journal of medicine. 2015;372(6):569–71. doi: 10.1056/NEJMcibr1415117. [DOI] [PubMed] [Google Scholar]
- 41.McClune BL, Polgreen LE, Burmeister LA, Blaes AH, Mulrooney DA, Burns LJ, et al. Screening, prevention and management of osteoporosis and bone loss in adult and pediatric hematopoietic cell transplant recipients. Bone marrow transplantation. 2011;46(1):1–9. doi: 10.1038/bmt.2010.198. [DOI] [PubMed] [Google Scholar]
- 42.Pundole X, Cheema HI, Petitto GS, Lopez-Olivo MA, Suarez-Almazor ME, Lu H. Prevention and treatment of bone loss and fractures in patients undergoing a hematopoietic stem cell transplant: a systematic review and meta-analysis. Bone marrow transplantation. 2017 doi: 10.1038/bmt.2016.312. [DOI] [PubMed] [Google Scholar]
- 43••.Bertaina A, Zorzoli A, Petretto A, Barbarito G, Inglese E, Merli P, et al. Zoledronic acid boosts gammadelta T-cell activity in children receiving alphabeta+ T and CD19+ cell-depleted grafts from an HLA-haplo-identical donor. Oncoimmunology. 2017;6(2):e1216291. doi: 10.1080/2162402X.2016.1216291. This trial shows that ZOL stimulates vδ2+ T cells and to a lesser extend vδ1+ T cells. ZOL treatment may associate with an improved outcome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nussbaumer O, Gruenbacher G, Gander H, Thurnher M. DC-like cell-dependent activation of human natural killer cells by the bisphosphonate zoledronic acid is regulated by gammadelta T lymphocytes. Blood. 2011;118(10):2743–51. doi: 10.1182/blood-2011-01-328526. [DOI] [PubMed] [Google Scholar]
- 45.Maniar A, Zhang X, Lin W, Gastman BR, Pauza CD, Strome SE, et al. Human gammadelta T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood. 2010;116(10):1726–33. doi: 10.1182/blood-2009-07-234211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.An G, Acharya C, Feng X, Wen K, Zhong M, Zhang L, et al. Osteoclasts promote immune suppressive microenvironment in multiple myeloma: therapeutic implication. Blood. 2016;128(12):1590–603. doi: 10.1182/blood-2016-03-707547. In this report the authors show that osteoclasts in MM contribute to the immunosuppressive microenvironment. Treatment with an anti-CD38 antibody shows hat osteoclastogenesis is impaired and T cell immunity is restored. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Charles JF, Aliprantis AO. Osteoclasts: more than 'bone eaters'. Trends Mol Med. 2014;20(8):449–59. doi: 10.1016/j.molmed.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA. 1994;271(12):907–13. [PubMed] [Google Scholar]
- 49••.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192(12):5451–8. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123(25):3855–63. doi: 10.1182/blood-2013-10-532531. Large cumulative report showing 30–50% remission in advanced AML using lymphodepleting chemotherapy, haploidentical NK cells and IL-2 with or with Treg depletion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waldmann TA. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. Cancer Immunol Res. 2015;3(3):219–27. doi: 10.1158/2326-6066.CIR-15-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rautela J, Huntington ND. IL-15 signaling in NK cell cancer immunotherapy. Current opinion in immunology. 2016;44:1–6. doi: 10.1016/j.coi.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200(7):825–34. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bergamaschi C, Bear J, Rosati M, Beach RK, Alicea C, Sowder R, et al. Circulating IL-15 exists as heterodimeric complex with soluble IL-15Ralpha in human and mouse serum. Blood. 2012;120(1):e1–8. doi: 10.1182/blood-2011-10-384362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55•.Mao Y, van Hoef V, Zhang X, Wennerberg E, Lorent J, Witt K, et al. IL-15 activates mTOR and primes stress-activated gene-expression leading to prolonged anti-tumor capacity of NK cells. Blood. 2016 doi: 10.1182/blood-2016-02-698027. This study shows by gene-expression analysis how IL-15 is superior towards IL-2 in stimulating NK cells and that mTOR is key in the signaling process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191(5):771–80. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cairo C, Sagnia B, Cappelli G, Colizzi V, Leke RG, Leke RJ, et al. Human cord blood gammadelta T cells expressing public Vgamma2 chains dominate the response to bisphosphonate plus interleukin-15. Immunology. 2013;138(4):346–60. doi: 10.1111/imm.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ribot JC, Ribeiro ST, Correia DV, Sousa AE, Silva-Santos B. Human gammadelta thymocytes are functionally immature and differentiate into cytotoxic type 1 effector T cells upon IL-2/IL-15 signaling. J Immunol. 2014;192(5):2237–43. doi: 10.4049/jimmunol.1303119. [DOI] [PubMed] [Google Scholar]
- 59.Garcia VE, Jullien D, Song M, Uyemura K, Shuai K, Morita CT, et al. IL-15 enhances the response of human gamma delta T cells to nonpeptide [correction of nonpetide] microbial antigens. J Immunol. 1998;160(9):4322–9. [PubMed] [Google Scholar]
- 60••.Dadi S, Chhangawala S, Whitlock BM, Franklin RA, Luo CT, Oh SA, et al. Cancer Immunosurveillance by Tissue-Resident Innate Lymphoid Cells and Innate-like T Cells. Cell. 2016;164(3):365–77. doi: 10.1016/j.cell.2016.01.002. In a mouse models these authors show that the non circulating lymphocytes are shaped by the tumor microenvironment. IL-15 promotes anti-tumor reactivity of these innate lymphoid cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Almeida AR, Correia DV, Fernandes-Platzgummer A, da Silva CL, da Silva MG, Anjos DR, et al. Delta One T Cells for Immunotherapy of Chronic Lymphocytic Leukemia: Clinical-Grade Expansion/Differentiation and Preclinical Proof of Concept. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22(23):5795–804. doi: 10.1158/1078-0432.CCR-16-0597. [DOI] [PubMed] [Google Scholar]
- 62.Van Acker HH, Anguille S, Willemen Y, Van den Bergh JM, Berneman ZN, Lion E, et al. Interleukin-15 enhances the proliferation, stimulatory phenotype, and antitumor effector functions of human gamma delta T cells. J Hematol Oncol. 2016;9(1):101. doi: 10.1186/s13045-016-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63••.Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33(1):74–82. doi: 10.1200/JCO.2014.57.3329. In this first clinical trial with recombinant IL-15, the authors show that r-IL15 most strongly promotes NK and γδT cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206(1):25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, et al. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha} Proceedings of the National Academy of Sciences of the United States of America. 2006;103(24):9166–71. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong HC, Jeng EK, Rhode PR. The IL-15-based superagonist ALT-803 promotes the antigen-independent conversion of memory CD8+ T cells into innate-like effector cells with antitumor activity. Oncoimmunology. 2013;2(11):e26442. doi: 10.4161/onci.26442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mortier E, Quemener A, Vusio P, Lorenzen I, Boublik Y, Grotzinger J, et al. Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective and potent agonist of IL-15 action through IL-15R beta/gamma. Hyperagonist IL-15 × IL-15R alpha fusion proteins. J Biol Chem. 2006;281(3):1612–9. doi: 10.1074/jbc.M508624200. [DOI] [PubMed] [Google Scholar]
- 68.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47(2):115–23. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Felices M, Lenvik TR, Davis ZB, Miller JS, Vallera DA. Generation of BiKEs and TriKEs to Improve NK Cell-Mediated Targeting of Tumor Cells. Methods Mol Biol. 2016;1441:333–46. doi: 10.1007/978-1-4939-3684-7_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gleason MK, Verneris MR, Todhunter DA, Zhang B, McCullar V, Zhou SX, et al. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol Cancer Ther. 2012;11(12):2674–84. doi: 10.1158/1535-7163.MCT-12-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gleason MK, Ross JA, Warlick ED, Lund TC, Verneris MR, Wiernik A, et al. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood. 2014;123(19):3016–26. doi: 10.1182/blood-2013-10-533398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72•.Vallera DA, Felices M, McElmurry R, McCullar V, Zhou X, Schmohl JU, et al. IL15 Trispecific Killer Engagers (TriKE) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing Persistence, In Vivo Expansion, and Enhanced Function. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22(14):3440–50. doi: 10.1158/1078-0432.CCR-15-2710. Here the authors show that adding an IL-15 linker to a CD16-CD33 BiKE creates a TriKE with more robust NK cell mediated anti-leukemia responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gillis C, Gouel-Cheron A, Jonsson F, Bruhns P. Contribution of Human FcgammaRs to Disease with Evidence from Human Polymorphisms and Transgenic Animal Studies. Frontiers in immunology. 2014;5:254. doi: 10.3389/fimmu.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Braakman E, van de Winkel JG, van Krimpen BA, Jansze M, Bolhuis RL. CD16 on human gamma delta T lymphocytes: expression, function, and specificity for mouse IgG isotypes. Cell Immunol. 1992;143(1):97–107. doi: 10.1016/0008-8749(92)90008-d. [DOI] [PubMed] [Google Scholar]
- 75.Schiller CB, Braciak TA, Fenn NC, Seidel UJ, Roskopf CC, Wildenhain S, et al. CD19-specific triplebody SPM-1 engages NK and gammadelta T cells for rapid and efficient lysis of malignant B-lymphoid cells. Oncotarget. 2016 doi: 10.18632/oncotarget.13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seidel UJ, Vogt F, Grosse-Hovest L, Jung G, Handgretinger R, Lang P. gammadelta T Cell-Mediated Antibody-Dependent Cellular Cytotoxicity with CD19 Antibodies Assessed by an Impedance-Based Label-Free Real-Time Cytotoxicity Assay. Frontiers in immunology. 2014;5:618. doi: 10.3389/fimmu.2014.00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer cell. 2015;27(4):450–61. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nature immunology. 2016;17(9):1025–36. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 79.Iwasaki M, Tanaka Y, Kobayashi H, Murata-Hirai K, Miyabe H, Sugie T, et al. Expression and function of PD-1 in human gammadelta T cells that recognize phosphoantigens. European journal of immunology. 2011;41(2):345–55. doi: 10.1002/eji.201040959. [DOI] [PubMed] [Google Scholar]
- 80.Wistuba-Hamprecht K, Martens A, Haehnel K, Geukes Foppen M, Yuan J, Postow MA, et al. Proportions of blood-borne Vdelta1+ and Vdelta2+ T-cells are associated with overall survival of melanoma patients treated with ipilimumab. Eur J Cancer. 2016;64:116–26. doi: 10.1016/j.ejca.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Albring JC, Inselmann S, Sauer T, Schliemann C, Altvater B, Kailayangiri S, et al. PD-1 checkpoint blockade in patients with relapsed AML after allogeneic stem cell transplantation. Bone marrow transplantation. 2017;52(2):317–20. doi: 10.1038/bmt.2016.274. [DOI] [PubMed] [Google Scholar]
- 82.Yared JA, Hardy N, Singh Z, Hajj S, Badros AZ, Kocoglu M, et al. Major clinical response to nivolumab in relapsed/refractory Hodgkin lymphoma after allogeneic stem cell transplantation. Bone marrow transplantation. 2016;51(6):850–2. doi: 10.1038/bmt.2015.346. [DOI] [PubMed] [Google Scholar]
- 83.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20(4):202–13. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- 84.Boeckh M, Nichols WG, Papanicolaou G, Rubin R, Wingard JR, Zaia J. Cytomegalovirus in hematopoietic stem cell transplant recipients: Current status, known challenges, and future strategies. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2003;9(9):543–58. doi: 10.1016/s1083-8791(03)00287-8. [DOI] [PubMed] [Google Scholar]
- 85.Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119(11):2665–74. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86••.Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42(3):443–56. doi: 10.1016/j.immuni.2015.02.008. Here the authors show that CMV infection induces a epigenetic diversification of adaptive NK cells, parallelling T cell differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Elmaagacli AH, Steckel NK, Koldehoff M, Hegerfeldt Y, Trenschel R, Ditschkowski M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118(5):1402–12. doi: 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- 88.Green ML, Leisenring WM, Xie H, Walter RB, Mielcarek M, Sandmaier BM, et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122(7):1316–24. doi: 10.1182/blood-2013-02-487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89•.Cichocki F, Cooley S, Davis Z, DeFor TE, Schlums H, Zhang B, et al. CD56dimCD57+NKG2C+ NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia. 2016;30(2):456–63. doi: 10.1038/leu.2015.260. In a large clinical cohort CMV induced adaptive NK cells results in reduced relapse rates after allo-HCT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dechanet J, Merville P, Lim A, Retiere C, Pitard V, Lafarge X, et al. Implication of gammadelta T cells in the human immune response to cytomegalovirus. The Journal of clinical investigation. 1999;103(10):1437–49. doi: 10.1172/JCI5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91•.Scheper W, van Dorp S, Kersting S, Pietersma F, Lindemans C, Hol S, et al. gammadeltaT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia. 2013;27(6):1328–38. doi: 10.1038/leu.2012.374. This report shows cross reactivity of CMV induced vδ1+ T cells with cancer cells. [DOI] [PubMed] [Google Scholar]
- 92••.Ravens S, Schultze-Florey C, Raha S, Sandrock I, Drenker M, Oberdorfer L, et al. Human gammadelta T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nature immunology. 2017 doi: 10.1038/ni.3686. This report utilizes NGS to show that γδT cells reconstitute quickly post allo-HCT. CMV reactivation results in adaptive clonal expansion of γδT cells. [DOI] [PubMed] [Google Scholar]
- 93.Laberko A, Bogoyavlenskaya A, Shelikhova L, Shekhovtsova Z, Balashov D, Voronin K, et al. Risk Factors for and the Clinical Impact of Cytomegalovirus and Epstein-Barr Virus Infections in Pediatric Recipients of TCR Alpha/Beta- and CD19-Depleted Grafts. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2016 doi: 10.1016/j.bbmt.2016.12.635. [DOI] [PubMed] [Google Scholar]
- 94.Knight A, Madrigal AJ, Grace S, Sivakumaran J, Kottaridis P, Mackinnon S, et al. The role of Vdelta2-negative gammadelta T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood. 2010;116(12):2164–72. doi: 10.1182/blood-2010-01-255166. [DOI] [PubMed] [Google Scholar]
- 95.Pitard V, Roumanes D, Lafarge X, Couzi L, Garrigue I, Lafon ME, et al. Long-term expansion of effector/memory Vdelta2-gammadelta T cells is a specific blood signature of CMV infection. Blood. 2008;112(4):1317–24. doi: 10.1182/blood-2008-01-136713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lalor SJ, McLoughlin RM. Memory gammadelta T Cells-Newly Appreciated Protagonists in Infection and Immunity. Trends in immunology. 2016;37(10):690–702. doi: 10.1016/j.it.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 97.Schleiss MR. Cytomegalovirus vaccines under clinical development. J Virus Erad. 2016;2(4):198–207. [PMC free article] [PubMed] [Google Scholar]
- 98.Kharfan-Dabaja MA, Boeckh M, Wilck MB, Langston AA, Chu AH, Wloch MK, et al. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2012;12(4):290–9. doi: 10.1016/S1473-3099(11)70344-9. [DOI] [PubMed] [Google Scholar]
- 99•.Nakamura R, La Rosa C, Longmate J, Drake J, Slape C, Zhou Q, et al. Viraemia, immunogenicity, and survival outcomes of cytomegalovirus chimeric epitope vaccine supplemented with PF03512676 (CMVPepVax) in allogeneic haemopoietic stem-cell transplantation: randomised phase 1b trial. Lancet Haematol. 2016;3(2):e87–98. doi: 10.1016/S2352-3026(15)00246-X. Clinical trial showing that administration of the CMV specific peptide vaccine early after allo-HCT is safe, results in less CMV viraemia and improved relape free survival. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schmitt M, Schmitt A, Wiesneth M, Hückelhoven A, Wu Z, Kuball J, Wang L, Schauwecker P, Hofmann S, Götz M, Michels B, Maccari B, Wuchter P, Mertens T, Schnitzler P, Döhner H, Ho AD, Bunjes D, Dreger P, Schrezenmeier H, Greiner J. Peptide Vaccination Against Cytomegalovirus (CMV) Elicits Immunological and Clinical Responses after Allogeneic Stem Cell Transplantation Even from a CMV Seronegative Donor. ASH; San Diego. 2016 [Google Scholar]
- 101.Goodier MR, Rodriguez-Galan A, Lusa C, Nielsen CM, Darboe A, Moldoveanu AL, et al. Influenza Vaccination Generates Cytokine-Induced Memory-like NK Cells: Impact of Human Cytomegalovirus Infection. J Immunol. 2016;197(1):313–25. doi: 10.4049/jimmunol.1502049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014;58(3):e44–100. doi: 10.1093/cid/cit684. [DOI] [PubMed] [Google Scholar]
- 103•.Djaoud Z, Guethlein LA, Horowitz A, Azzi T, Nemat-Gorgani N, Olive D, et al. Two alternate strategies for innate immunity to Epstein-Barr virus: One using NK cells and the other NK cells and gammadelta T cells. J Exp Med. 2017 doi: 10.1084/jem.20161017. Here the authors show that EBV infections results in differential NK and γδT cell mediated innate immune responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gale RP, Fuchs EJ. Is there really a specific graft-versus-leukaemia effect? Bone marrow transplantation. 2016;51(11):1413–5. doi: 10.1038/bmt.2016.183. [DOI] [PubMed] [Google Scholar]
- 105.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nature reviews Immunology. 2012;12(4):269–81. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nature reviews Cancer. 2016;16(9):566–81. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de Witte MA, Kierkels GJ, Straetemans T, Britten CM, Kuball J. Orchestrating an immune response against cancer with engineered immune cells expressing alphabetaTCRs, CARs, and innate immune receptors: an immunological and regulatory challenge. Cancer immunology, immunotherapy: CII. 2015;64(7):893–902. doi: 10.1007/s00262-015-1710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thomas ED, Lochte HL, Jr, Cannon JH, Sahler OD, Ferrebee JW. Supralethal whole body irradiation and isologous marrow transplantation in man. The Journal of clinical investigation. 1959;38:1709–16. doi: 10.1172/JCI103949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Singer M, Wang C, Cong L, Marjanovic ND, Kowalczyk MS, Zhang H, et al. A Distinct Gene Module for Dysfunction Uncoupled from Activation in Tumor-Infiltrating T Cells. Cell. 2016;166(6):1500–11 e9. doi: 10.1016/j.cell.2016.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110•.Horowitz A, Strauss-Albee DM, Leipold M, Kubo J, Nemat-Gorgani N, Dogan OC, et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Science translational medicine. 2013;5(208):208ra145. doi: 10.1126/scitranslmed.3006702. First report using CyTOF to perform a more comprehensive analysis of NK cell diversity as compared to classical immuno flow cytometry. [DOI] [PMC free article] [PubMed] [Google Scholar]


