Abstract
Objective
To quantitatively examine the regional densities and hemispheric distribution of the 43-kDa transactive response DNA-binding protein (TDP-43) inclusions, neurons, and activated microglia in a left-handed patient with right hemisphere language dominance and logopenic-variant primary progressive aphasia (PPA).
Methods
Phosphorylated TDP-43 inclusions, neurons, and activated microglia were visualized with immunohistochemical and histologic methods. Markers were quantified bilaterally with unbiased stereology in language- and memory-related cortical regions.
Results
Clinical MRI indicated cortical atrophy in the right hemisphere, mostly in the temporal lobe. Significantly higher densities of TDP-43 inclusions were present in right language-related temporal regions compared to the left or to other right hemisphere regions. The memory-related entorhinal cortex (ERC) and language regions without significant atrophy showed no asymmetry. Activated microglia displayed extensive asymmetry (R > L). A substantial density of neurons remained in all areas and showed no hemispheric asymmetry. However, perikaryal size was significantly smaller in the right hemisphere across all regions except the ERC. To demonstrate the specificity of this finding, sizes of residual neurons were measured in a right-handed case with PPA and were found to be smaller in the language-dominant left hemisphere.
Conclusions
The distribution of TDP-43 inclusions and microglial activation in right temporal language regions showed concordance with anatomic distribution of cortical atrophy and clinical presentation. The results revealed no direct relationship between density of TDP-43 inclusions and activated microglia. Reduced size of the remaining neurons is likely to contribute to cortical atrophy detected by MRI. These findings support the conclusion that there is no obligatory relationship between logopenic PPA and Alzheimer pathology.
Primary progressive aphasia (PPA) is a clinical dementia syndrome characterized by progressive impairment of language function.1,2 In the early stages of the disease, memory, reasoning, and visuospatial abilities remain relatively intact. As the disease progresses, dissolution of language function is accompanied by focal atrophy of major language-related cortical areas in the dominant (usually left) hemisphere.3,4
Dementia syndromes are associated with sites of peak atrophy or hypometabolism within components of the relevant neurocognitive network,5 and each is associated with a heterogeneous set of neuropathologic entities. Brains of patients with PPA display pathologies that include Alzheimer disease (AD), frontotemporal lobar degeneration (FTLD) with tauopathy (FTLD-tau), and FTLD with hyperphosphorylated aggregates of the 43-kDa transactive response DNA-binding protein (TDP-43) (FTLD-TDP).6
For PPA associated with AD pathology (PPA-AD), we previously reported atypical distributions of neurofibrillary tangles that mirror the asymmetry and distribution of peak atrophy sites.3,7,8 We also reported similar results in a cohort of right-handed patients with PPA with FTLD-TDP pathology and mutations of the progranulin (GRN) gene.9
The patient in this study was a left-handed PPA participant with postmortem FTLD-TDP pathology, in whom language function was lateralized to the right hemisphere as revealed by fMRI.10 The clinical characteristics of this patient, including comprehensive neuropsychological findings, have been described in detail previously.10 Here, we investigated the postmortem relationships among pathologic TDP-43 inclusions, neuronal loss, microglial activation, and the distribution of atrophy as revealed by imaging during life and at autopsy.
Methods
Case material
The patient's PPA was characterized by impaired word retrieval and abnormal repetition but preserved word comprehension and grammar,10 which would have fit previously published criteria for logopenic PPA.11 The modified Edinburgh questionnaire revealed a handedness score of −100, demonstrating strong left-handedness. The patient died at the age of 65, after an 8-year course,10 and had no known genetic mutations.
We had limited access to antemortem structural MRI scans. The MRI cuts included in our earlier report10 showed severe asymmetric atrophy predominantly within the right lateral temporal cortex, especially in the superior temporal gyrus (STG) and medial temporal gyrus. The inferior temporal gyrus (ITG), inferior parietal lobule (IPL), and inferior frontal gyrus (IFG) on the right are not fully represented in the existing views but also seem to show asymmetric atrophy on the right.
The fresh brain weight at autopsy was 1,040 g. Gross examination after autopsy showed asymmetric atrophy, with mild atrophy in the left frontal, temporal, and parietal lobes and more severe atrophy in the homologous areas on the right, particularly notable in the temporal lobe (figure 1). There was severe cerebrovascular atherosclerosis, with no gross infarcts or hemorrhages. Severe dilatation of the lateral ventricles, greater on the right, was observed, and no large or lacunar infarcts were seen.
Figure 1. Gross photographs of coronal sections.
Coronal sections show asymmetric atrophy, greater on the right. Severe atrophy of the right temporal cortex and moderate atrophy of the right parietal cortex are seen.
Postmortem evaluation of the brain revealed frequent TDP-43 inclusions, consistent with the pathologic diagnosis of FTLD-TDP.
Patient consent
Written informed consent for brain donation was obtained from the guardian.
Tissue processing
Immediately after autopsy, the brain was placed in formalin at room temperature. After fixation, the hemispheres were cut into 2- to 3-cm slabs and taken through sucrose gradients (10%–40%) for cryoprotection. Whole-hemisphere blocks containing regions of interest were cut at 40-µm thickness on a sliding microtome and stored in 0.1 mol/L phosphate buffer at 4°C until used. Every 1 in 24 sections spanning each cortical region of interest was stained immunohistochemically using the avidin-biotin peroxidase method employing the Vecstastain Elite Kit (Vector Laboratories, Burlingame, CA), and diaminobenzidine as chromogen.
Specific antibodies to TDP-43 phosphorylated at serine residues 409/410 (pS409/410-2, rabbit polyclonal, 1:3,000, CosmoBio, Tokyo, Japan), neuronal nuclear protein (NeuN; clone A60, mouse monoclonal, 1:1,000, EMD Millipore, Burlington, MA), and the major histocompatibility class II cell surface receptor HLA-DR (mouse monoclonal, 1:1,000, Dako, Glostrup, Denmark) were used to visualize TDP-43 inclusions, neurons, and activated microglia, respectively.
The Cresyl violet (Nissl) stain was used to visualize neuronal perikarya.
Unbiased stereologic quantification
Unbiased stereologic methods were used to estimate the density of TDP-43 inclusions, neurons, and activated microglia using bright-field microscopy. The following 6 selected bilateral regions were analyzed at ×60 magnification by a viewer blinded to the hemisphere: IFG, middle frontal gyrus (MFG), IPL, ITG, STG, and entorhinal cortex (ERC). Stereologic analysis was conducted employing the optical fractionator method in the StereoInvestigator software (MBF Biosciences, MicroBrightField, Inc, Williston, VT).
The number of sections quantified varied depending on the extent of each region (5–21 sections). Each region of interest was delineated from the cortical surface to the white matter junction at ×1 magnification, and immunoreactive profiles were quantified at ×60 magnification. The top and bottom 2 μm of each section were used as guard height. All cortical layers were collectively assessed. Densities of TDP-43–immunoreactive neuronal cytoplasmic inclusions (NCIs), neuronal intranuclear inclusions (NIIs), and dystrophic neurites (DNs) were determined separately. NeuN-positive neurons and HLA-DR–positive activated microglia were also quantified across all cell layers. Because HLA-DR is a cell-surface receptor that is upregulated in activated microglia regardless of morphology (e.g., hypertrophic, ramified), all HLA-DR–positive microglia were quantified. Stereological counts were expressed as mean densities of TDP-43 inclusions, neurons, or activated microglia per cubic millimeter on the basis of planimetric calculation of volume by the optical fractionator software. The adopted stereologic parameters resulted in a coefficient of error <0.1. Mean densities were compared between regions and hemispheres because PPA is characterized by asymmetric atrophy patterns in language-related regions of the language-dominant hemisphere.
Previous studies have shown that NeuN may lose antigenicity in the presence of neuronal injury,12,13 and staining may be influenced by tissue fixation and shrinkage. To ensure that potential staining artifacts did not affect counts, matched Cresyl violet–stained sections were stereologically quantified for neurons across all regions. As in NeuN-stained sections, stereologic counts were compared between hemispheres and regions within the Cresyl violet–stained sections and additionally to those of NeuN-stained sections.
Measurement of perikaryal size
The size of pyramidal neurons was separately measured in layers III and V across all regions and hemispheres in Cresyl violet–stained sections because NeuN immunoreactivity does not consistently fill the cytoplasm. To measure neuronal size, 6 to 10 photomicrographs were obtained at ×20 magnification from combined layers III and V per each slide and analyzed using the NIH image analysis software ImageJ (version 1.451, http://imagej.nih.gov/ij/). The tracing function was used to measure the area of soma at 5.5 pixels/μm (image size = 1,600 × 1,200 pixels). Mean total area of neuronal soma from layers III and V was calculated and assessed for differences across regions and hemispheres.
As a measure of the specificity of our results, the soma size measurements from this left-handed case were compared to those of a randomly chosen right-handed PPA-TDP case with FTLD-TDP type A pathology (table) and known patterns of atrophy (left > right) as visualized by quantitative MRI.
Table.
Patient characteristics
Percentage of neurons containing NCIs or NIIs
The percentage of neurons containing TDP-43 inclusions was calculated in each region with the following equation: (density of NCI + NII)/(density of neurons). Neurites (present in axons or dendrites) were excluded from the percent analysis because multiple fragments could be present per cell. All layers were collectively assessed.
Statistical analysis
Counts per slide were used to compare TDP-43 inclusion, microglia, and neuron densities across areas and hemispheres with analysis of variance. Bonferroni post hoc tests were performed to correct for multiple comparisons. Statistical analyses were completed with the GraphPad Prism software (version 7.03, GraphPad Software, Inc, La Jolla, CA).
Results
Densities of TDP-43 inclusions, neurons, and activated microglia were analyzed in 6 bilateral cortical regions. TDP-43 inclusions and activated microglia were present in all cortical areas examined. While TDP-43–positive NCIs, NIIs, and DNs were all present, DNs were present in significantly higher proportions than NCIs and NIIs (figure 2, A and B), consistent with FTLD-TDP type A.14 Cortical DNs were present in 54- to nearly 1,000-fold higher densities than NCIs and 7- to 10-fold higher densities than NIIs. HLA-DR immunoreactivity was visualized in enlarged, activated microglia and their processes, and NeuN immunoreactivity was visualized in neuronal nuclei and frequently also in the cytoplasm (figure 2, C–F).
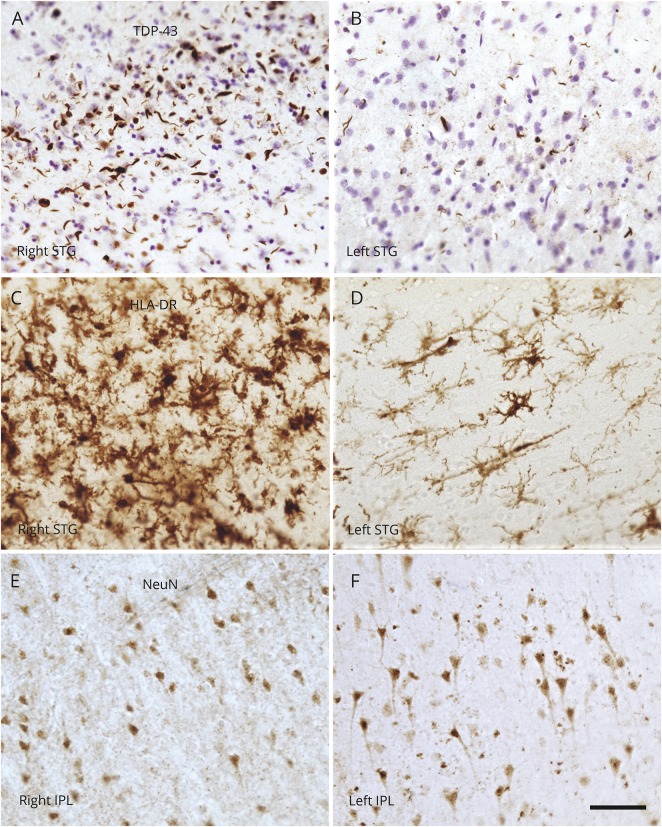
Figure 2. Photomicrographs of TDP-43 inclusions, activated microglia, and neurons in each hemisphere.
(A and B) Distribution of TDP-43 inclusions in the right and left STG, respectively. (C and D) Photomicrographs of activated microglia, visualized with an antibody to HLA-DR, in the (C) right and (D) left STG. The dramatic differences in densities of pathologic markers, which heavily favor the right hemisphere, are noteworthy. NeuN-positive neurons are visualized in the (E) right and (F) left IPL in relatively equal densities. Note the differences in soma size (R < L). Scale bar = 10 μm. IPL = inferior parietal lobule; NeuN = neuronal nuclear protein; STG = superior temporal gyrus; TDP-43 = 43-kDa transactive response DNA-binding protein.
Hemispheric asymmetry of TDP-43 pathology corresponds to pattern of atrophy
Consistent with the severe atrophy of the right temporal lobe, the highest densities of TDP-43 inclusions were found in the STG and ITG in the right hemisphere. Densities of TDP-43 inclusions in the same regions also displayed the most drastic asymmetries compared to the corresponding left hemisphere regions (≈5- and 6-fold more in the right STG and ITG, respectively, compared to the left) (p < 0.0001) (figure 3A). The ERC, a memory-related region located in the temporal lobe, also displayed asymmetry in its distribution of TDP-43 pathology, with more on the right, but this difference was not statistically significant (p > 0.05).
Figure 3. Bilateral densities of TDP-43 inclusions, activated microglia, and neurons across 6 cortical regions.
(A) Counts of TDP-43 inclusions showed heavy asymmetry in the temporal gyri, which aligned with patterns of cortical atrophy as seen by structural MRI and postmortem examination. These numbers represent all forms of TDP-43 inclusions, consisting of DNs, NCIs, and NIIs. (B) Stereologic estimates of activated microglia densities revealed distinct asymmetry favoring the right hemisphere across all investigated regions. (C) Neuronal densities revealed little to no asymmetry. Bars represent mean stereologic counts per mm3 and standard errors; *p < 0.01, **p < 0.001, ***p < 0.0001. DN = dystrophic neurite; ERC = entorhinal cortex; IFG = inferior frontal gyrus; IPL = inferior parietal lobule; ITG = inferior temporal gyrus; MFG = middle frontal gyrus; NCI = neuronal cytoplasmic inclusion; NII = neuronal intranuclear inclusion; STG = superior temporal gyrus; TDP-43 = 43-kDa transactive response DNA-binding protein.
In contrast, a lack of asymmetry or a slightly reversed asymmetry was observed in the IFG, MFG, and IPL, with no statistically significant differences in densities between the hemispheres (p > 0.05). In each hemisphere, each of the language-related regions analyzed (IFG, MFG, IPL, and STG) contained significantly greater numbers of inclusions compared with the memory-related ERC (p < 0.05).
Asymmetric distribution of activated microglia favors the language-dominant hemisphere
Densities of activated microglia were relatively high in all cortical areas, with slightly lower density in the memory-related ERC. However, microglial activation was significantly greater in the right hemisphere across all regions (p < 0.001) except the IPL and ERC (p > 0.05) compared to the left (figure 3B). This asymmetry was most pronounced in the temporal language-related regions.
The selective preference of activated microglia for the right hemisphere is consistent with the anatomic distribution of atrophy demonstrated on in vivo MRI, which had shown striking shrinkage in the perisylvian network of the right hemisphere.10 While this right hemisphere predominance of activated microglia did not directly correlate with TDP-43 inclusion densities, the highest extent of asymmetry for both pathologic markers was located in the STG, as well as the ITG, a region that is also at the center of the most prominent atrophy as observed in vivo and at postmortem (figure 3, A and B).
Perikaryal size is smaller in the language-dominant hemisphere
In contrast to our counts of TDP-43 inclusions and activated microglia, neuronal densities did not show significant patterns of hemispheric asymmetry (p > 0.05) in NeuN- (figures 2, E and F, and 3C) or Cresyl violet–stained sections. While counts from adjacent Cresyl violet–stained sections, like the NeuN-positive tissue, showed a lack of significant variation across regions (p > 0.05) and absence of asymmetry (p > 0.05), the overall densities were smaller compared to counts from NeuN-positive sections. These results validated the findings obtained with the NeuN stain; therefore, neuronal densities from the NeuN sections were used for the remainder of our analyses.
Of interest, significant densities of neurons were present even in regions of prominent atrophy, reaching nearly 15,000 per 1 mm3 (figure 3C). It is noteworthy that the highest total neuronal densities were found in the MFG, IPL, and ERC, regions that showed relatively the least atrophy at postmortem examination. In contrast, the average soma sizes of layer III and V pyramidal neurons were significantly smaller in the right hemisphere compared to the left (p < 0.0001) in all regions except the memory-related ERC, which showed no size difference (p > 0.05) (figure 3, A and B). In the right-handed participant with left hemisphere atrophy, the reverse pattern was seen in both layers III and V, including the ERC (p < 0.0001) (figure 4).
Figure 4. Bilateral soma size in left- and right-handed patients.
Soma sizes of (A) layer III and (B) layer V pyramidal neurons are smaller in the right hemisphere compared to the left across all language-related cortical regions in the left-handed patient. (C and D) The reverse pattern in layers III and V, respectively, in the right-handed individual with left hemisphere atrophy. Note: Bars represent average soma size in μm2 and standard errors. *** p < 0.0001. ERC = entorhinal cortex; IFG = inferior frontal gyrus; IPL = inferior parietal lobule; ITG = inferior temporal gyrus; MFG = middle frontal gyrus; STG = superior temporal gyrus.
In the right STG and ITG, 86% to 89% of the remaining neurons contained an NCI or NII (figure 2D). In contrast, only 29% to 37% of cells in the other right hemisphere regions (IFG, MFG, IPL, and ERC) contained an NCI or NII. This also is in stark contrast to the 10% to 19% of affected neurons in the left temporal regions that contained an NCI or NII.
Discussion
This study reports quantitative histopathologic data in a left-handed patient with PPA, right hemisphere language dominance, and asymmetric atrophy of the right hemisphere.10 In keeping with the logopenic PPA pattern, the right hemisphere atrophy was located predominantly in lateral temporal components of the language network. We found that the right hemisphere, and specifically the lateral temporal cortices, contained higher densities of abnormal TDP-43 precipitates and activated microglia, as well as neurons with smaller perikaryal volumes. These results imply that TDP-43 proteinopathy, microglial activation, and shrinkage of perikarya constitute 3 major substrates of regionally selective atrophy and dysfunction. Although neuronal numbers did not display asymmetry, the average soma size was significantly smaller in all right hemisphere language–related regions, and a higher percentage contained abnormal TDP-43 inclusions in the right hemisphere temporal cortices. In the absence of normative baseline values, we cannot rule out the possibility that there was substantial neuronal loss in both hemispheres. However, cellular shrinkage in the reverse pattern in the right-handed patient suggests that cellular atrophy is a byproduct of disease, rather than the result of normative differences of neuronal size in healthy tissue. Together, our findings suggest that the residual neuronal populations in language areas experience varying degrees of vulnerability to abnormal TDP-43 inclusions. Without baseline values of normative neuronal densities from control brains, the extent of neuron loss cannot be properly evaluated. However, the finding of substantial population of neurons remaining in regions of prominent atrophy and high pathologic density provides an explanation for preservation of fMRI activation at atrophy sites at the same time that the phonological loop function is disturbed.10 Reduced neuronal size in the right hemisphere is a likely substrate for atrophy in life and at autopsy.
One aim of this study was to determine whether microglia, the primary inflammatory mediators in the CNS, show asymmetric and variable densities across language-related cortical regions. Activated microglia are responsible for removing cellular debris, bacteria, protein aggregates, and other elements that may endanger the CNS.15,16 In the present study, we observed asymmetric densities of activated microglia across all cortical regions but without pronounced differences between areas. Some of the HLA-DR positivity may be attributed to increased microglial activation during normal aging, primarily as a response to age-related pathology17; in fact, preliminary data from our laboratory have shown an age-related increase of microglial activation in the white matter of normal brains. However, we have also found that in regions of greater atrophy in PPA-TDP, there is a substantial increase of activated microglia in the white matter (manuscript under review).
The highest burden and asymmetry of TDP-43 inclusions were found in the STG and ITG, both located in the most severely atrophied portion of the perisylvian language network. These findings complement our recent observations highlighting concordant relationships between cortical atrophy, accumulation of TDP-43 pathology, and microglial activation in 4 patients with PPA-TDP, all of whom were right-handed, with GRN mutations.9 Two of those patients had agrammatic PPA, and the other 2 had logopenic PPA. The distribution of pathology was concordant with each clinical PPA phenotype such that the cortical region of highest pathologic burden aligned with the specific regions known to atrophy in a given clinical subtype; in the cases with agrammatic PPA, the highest densities of TDP-43 inclusions were observed in left IFG or MFG, and in participants with logopenic PPA, the highest density of inclusions was seen in the left IPL. In the left-handed patient reported here, the highest density and asymmetry of TDP-43 inclusions were observed in the STG, a core language region known to be a site of peak atrophy in logopenic PPA.1 These findings highlight the principle that it is the anatomic distribution of pathology that dictates clinical syndrome rather than its molecular nature.18 It is also interesting that this patient with logopenic PPA had FTLD-TDP, providing further support for the contention that the association of logopenic PPA with AD is highly probable but not obligatory.1
The results of this study offer a quantitative characterization of the residual neurons that we had reported in areas of cortical atrophy in PPA.18 Although many of these are smaller and contain abnormal inclusions, they are likely the source of retained functional activation that can be observed within areas of atrophy in PPA.18 We also provide a quantitative mapping of the regional and hemispheric distribution of TDP-43 pathology, which shows significant relationship to microglial activation and to functional anatomy in a left-handed patient with PPA with right hemisphere language dominance.
Glossary
- AD
Alzheimer disease
- DN
dystrophic neurite
- ERC
entorhinal cortex
- FTLD
frontotemporal lobar degeneration
- IFG
inferior frontal gyrus
- IPL
inferior parietal lobule
- ITG
inferior temporal gyrus
- MFG
middle frontal gyrus
- NCI
neuronal cytoplasmic inclusion
- NeuN
neuronal nuclear protein
- NII
neuronal intranuclear inclusion
- PPA
primary progressive aphasia
- STG
superior temporal gyrus
- TDP-43
43-kDa transactive response DNA-binding protein
Author contributions
G. Kim contributed to the design and conceptualization of the study, analysis and interpretation of data, and drafting the manuscript. S. Vahedi contributed to the design and conceptualization of the study, analysis and interpretation of data, and revising the manuscript for intellectual content. T. Gefen contributed to revising the manuscript for intellectual content. S. Weintraub is responsible for the clinical diagnosis and contributed to revising the manuscript for intellectual content. E. Bigio is responsible for the pathologic diagnosis and contributed to revising the manuscript for intellectual content. M-M. Mesulam contributed to the design and conceptualization of the study and revising the manuscript for intellectual content. C. Geula contributed to the design and conceptualization of the study, analysis and interpretation of data, and drafting and revising the manuscript for intellectual content.
Study funding
This work was supported by grants from the National Institute of Neurologic Disorders and Stroke (NS085770), the National Institute on Deafness and Other Communication Disorders (DC008552), the Louis Family Foundation, and the Florane and Jerome Rosenstone Fellowship and by an Alzheimer's Disease Center Grant from the National Institute on Aging (AG013854).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH. Asymmetry and heterogeneity of Alzheimer's and frontotemporal pathology in primary progressive aphasia. Brain 2014;137:1176–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mesulam M. Slowly progressive aphasia without generalized dementia. Ann Neurol 1982;11:592–598. [DOI] [PubMed] [Google Scholar]
- 3.Sonty SP, Mesulam MM, Weintraub S, Johnson NA, Parrish TB, Gitelman DR. Altered effective connectivity within the language network in primary progressive aphasia. J Neurosci 2007;27:1334–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mesulam MM. Primary progressive aphasia. Ann Neurol 2001;49:425–432. [PubMed] [Google Scholar]
- 5.Weintraub S, Mesulam M. With or without FUS, it is the anatomy that dictates the dementia phenotype. Brain 2009;132:2906–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bigio EH, Mishra M, Hatanpaa KJ, et al. TDP-43 pathology in primary progressive aphasia and frontotemporal dementia with pathologic Alzheimer disease. Acta Neuropathol 2010;120:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gefen T, Gasho K, Rademaker A, et al. Clinically concordant variations of Alzheimer pathology in aphasic versus amnestic dementia. Brain 2012;135:1554–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonty SP, Mesulam MM, Thompson CK, et al. Primary progressive aphasia: PPA and the language network. Ann Neurol 2003;53:35–49. [DOI] [PubMed] [Google Scholar]
- 9.Kim G, Ahmadian SS, Peterson M, et al. Asymmetric pathology in primary progressive aphasia with progranulin mutations and TDP inclusions. Neurology 2016;86:627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mesulam MM, Weintraub S, Parrish T, Gitelman D. Primary progressive aphasia: reversed asymmetry of atrophy and right hemisphere language dominance. Neurology 2005;64:556–557. [DOI] [PubMed] [Google Scholar]
- 11.Gorno-Tempini M, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collombet JM, Masqueliez C, Four E, et al. Early reduction of NeuN antigenicity induced by soman poisoning in mice can be used to predict delayed neuronal degeneration in the hippocampus. Neurosci Lett 2006;398:337–342. [DOI] [PubMed] [Google Scholar]
- 13.Unal-Cevik I, Kilinc M, Gursoy-Ozdemir Y, Gurer G, Dalkara T. Loss of NeuN immunoreactivity after cerebral ischemia does not indicate neuronal cell loss: a cautionary note. Brain Res 2004;1015:169–174. [DOI] [PubMed] [Google Scholar]
- 14.Mackenzie IR, Baborie A, Pickering-Brown S, et al. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol 2006;112:539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gehrmann J, Banati RB, Kreutzberg GW. Microglia in the immune surveillance of the brain: human microglia constitutively express HLA-DR molecules. J Neuroimmunol 1993;48:189–198. [DOI] [PubMed] [Google Scholar]
- 16.Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol 2017;35:441–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Streit W, Xue Q, Tischer J, Bechmann I. Microglial pathology. Acta Neuropathol Commun 2014;2:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mesulam MM, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol 2014;10:554–569. [DOI] [PMC free article] [PubMed] [Google Scholar]