Abstract
Objective
To examine the association between physical comorbidities and disability progression in multiple sclerosis (MS).
Methods
We conducted a retrospective cohort study using linked health administrative and clinical databases in 2 Canadian provinces. Participants included adults with incident MS between 1990 and 2010 who entered the cohort at their MS symptom onset date. Comorbidity status was identified with validated algorithms for health administrative data and was measured during the 1 year before study entry and throughout the study period. The outcome was the Expanded Disability Status Scale (EDSS) score as recorded at each clinic visit. We used generalized estimating equations to examine the association between physical comorbidities and EDSS scores over time, adjusting for sex, age, cohort entry year, use of disease-modifying drugs, disease course, and socioeconomic status. Meta-analyses were used to estimate overall effects across the 2 provinces.
Results
We identified 3,166 individuals with incident MS. Physical comorbidity was associated with disability; with each additional comorbidity, there was a mean increase in the EDSS score of 0.18 (95% confidence interval [CI] 0.09–0.28). Among specific comorbidities, the presence of ischemic heart disease (IHD) or epilepsy was associated with higher EDSS scores (IHD 0.31, 95% CI 0.01–0.61; epilepsy 0.68, 95% CI 0.11–1.26).
Conclusions
Physical comorbidities are associated with an apparent increase in MS disability progression. Appropriate management of comorbidities needs to be determined to optimize outcomes.
Multiple sclerosis (MS) is an immune-mediated disease of the CNS that typically causes affected individuals to progressively accumulate disability over time,1 even when treated with disease-modifying drugs (DMDs). Heterogeneity in disability outcomes is recognized in MS but remains poorly understood. Ethnicity, social factors, and comorbidity have all been proposed as contributors to this heterogeneity.2
Physical comorbidities are common in MS. A meta-analysis reported that 18.6% of the MS population had hypertension, 10% had hyperlipidemia, and 10% had chronic lung disease.3 Several comorbidities, including hypertension, ischemic heart disease (IHD), and seizure disorders, occur more commonly in the MS population than in the general population.3 Comorbidities have been associated with delays in MS diagnosis4 and initiation of DMDs5 and increased hospitalization rates.6
However, information remains limited regarding the effects of physical comorbidities on disability progression in MS.7 Previous studies have been limited by small sample sizes,8 the use of self-reported data,4,9 and cross-sectional designs that do not establish temporality.4,10 If comorbidities are shown to adversely affect progression, there are implications for prognostication and treatment. Therefore, we examined the effect of physical comorbidities on MS disability progression in 2 populations. We hypothesized that the presence of comorbidities would be associated with greater disability progression in MS.
Methods
Study design and data sources
We followed a common protocol to conduct this retrospective cohort study in 2 Canadian provinces, British Columbia and Nova Scotia, with a combined population of 5.5 million in 2012.11
We used prospectively collected, linked clinical and administrative (health claims) data in each province. Clinical information was gathered from 2 clinical databases, the British Columbia MS clinic database and the Dalhousie MS Research Unit (DMSRU) database. The British Columbia MS database provided data captured from all patients with MS first registered at 1 of the 4 MS clinics in British Columbia, the only source of specialty MS care, between 1980 and 2004. The DMSRU database provided data for all patients with MS who attended the DMSRU at least once from 1980 to 2010. The DMSRU is the only clinic in Nova Scotia providing specialized MS care and captures 85% to 90% of patients with MS in Nova Scotia. Clinical information in each database included the date of MS symptom onset, disease course at onset (relapsing remitting or primary progressive MS), and disability as indicated by the Expanded Disability Status Scale (EDSS) score12 measured at clinic visits.
Information captured in the administrative databases within each province is summarized in table e-1, http://links.lww.com/WNL/A87. The linked administrative data for each province comprised population registry files, physician claims, hospital claims,13–15 and socioeconomic information derived via an algorithm developed by Statistics Canada that assigns quintiles of neighborhood income based on postal code and census data.16 Provincial prescription claims data were available in British Columbia only from January 1, 1996, on.17 In Nova Scotia, all prescriptions for the available first-line injectable DMDs during the study period were captured from the DMSRU database through the Queen Elizabeth II Health Sciences Centre, Halifax, the only facility where prescriptions for these therapies can be accessed in that province. Data linkage in each province was performed at the individual level with encrypted unique personal heath numbers.
Study cohort and follow-up
Using the British Columbia MS and DMSRU clinical databases, we identified adults with MS as confirmed with the prevailing diagnostic criteria at the time the individual was diagnosed.18–20 The study cohorts included cases with incident MS with a symptom onset date (i.e., date at cohort entry) from April 1, 1991, on in British Columbia and 1991 on in Nova Scotia. These dates reflect the first availability of diagnostic codes in the hospital and physician services databases (table e-1). We also required provincial residency for ≥270 cumulative days before cohort entry. Combined, these criteria allowed sufficient comorbidity-related data to be available. Participants were followed up from cohort entry until the last available EDSS assessment before the end of the study, which was December 31, 2008, in British Columbia, and December 31, 2010, in Nova Scotia. Table e-2, http://links.lww.com/WNL/A87, shows participant selection.
Outcome
The outcome was the EDSS score as recorded by a neurologist at each clinic visit. Because it is not fully understood which comorbidities might or might not contribute to disability progression in MS and given that the EDSS was originally designed to measure MS-specific disability, we did not censor or remove scores based on a specific disease or condition.
Measurement of comorbidity status
The comorbidities of interest included diabetes mellitus, hypertension, hyperlipidemia, heart disease, chronic lung disease, and epilepsy. These were selected on the basis of the availability of health administrative case definitions with adequate validity,21–23 potential clinical relevance in the MS population, and recent recommendations for comorbidity research.7 We applied previously validated algorithms21 to identify whether individuals ever met the case definition for an individual comorbidity using data available from the 1-year period before cohort entry until the study end. We used the first claim for the comorbidity as the date of first clinical recognition. This date was also used to determine whether a comorbidity was present or absent at cohort entry (baseline) or whether it was first identified at any time after cohort entry. Because these are chronic conditions, once a comorbidity was evident, it was considered to be present for the rest of follow-up. Comorbidity status at cohort entry and at each EDSS assessment date after cohort entry was examined in 2 ways: (1) as the total number of comorbidities listed above, as a summary measure of comorbidity burden (treated as a continuous variable), and (2) the presence vs absence of each individual comorbidity to assess whether the effects of individual comorbidities differ.
Statistical analyses
The associations between comorbidity status and the study outcome were examined with a generalized linear model with generalized estimating equations (GEEs), choosing a normal distribution with an identity link. We selected this model rather than a mixed linear model because we were interested in population averages; it is less sensitive than mixed models to variance structure specification, has fewer assumptions, and is able to account for correlations between repeated EDSS scores within individuals. This approach also allowed us to use all available EDSS data, maximizing statistical power, unlike survival analysis. Model assumptions were tested and met.24 The EDSS score was treated as a continuous variable. We used an exchangeable correlation structure because this correlation structure best fit the data. Comorbidity status was defined at the time each EDSS score was recorded as defined above; thus, it varied over the course of the study. Potential confounders that did not change over time included sex, disease course at cohort entry, cohort entry year, and socioeconomic status (SES). If the SES quintile at cohort entry was not available, the SES measure closest to the entry date was used. We also considered potential confounders that could change between 1 EDSS assessment date and another, including increasing age in months and DMD use. Age was used as the time scale; therefore, the EDSS changes were interpreted as averages per year of age. Use of a DMD at the time of the EDSS assessment was based on the date of dispensation and days’ supply fields as recorded in the British Columbia prescription database or on the initiation and cessation dates from the DMSRU database.
An additional analysis was conducted to examine MS disability progression associated with the use of comorbidity-related drug treatment (table e-3, http://links.lww.com/WNL/A87).21–23 This analysis was limited to British Columbia because of the lack of availability of prescription claims in Nova Scotia and relied on the dispensing dates and days’ supply fields in the British Columbia prescription database. Comorbidity status at each EDSS assessment date was classified as absent (reference group), present with pharmacologic treatment, and present without pharmacologic treatment.
Because of privacy regulations, which prevent line-level data from leaving the province of origin, analyses were performed in parallel at each site. Results from each site were pooled, and meta-analyses were conducted with random-effects models.25 We present unadjusted and adjusted coefficients with 95% confidence intervals (CIs). A positive coefficient represents the average additional disability burden associated with an increase in the number or the presence of comorbidities. Heterogeneity between sites was estimated with the Cochran Q distributed as a χ2 with k (number of studies) minus 1 df, which indicates the percentage of variance based on the summed squared deviations of the coefficients, with the contribution from each site weighted in the meta-analysis.26 Statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS, Chicago, IL),27 R (version 3.1.2),28 and SAS version 9.3 (SAS Institute Inc, Cary, NC).
Standard protocol approvals, registrations, and patient consents
The Research Ethics boards at each site approved the study. Access to administrative data was approved by the relevant body within each province (British Columbia Ministry of Health and Data Stewardship Committee and the Nova Scotia Department of Health and Wellness).
Results
Study cohort
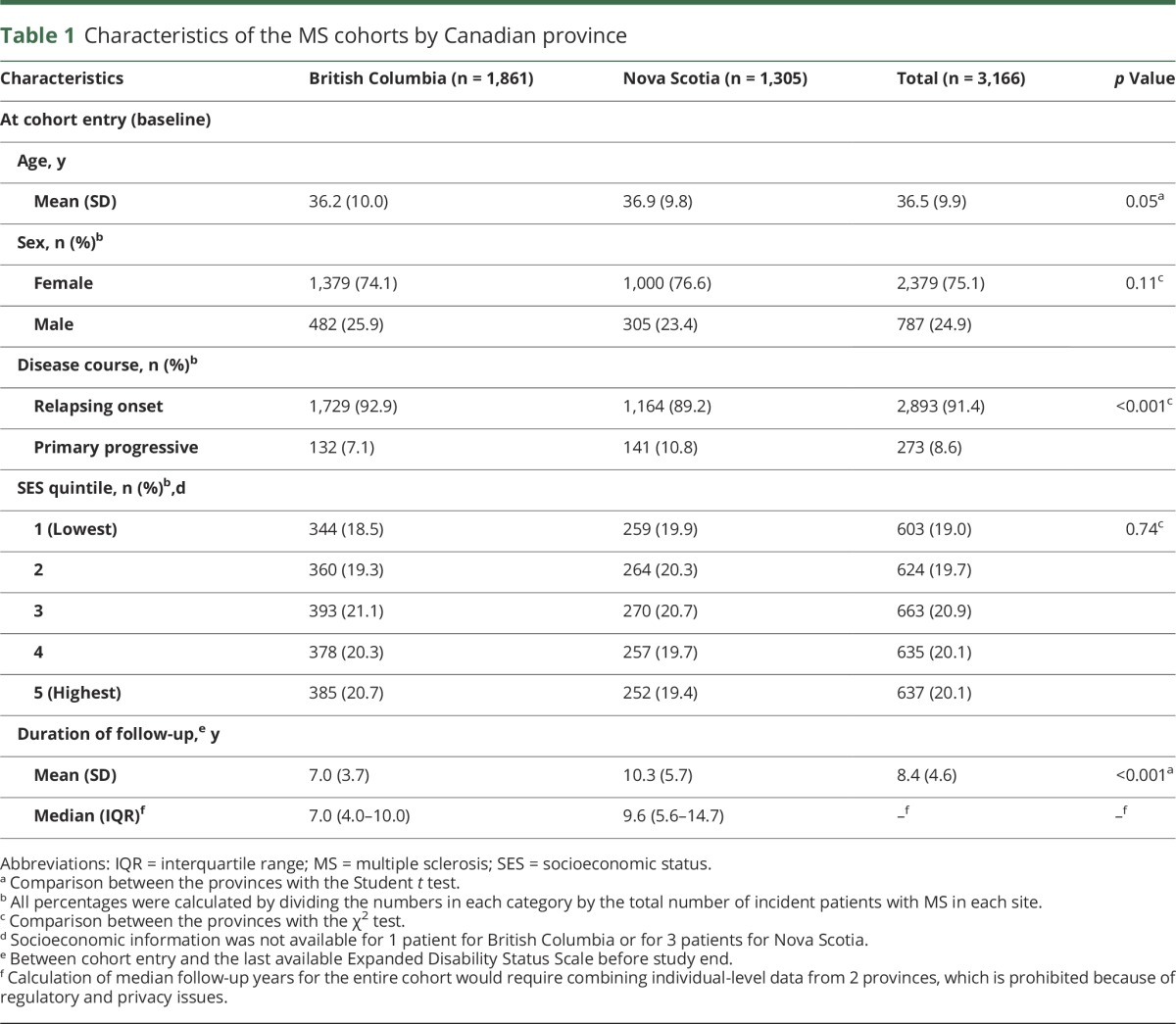
Across the 2 provinces, we identified 3,166 individuals with incident MS during the study period. The distribution of baseline age, sex, and SES was consistent across provinces (table 1). The proportion of individuals with primary progressive MS differed by 3% between Nova Scotia and British Columbia, and follow-up in Nova Scotia averaged 2.3 years longer than in British Columbia, consistent with the greater number of years of data available.
Table 1.
Characteristics of the MS cohorts by Canadian province
Comorbidity
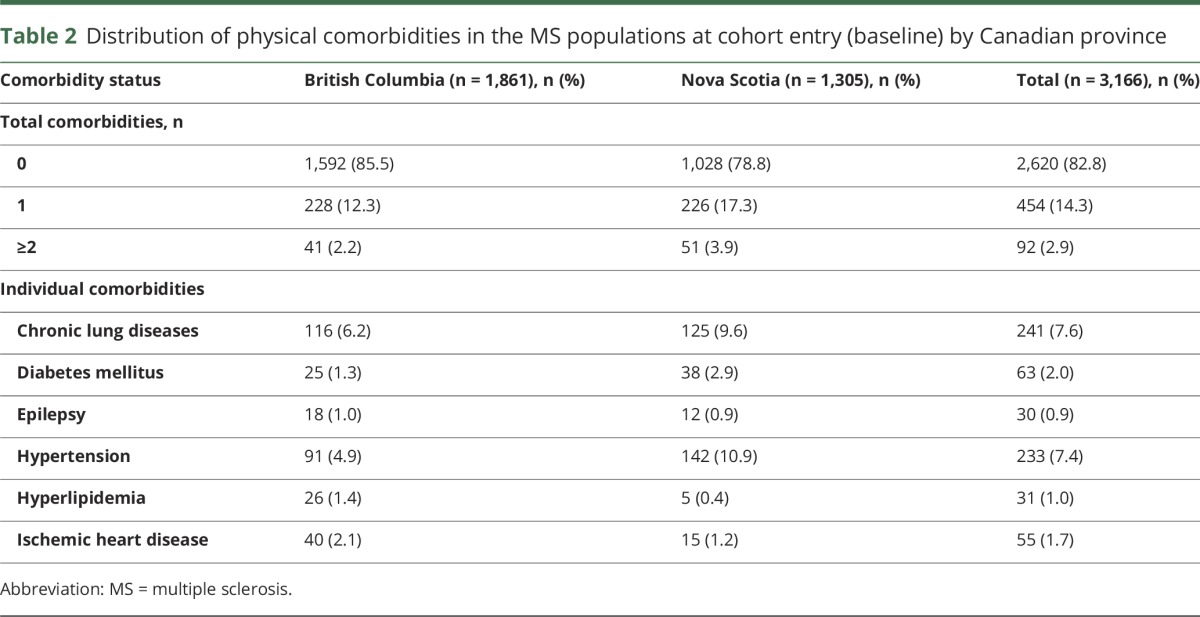
At cohort entry (baseline), a total of 2,620 (82.8%) participants had no evidence of physical comorbidities, 454 (14.3%) had 1 comorbidity, and 92 (2.9%) had ≥2 comorbidities. Among the preexisting individual comorbidities, chronic lung disease and hypertension were most common, followed by IHD and diabetes mellitus (table 2).
Table 2.
Distribution of physical comorbidities in the MS populations at cohort entry (baseline) by Canadian province
Comorbidities and disability progression
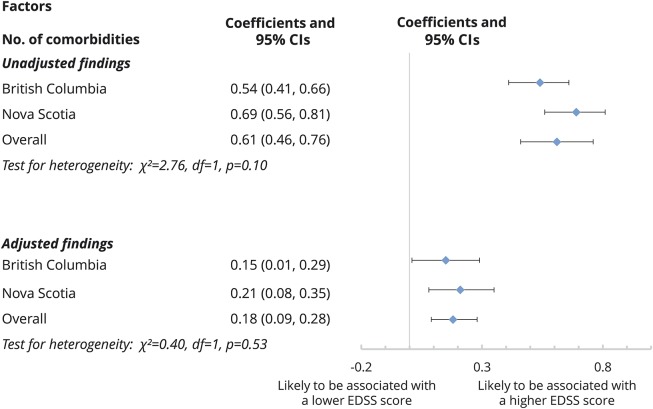
From the unadjusted GEE models, the addition of 1 comorbidity was associated with a mean increase in the EDSS score of 0.61 (95% CI 0.46–0.76) (figure 1). The results of the multivariable GEE models revealed that with each additional comorbidity there was a mean increase of 0.18 (95% CI 0.09–0.28) in the EDSS score over the study period (figure 1).
Figure 1. Association between an increase in the number of physical comorbidities and MS disability (EDSS) over the study period.
A positive coefficient represents the additional disability burden of 1 comorbidity on average. The physical comorbidities of interest included diabetes mellitus, hypertension, hyperlipidemia, heart disease, chronic lung disease, and epilepsy. Results were obtained from generalized estimating equation models. Sex, disease course, cohort entry year, and socioeconomic status were included in the adjusted model. Age was the time scale. CI = confidence interval; EDSS = Expanded Disability Status Scale; MS = multiple sclerosis.
Male sex, increasing age, and a progressive vs a relapsing-onset course were also associated with higher EDSS scores. In an examination of the effects of the number of comorbidities on progression, for example, the adjusted coefficient was 0.18 for male sex (95% CI 0.09–0.28), 0.10 for 1 year of age (95% CI 0.08–0.12), and 0.92 for progressive vs relapsing-onset MS (95% CI 0.66–1.17). In contrast, the highest vs lowest quintile of SES was associated with lower EDSS scores (−0.39, 95% CI −0.57 to −0.20). The use of a DMD was not associated with a higher EDSS score (0.04, 95% CI −0.28 to 0.35).
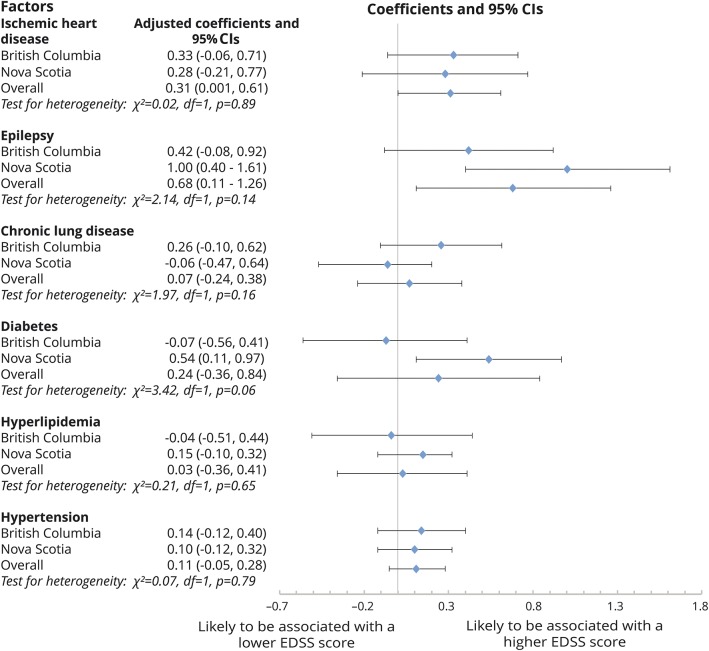
In an examination of each comorbidity individually in unadjusted models, the presence of IHD, epilepsy, hypertension, and hyperlipidemia was associated with higher EDSS scores compared to the absence of the condition (figure e-1, http://links.lww.com/WNL/A86). When all individual comorbidities were included in a multivariable model, IHD and epilepsy remained associated with higher EDSS scores after adjustment for covariates (figure 2). Diabetes mellitus was associated with higher EDSS scores in Nova Scotia but not in British Columbia; the combined result did not reach statistical significance (0.24, 95% CI −0.36 to 0.84). The presence of other individual comorbidities (chronic lung disease, hyperlipidemia, and hypertension) was not associated with the EDSS scores compared to the absence of these comorbidities.
Figure 2. Association between each individual physical comorbidity and MS disability (EDSS) over the study period: findings based on the multivariable GEE model.
Reference groups were patients without the relevant comorbidity. This multivariable model was adjusted for sex, disease course, cohort entry year, use of a disease-modifying drug, and socioeconomic status. Age was the time scale. CI = confidence interval; EDSS = Expanded Disability Status Scale; GEE = generalized estimating equation; MS = multiple sclerosis.
Effects of treatment for comorbidity in the British Columbia cohort
A total of 87 (4.7%) participants in the British Columbia cohort received at least 1 medication for diabetes mellitus, 134 (7.2%) received antihypertensive drugs, 42 (2.3%) received pharmacotherapy for IHD, 27 (1.5%) received anticonvulsants, and 38 (2.0%) received pharmacotherapy for hyperlipidemia during the follow-up period.
The effects of comorbidity on disability progression did not differ on the basis of treatment status (table e-4, http://links.lww.com/WNL/A87). Adjusted coefficients for the drug-treated and untreated comorbidity groups exhibited largely the same direction of effect. However, findings should be interpreted cautiously because of the small number of affected participants.
Discussion
In this multicenter cohort study of 3,166 individuals with incident MS, we found that physical comorbidities were associated with an apparent increase in disability progression in MS indicated by an increase in average EDSS scores. Specifically, we found that the greater the number of comorbidities was, the higher the level of disability was. The magnitude of the effect was substantial. In Rochester County, Minnesota, the average change in EDSS per year is 0.0929; thus, having 1 comorbidity confers an increase in disability as large as 2 years of expected progression over time. In an examination of individual comorbidities, IHD and epilepsy were associated with higher EDSS scores, amounting to the degree of progression occurring over 3 and 6 years, respectively. These findings are consistent with adverse effects of comorbidity on outcomes in other chronic neurologic diseases and in MS. For instance, vascular comorbidities are associated with progression of cognitive impairment and risk of Alzheimer disease.30 All of the comorbidities examined here have been associated with increased health care use and mortality in MS.6
Our findings are consistent with a previous study using the North American Research Committee on Multiple Sclerosis Registry in which the number of vascular comorbidities was associated with an increased risk of gait disability.9 Among the vascular comorbidities evaluated, we found that IHD was associated with greater disability progression. The association between vascular disease such as IHD and MS progression may reflect the increased peripheral inflammation associated with vascular diseases, which may accelerate neurodegeneration and brain atrophy,31,32 thereby increasing disability progression. However, of the other vascular comorbidities we examined (hypertension, diabetes mellitus, hyperlipidemia), none was found to have a statistically significant association with disability after adjustment for confounders. This result is consistent with a study of a US population with MS in which comorbidities were identified on the basis of prescription medications in an electronic medical record and no significant effect of hyperlipidemia on walking speed and self-reported disability was seen.33 However, our finding is contrary to others.8,34 For instance, in a study based on another US population, the investigators examined the effect of specific lipid parameters measured in serum and reported modest effects on disease progression in MS.34 These differences may, in part, reflect differences in the how the comorbidity was measured and differences in outcome measurement. The types of treatments received for the comorbidities also may have differed across studies. In addition, our findings may reflect the small number of individuals affected. Further evaluation of the effects of vascular comorbidities in larger samples is needed.
Our finding regarding the adverse effect of epilepsy on disability progression is consistent with results observed in a Turkish population10 in which individuals with MS and epileptic seizures had greater disability, as measured by the EDSS, than those without seizures. A Chilean study did not observe a significant association between epilepsy and EDSS scores, possibly because of a small sample size, although individuals with poor epilepsy control were found to have lower brain volumes and worse cognitive performance.35 Several hypotheses regarding the adverse effects of epilepsy in MS have been proposed. Some suggest that epilepsy increases inflammatory cortical lesions, as observed on MRI,36 while others suggest that frequent seizures are associated with cortical atrophy and neurodegeneration.37
We did not observe effects of comorbidity-related drug treatment on the association between comorbidity and disability progression, and we are not aware of comparable studies in the literature. It is possible that patients with comorbidity-related treatment may have more active or severe diseases, which may require drug therapy, compared to those who did not require treatment for their comorbidities. In addition, the treatments for comorbidities could have effects on MS that are independent of their effects on the comorbidities. Statins, for example, have pleiotropic effects and may have beneficial effects on disability progression and brain atrophy in MS.38 However, the number of individuals included in these subgroup analyses was relatively small, which limited our ability to detect an effect. Further study is needed to understand the effects of comorbidity treatment on MS outcomes.
Our findings regarding the effects of increasing age, male sex, and a progressive vs relapsing course on disability progression are consistent with the literature.39 We observed that the highest SES quintile was associated with lower EDSS scores compared to the lowest SES quintile independently of comorbidity status. This finding adds to the emerging literature that indicates that SES may be an important factor in disability outcomes.9 This may reflect the observation that lower SES can be associated with reduced self-management skills and adverse health behaviors40 such as smoking, which might also affect disability progression in MS.2
The strengths of our study include that it was population based, spanned 2 geographic regions, and used validated algorithms to determine comorbidity status. Our algorithms for comorbidity were highly specific, but some algorithms such as those for IHD were only moderately sensitive.22 This suggests that we did not capture all cases of each comorbidity, which might bias our findings toward the null. Indicators of comorbidity severity were not available; this would be of value to explore in future studies. Because we used an incident MS cohort, study participants were young with relatively low levels of disability, and the absolute number of individuals affected by comorbidity was small, limiting power to detect an effect. The date of the first clinical recognition of a comorbidity could be misclassified in individuals who infrequently access the health system, potentially underestimating or overestimating the effect on disability progression. In some individuals, epilepsy may develop secondary to MS; therefore, we cannot exclude the possibility that in some cases MS disease progression itself caused epilepsy. We were unable to consider all potential comorbidities that could directly or indirectly affect physical disability; future studies should consider a broader range of comorbidities. Only a small number of participants received drug treatment for each comorbidity, which limited our ability to examine the effects of comorbidity drug treatment. Finally, in the presence of comorbidity, an increase in EDSS score may not necessarily reflect MS-specific disability progression.
This multiprovince, population-based cohort study showed apparent adverse effects of comorbidities on disability progression in MS. On average, as the number of current comorbidities increased, EDSS scores were higher, suggesting an apparent increase in disability progression and that clinicians should pay particular attention to individuals with a high burden of comorbidity. Comorbid IHD and epilepsy were associated with increased EDSS scores. Future work should evaluate the mechanisms underlying these associations and evaluate whether interventions for preventing and treating comorbidities in MS could improve disability outcomes.
Acknowledgment
All inferences, opinions, and conclusions drawn in this publication are those of the authors and do not reflect the opinions or policies of the Data Stewards. No official endorsement by Population Data BC or PharmaNet is intended or should be inferred. Some data used in this report were made available by Health Data Nova Scotia of Dalhousie University. Although this research is based on data obtained from the Nova Scotia Department of Health and Wellness, the observations and opinions expressed of those of the authors and do not represent those of either Health Data Nova Scotia or the Department of Health and Wellness. CONTRIBUTORS: Patricia Caetano, PhD (University of Manitoba, policy consultant); Nicholas Hall, BSc (University of Manitoba, study coordinator); Yan Wang (Dalhousie University, analytic support); and Larry Svenson, BSc (University of Alberta, Collaborator).
Glossary
- CI
confidence interval
- DMD
disease-modifying drug
- DMSRU
Dalhousie MS Research Unit
- EDSS
Expanded Disability Status Scale
- GEE
generalized estimating equation
- IHD
ischemic heart disease
- MS
multiple sclerosis
- SES
socioeconomic status
Contributor Information
Collaborators: CIHR Team in the Epidemiology and Impact of Comorbidity on Multiple Sclerosis, T. Zhang, James Blanchard, Lawrence Elliott, Joanne Profetto-McGrath, Nathalie Jette, and Scott Patten
Author contributions
The corresponding author (Tingting Zhang) takes responsibility for the integrity of the data and the accuracy of the data analysis. The analysts and principal investigators at each site had full access to the data at each site (British Columbia: Helen Tremlett, Feng Zhu, Tingting Zhang; Nova Scotia: John Fisk, Yan Wang). Ruth Ann Marrie, John Fisk, and Helen Tremlett designed the study and obtained funding. Tingting Zhang, Feng Zhu, and Yan Wang performed the statistical analysis. All authors contributed to the interpretation of the data. Tingting Zhang drafted the manuscript. All authors revised the manuscript and approved the final version to be published.
Study funding
This study was supported by the Canadian Institutes of Health Research (CBG 101829), the Rx & D Health Research Foundation, a Don Paty Career Development award from the Multiple Sclerosis Society of Canada (to R.A.M.), a Manitoba Research Chair from Research Manitoba (to R.A.M.), and the Waugh Family Chair in Multiple Sclerosis (to R.A.M.). The funding sources had no role in the study design, collection, analysis or interpretation of the data or in the decision to submit the article for publication. Dr. Zhang received a Post-Doctoral Fellowship Award from the Multiple Sclerosis Society of Canada (2014–2016).
Disclosure
T. Zhang reports no disclosures relevant to the manuscript. H. Tremlett is the Canada Research Chair for Neuroepidemiology and Multiple Sclerosis. She currently receives research support from the National Multiple Sclerosis Society, the Canadian Institutes of Health Research, the Multiple Sclerosis Society of Canada, and the Multiple Sclerosis Scientific Research Foundation. In addition, in the last 5 years she has received research support from the Multiple Sclerosis Society of Canada (Don Paty Career Development Award), the Michael Smith Foundation for Health Research (Scholar Award), and the UK MS Trust, as well as speaker honoraria and/or travel expenses to attend conferences from the Consortium of MS Centres (2013), the National MS Society (2012, 2014, 2016), Teva Pharmaceuticals (2011), ECTRIMS (2011, 2012, 2013, 2014, 2015, 2016), the UK MS Trust (2011), the Chesapeake Health Education Program, US Veterans Affairs (2012), Novartis Canada (2012), Biogen Idec (2014), and the American Academy of Neurology (2013, 2014, 2015, 2016). All speaker honoraria are either declined or donated to an MS charity or to an unrestricted grant for use by her research group. F. Zhu and E. Kingwell report no disclosures relevant to the manuscript. J. Fisk receives research grant and training program support from the Canadian Institutes of Health Research, the National Multiple Sclerosis Society, and the Multiple Sclerosis Society of Canada and has received speaker honoraria from EMD Serono (2013, 2014). V. Bhan has received honoraria and consulting fees from Biogen Idec, EMD Serono, Genzyme, Novartis, Roche, and Teva Neurosciences. T. Campbell has received honoraria from Biogen Idec Canada, Teva Canada Innovation, EMD Serono, Genzyme, Novartis, and Roche; support for travel from Biogen Idec, Teva Canada Innovation, EMD Serono, and Genzyme; grant support from Biogen Idec Canada Inc and Novartis; and research support from Biogen Idec Canada Inc. K. Stadnyk and R. Carruthers report no disclosures relevant to the manuscript. C. Wolfson receives research funding from the Multiple Sclerosis Society of Canada, Canadian Institutes of Health Research, and Multiple Sclerosis International Federation and has received speaking honorarium from Novartis. S. Warren reports no disclosures relevant to the manuscript. R. Marrie receives research funding from the Canadian Institutes of Health Research, Research Manitoba, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, National Multiple Sclerosis Society, and Rx & D Health Research Foundation and has conducted clinical trials funded by Sanofi-Aventis. Go to Neurology.org/N for full disclosures.
References
- 1.Leary SM, Porter B, Thompson AJ. Multiple sclerosis: diagnosis and the management of acute relapses. Postgrad Med J 2005;81:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKay KA, Jahanfar S, Duggan T, Tkachuk S, Tremlett H. Factors associated with onset, relapses or progression in multiple sclerosis: a systematic review. Neurotoxicology 2017;61:189–212. [DOI] [PubMed] [Google Scholar]
- 3.Marrie RA, Cohen J, Stuve O, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult Scler 2015;21:263–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marrie RA, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. Comorbidity delays diagnosis and increases disability at diagnosis in MS. Neurology 2009;72:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang T, Tremlett H, Leung S, et al. Examining the effects of comorbidities on disease-modifying therapy use in multiple sclerosis. Neurology 2016;86:1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marrie RA, Elliott L, Marriott J, Cossoy M, Tennakoon A, Yu N. Comorbidity increases the risk of hospitalizations in multiple sclerosis. Neurology 2015;84:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marrie RA, Miller A, Sormani MP, et al. Recommendations for observational studies of comorbidity in multiple sclerosis. Neurology 2016;86:1446–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tettey P, Simpson S Jr, Taylor B, et al. An adverse lipid profile is associated with disability and progression in disability, in people with MS. Mult Scler 2014;20:1737–1744. [DOI] [PubMed] [Google Scholar]
- 9.Marrie RA, Rudick R, Horwitz R, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology 2010;74:1041–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durmus H, Kurtuncu M, Tuzun E, et al. Comparative clinical characteristics of early- and adult-onset multiple sclerosis patients with seizures. Acta Neurol Belg 2013;113:421–426. [DOI] [PubMed] [Google Scholar]
- 11.Statistics Canada. Population by year, by province and territory [online]. Available at: http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo02a-eng.htm. Accessed January 29, 2016.. [Google Scholar]
- 12.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 13.Canadian Institute for Health Information [creator] (2012). Discharge Abstract Database (Hospital Separations). Population Data BC [publisher]. Data Extract. MOH (2012). Available at: http://www.popdata.bc.ca/data. Accessed June 8, 2017 [online]. [Google Scholar]
- 14.British Columbia Ministry of Health [creator] (2012). Medical Services Plan (MSP) Payment Information File. V2. Population Data BC [publisher]. Data Extract. MOH (2012). Available at: http://www.popdata.bc.ca/data. Accessed June 8, 2017 [online]. [Google Scholar]
- 15.British Columbia Ministry of Health [creator] (2012). Consolidation File (MSP Registration & Premium Billing). V2. Population Data BC [publisher]. Data Extract. MOH (2012). Available at: http://www.popdata.bc.ca/data. Accessed June 8, 2017 [online]. [Google Scholar]
- 16.Wilkins R, Berthelot JM, Ng E. Trends in mortality by neighbourhood income in urban Canada from 1971 to 1996. Health Rep 2002;13(suppl):1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.British Columbia Ministry of Health [creator] (2012). PharmaNet. BC Ministry of Health [publisher]. Data Extract. Data Stewardship Committee (2012). Available at: http://www.popdata.bc.ca/data. Accessed June 8, 2017. [Google Scholar]
- 18.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121–127. [DOI] [PubMed] [Google Scholar]
- 19.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983;13:227–231. [DOI] [PubMed] [Google Scholar]
- 20.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.” Ann Neurol 2005;58:840–846. [DOI] [PubMed] [Google Scholar]
- 21.Marrie RA, Yu BN, Leung S, et al. The utility of administrative data for surveillance of comorbidity in multiple sclerosis: a validation study. Neuroepidemiology 2013;40:85–92. [DOI] [PubMed] [Google Scholar]
- 22.Marrie RA, Yu BN, Leung S, et al. Prevalence and incidence of ischemic heart disease in multiple sclerosis: a population-based validation study. Mult Scler Relat Disord 2013;2:355–361. [DOI] [PubMed] [Google Scholar]
- 23.Marrie RA, Yu BN, Leung S, et al. Rising prevalence of vascular comorbidities in multiple sclerosis: validation of administrative definitions for diabetes, hypertension, and hyperlipidemia. Mult Scler 2012;18:1310–1319. [DOI] [PubMed] [Google Scholar]
- 24.Wang M. Generalized estimating equations in longitudinal data analysis: a review and recent developments. Adv Stat 2014;2014:11. [Google Scholar]
- 25.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- 26.Gavaghan DJ, Moore RA, McQuay HJ. An evaluation of homogeneity tests in meta-analyses in pain using simulations of individual patient data. Pain 2000;85:415–424. [DOI] [PubMed] [Google Scholar]
- 27.IBM Corp. IBM SPSS Statistics for Windows, version 22.0. Armonk, NY: IBM Corp; 2012. [Google Scholar]
- 28.R Core Team. R: A Language and Environment for Statistical Computing [online]. Vienna, Austria: R Foundation for Statistical Computing; 2016. Available at: http://www.R-project.org/. Accessed January 29, 2016. [Google Scholar]
- 29.Pittock SJ, Mayr WT, McClelland RL, et al. Change in MS-related disability in a population-based cohort: a 10-year follow-up study. Neurology 2004;62:51–59. [DOI] [PubMed] [Google Scholar]
- 30.Duron E, Hanon O. Vascular risk factors, cognitive decline, and dementia. Vasc Health Risk Manag 2008;4:363–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tettey P, Simpson S Jr, Taylor BV, van der Mei IA. Vascular comorbidities in the onset and progression of multiple sclerosis. J Neurol Sci 2014;347:23–33. [DOI] [PubMed] [Google Scholar]
- 32.Alexander JS, Zivadinov R, Maghzi AH, Ganta VC, Harris MK, Minagar A. Multiple sclerosis and cerebral endothelial dysfunction: mechanisms. Pathophysiology 2011;18:3–12. [DOI] [PubMed] [Google Scholar]
- 33.Conway DS, Thompson NR, Cohen JA. Influence of hypertension, diabetes, hyperlipidemia, and obstructive lung disease on multiple sclerosis disease course. Mult Scler 2017;23:277–285. [DOI] [PubMed] [Google Scholar]
- 34.Weinstock-Guttman B, Zivadinov R, Mahfooz N, et al. Serum lipid profiles are associated with disability and MRI outcomes in multiple sclerosis. J Neuroinflammation 2011;8:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uribe-San-Martin R, Ciampi-Diaz E, Suarez-Hernandez F, Vasquez-Torres M, Godoy-Fernandez J, Carcamo-Rodriguez C. Prevalence of epilepsy in a cohort of patients with multiple sclerosis. Seizure 2014;23:81–83. [DOI] [PubMed] [Google Scholar]
- 36.Calabrese M, Grossi P, Favaretto A, et al. Cortical pathology in multiple sclerosis patients with epilepsy: a 3 year longitudinal study. J Neurol Neurosurg Psychiatry 2012;83:49–54. [DOI] [PubMed] [Google Scholar]
- 37.Kelley BJ, Rodriguez M. Seizures in patients with multiple sclerosis: epidemiology, pathophysiology and management. CNS Drugs 2009;23:805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chataway J, Schuerer N, Alsanousi A, et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet 2014;383:2213–2221. [DOI] [PubMed] [Google Scholar]
- 39.Tremlett H, Zhao Y, Rieckmann P, Hutchinson M. New perspectives in the natural history of multiple sclerosis. Neurology 2010;74:2004–2015. [DOI] [PubMed] [Google Scholar]
- 40.Pampel FC, Krueger PM, Denney JT. Socioeconomic disparities in health behaviors. Annu Rev Sociol 2010;36:349–370. [DOI] [PMC free article] [PubMed] [Google Scholar]