Abstract
Purpose
This phase I trial evaluated epigenetic modulation of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor by using a histone deacetylase abexinostat in combination with pazopanib to enhance response and reverse resistance.
Patients and Methods
Pazopanib was administered once a day on days 1 to 28 and abexinostat was administered orally twice a day on days 1 to 5, 8 to 12, and 15 to 19 (schedule A) or on days 1 to 4, 8 to 11, and 15 to 18 (schedule B). Dose escalation (3 + 3 design) in all solid tumors was followed by dose expansion in renal cell carcinoma (RCC).
Results
Fifty-one patients with RCC (N = 22) were enrolled, including 30 (59%) with one or more lines of prior VEGF-targeting therapy. Five dose-limiting toxicities, including fatigue (n = 2), thrombocytopenia (n = 2), and elevated AST/ALT (n = 1), were observed with schedule A; one dose-limiting toxicity was observed (elevated AST/ALT) was observed with schedule B. Grade ≥ 3 related adverse events included fatigue (16%), thrombocytopenia (16%), and neutropenia (10%). The recommended phase II dose was established as abexinostat 45 mg/m2 twice a day administered per schedule B plus pazopanib 800 mg/d. Objective response rate was 21% overall and 27% in the RCC subset. Median duration of response was 9.1 months (1.2 to > 49 months). Eight patients (16%) had durable control of disease for > 12 months. Durable tumor regressions were observed in seven (70%) of 10 patients with pazopanib-refractory disease, including one patients with RCC with ongoing response > 3.5 years. Peripheral blood histone acetylation and HDAC2 gene expression were associated with durable response to treatment.
Conclusion
Abexinostat is well tolerated in combination with pazopanib, allowing prolonged exposure and promising durable responses in pazopanib- and other VEGF inhibitor-refractory tumors, which supports epigenetically mediated reversal of treatment resistance.
INTRODUCTION
Pazopanib is a multityrosine kinase inhibitor of vascular endothelial growth factor receptor (VEGFR) and other growth factor receptors and is approved for use in renal cell carcinoma (RCC) and soft tissue sarcoma1-3; however, treatment resistance to pazopanib is inevitable, and continued VEGF pathway blockade in RCC has modest activity and durable responses are uncommon.4,5 Agents that reverse resistance and/or prolong sensitivity to VEGF-targeting treatment would translate into a significant clinical benefit in these malignancies.
Proangiogenic, VEGF-driven tumors adapt to the presence of angiogenesis inhibitors to functionally evade therapeutic effect. One of the implicated mechanisms is hypoxia-driven, histone deacetylase (HDAC) –mediated overexpression and post-translational stabilization of hypoxia-inducible factor (HIF)-1α, a potent proangiogenic factor that directly regulates VEGF expression.6 HDAC inhibition significantly downregulates HIF-1α protein expression in hypoxic conditions; combined pazopanib and HDAC inhibition showed additive or synergistic effect across a variety of VEGF-driven tumors as well as reversal of resistance when added to pazopanib-resistant cancer cell lines.7
These observations made with other HDAC inhibitors—combined with studies that have demonstrated that single-agent abexinostat is a potent, pan-HDAC inhibitor with favorable pharmacokinetic profile and no expected drug-drug interactions with pazopanib on the basis of differing metabolic pathways (pazopanib predominantly via CYP3A4 and abexinostat via glucuronidation)—led to the initiation of a phase Ib study of abexinostat plus pazopanib in patients with advanced solid tumor malignancies, with an expansion cohort in RCC.8,9 A key objective was to test for potential resistance reversal in tumors that were refractory to prior pazopanib and other VEGF-targeting therapies.
PATIENTS AND METHODS
Patient Population
Patients were required to have an Eastern Cooperative Oncology Group performance status of ≤ 1, absolute neutrophil count > 1.5 × 109/L, total bilirubin < 1.5× upper limit of normal, creatinine < 1.5× upper limit of normal or creatinine clearance > 50 mL/min, and blood pressure < 140/90 mm Hg with use of antihypertensive therapy as indicated. Any number of prior lines of systemic therapy was allowed, including prior pazopanib. Patients with advanced solid tumor malignancies were enrolled in dose escalation; dose expansion was restricted to patients with RCC of any histologic subtype. Measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria was required in dose expansion. Key exclusion criteria were recent major surgery or radiation, untreated brain metastases, or recent major cardiovascular or thrombotic event.
Study approval was obtained from the institutional review board at the University of California, San Francisco, and regulatory authorities (clinical trial information: NCT01543763). All patients gave written informed consent. The study followed the Declaration of Helsinki and good clinical practice guidelines.
Study Design
The study was designed as a phase Ib, open-label, dose-escalation/expansion trial of abexinostat in combination with pazopanib. There was a 1-week run-in period with abexinostat, followed by combination dose administration on a 28-day treatment cycle, continuing until disease progression, unacceptable toxicity, or study withdrawal. Abexinostat was administered orally twice a day on days 1 to 5, 8 to 12, and 15 to 19 (schedule A) and later amended as a result of observed toxicity to evaluate a 4-day-per-week schedule: days 1 to 4, 8 to 11, and 15 to 18 (schedule B). Pazopanib was administered orally once a day.
Dose escalation proceeded using a 3 + 3 design. A dose-limiting toxicity (DLT) was defined as any grade ≥ 3 nonhematologic, treatment-related, adverse event; grade 4 neutropenia lasting > 7 days or grade ≥ 3 febrile neutropenia; grade 3 thrombocytopenia with bleeding or grade 4 thrombocytopenia; and failure to administer at least 75% of doses in cycle one because of treatment-related toxicity. Patients who missed > 25% of doses as a result of reasons other than treatment-related toxicity were replaced.
Safety and Efficacy Assessments
Clinical and laboratory assessments were conducted at baseline and once a week during cycle one and once per cycle thereafter. Tumor assessment was performed every two cycles for the first six cycles, then every three cycles thereafter. Adverse events were graded by using Common Toxicity Criteria version 4.0.
Pharmacodynamic Assessments
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood collected predose and 4 hours postdose on cycle one day −7 and day −4. PBMC histone H4 acetylation—normalized to pan-H3 expression—was determined by Western blot as previously described.10 PBMC HDAC2 and HDAC6 gene expression levels, normalized to the housekeeper gene GAPDH (glyceraldehyde 3-phosphate dehydrogenase), were determined at baseline. Plasma VEGF levels were measured predose on cycle one day −7, day −4, and day +4.
Pharmacokinetic Assessments
Abexinostat plasma concentrations were measured predose and up to 4 hours postdose during the lead-in phase on cycle one day −7 and day −4, as well as in combination with pazopanib on cycle one day 4. Plasma concentrations were determined by using high-performance liquid chromatography–tandem mass spectrometry (lower limit of quantification = 1 ng/mL).
Individual pharmacokinetic parameters for maximum serum concentration , area under the concentration-time curve (AUC) at day −4 and day 4 and were estimated using a previously published pharmacokinetic model of abexinostat.11 Nonlinear mixed-effects modeling was used for analysis (NONMEM VII Software; ICON Development Solutions, San Antonio, TX).
Study Objectives and Statistical Methods
The primary study objective was to determine the maximally tolerated dose and recommended phase II dose and schedule of abexinostat in combination with pazopanib. Secondary objectives included efficacy, safety, and characterization of pharmacokinetic and pharmacodynamic profiles. Pearson’s method was used to determine the correlation between PBMC histone acetylation, abexinostat plasma concentration, and plasma VEGF levels. Kruskal-Wallis and Mann-Whitney tests were used to compare baseline PBMC HDAC2 and HDAC6 expression, as well as change from baseline in total WBC count, in subgroups of patients defined by duration of response (< 6 months or no response v 6 to 12 months v > 12 months). Kaplan-Meier product limit method and log-rank test were used to compare progression-free survival between low versus high PBMC HDAC2 expressors.
RESULTS
Study Population
Fifty-one patients enrolled, including 36 patients with any solid tumor malignancy in dose escalation and 15 patients with RCC in dose expansion. Baseline characteristics are listed in Table 1. The majority of patients (72%) had received two or more lines of therapy in the locally advanced or metastatic setting. Thirty patients (59%) had experienced disease progression on one or more prior VEGF-targeting agents, and of those patients, 10 (20%) had experienced prior progression on pazopanib, including five patients with primary refractory disease. Median interval between last dose of prior VEGF-targeting therapy and start of study treatment was 3.8 months (range, 0.5 to 110 months).
Table 1.
Baseline Patient Characteristics and Patient Disposition
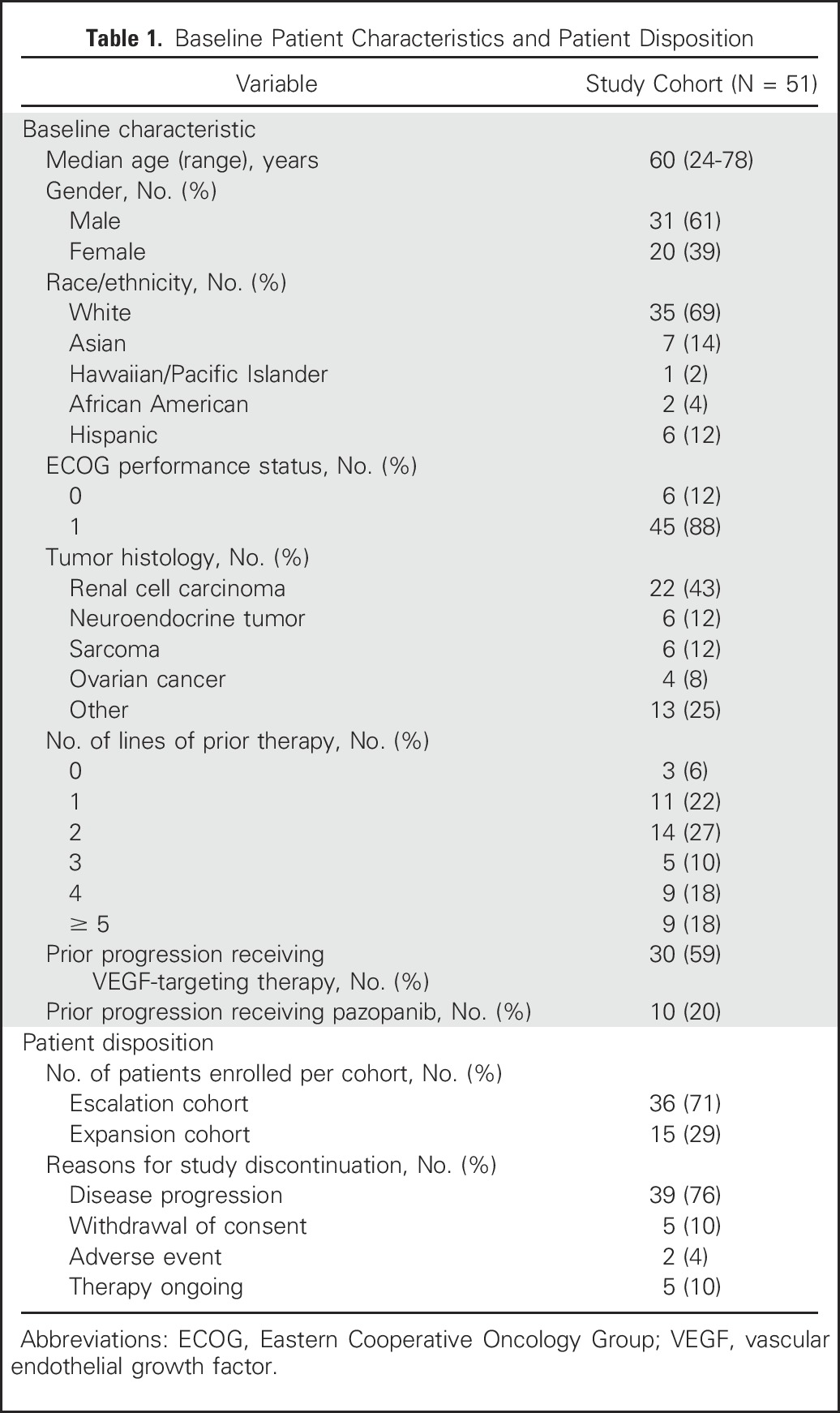
Patient disposition is listed in Table 1. The majority (76%) of patients discontinued study therapy as a result of disease progression. Five patients withdrew consent and five remain on study treatment with ongoing response or stable disease for > 12 months.
Determination of Maximally Tolerated Dose and Recommended Phase II Dose
With schedule A of abexinostat dosing, a total of 22 patients were enrolled across four dose levels (Table 2). Among 20 evaluable patients, five (25%) experienced DLTs, which were fatigue (n = 2), thrombocytopenia (n = 2), and elevated liver transaminases with fever (n = 1). The maximum tolerated dose of abexinostat with schedule A dose administration was determined to be 30 mg/m2 twice a day in combination with pazopanib 600 mg once a day.
Table 2.
Dose-Limiting Toxicities and Determination of Maximally Tolerated and Recommended Phase II Dose
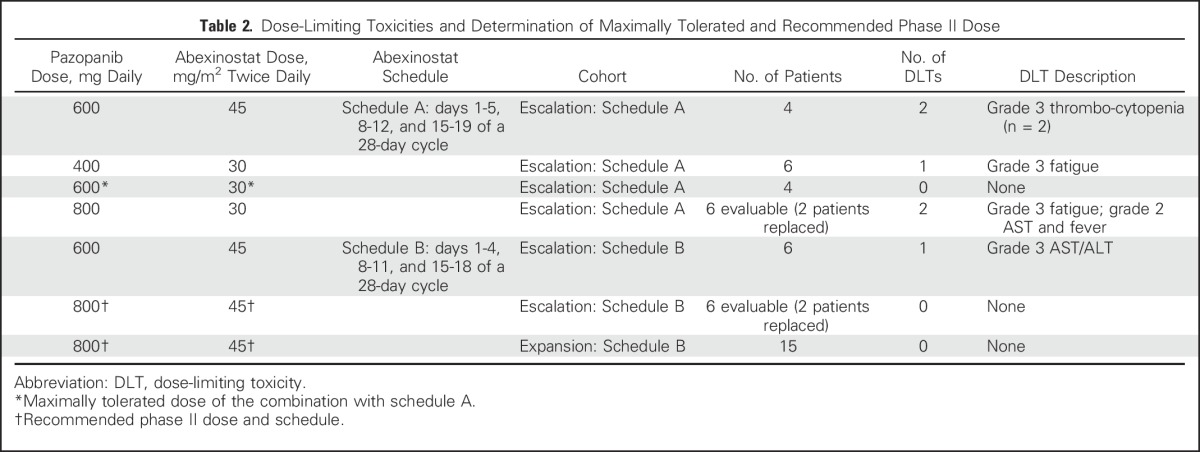
As a result of the observed toxicities with this schedule, an alternative dosing schedule was used with the goal of maintaining high cumulative weekly dosing of abexinostat with a shorter dose administration interval: 4 days per week (schedule B). With schedule B, only one (8%) in 12 patients experienced a DLT (grade 3 elevated AST/ALT), and the maximally tolerated dose was not reached (Table 2). The recommended phase II dose and schedule was pazopanib 800 mg once a day and abexinostat 45 mg/m2 twice a day for 4 and 7 days, respectively, and 3 and 4 weeks, respectively, thus reaching the approved dose of pazopanib and recommended phase II dose of single-agent abexinostat.9 No DLTs were seen in the expansion cohort, validating the recommended phase two dose level/schedule.
Safety Results
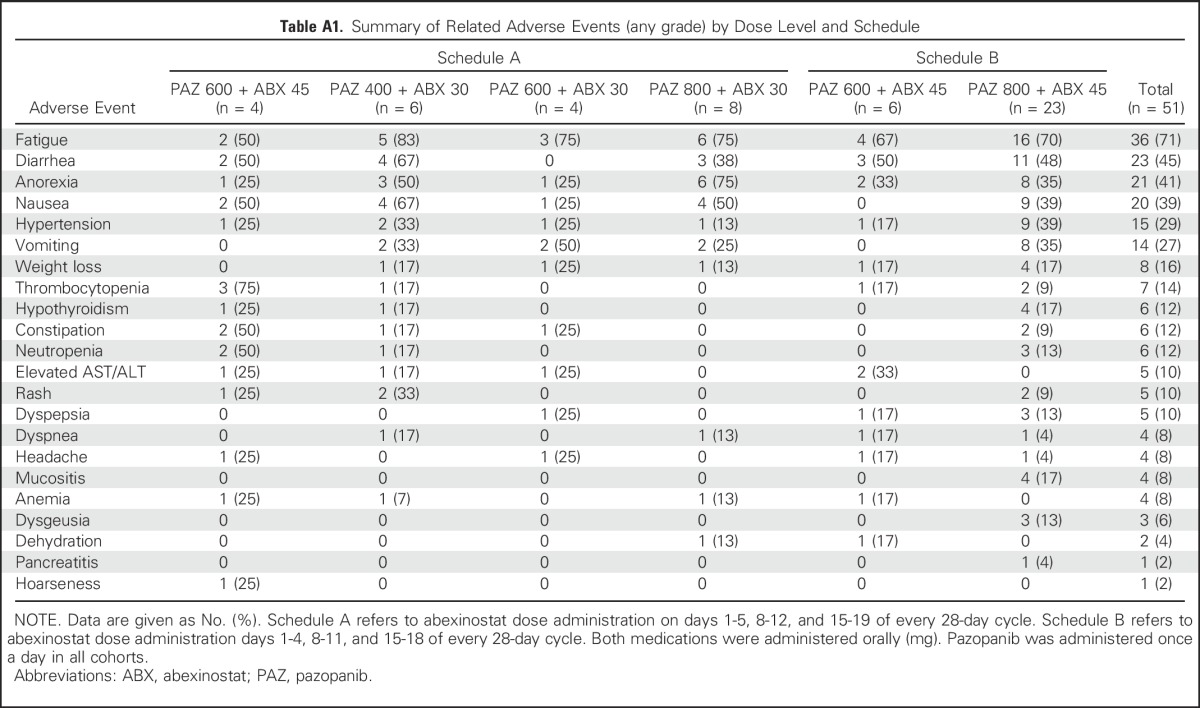
The most common treatment-related adverse events of any grade were as follows: fatigue (71%), diarrhea (45%), decreased appetite (41%), and nausea (39%) (Appendix Table A1, online only). Grade ≥ 3 adverse events were fatigue (16%), thrombocytopenia (16%), neutropenia (10%), anemia (10%), diarrhea (10%), and elevated AST/ALT (4%). There were no episodes of febrile neutropenia or clinically significant bleeding. One patient experienced grade 3 hypertension. There were no treatment-related grade 5 adverse events. Dose reductions and/or interruptions were fairly common (43%); however, only two patients (4%) discontinued study treatment because of adverse events, and prolonged dose administration was feasible in patients who experienced long-term response.
Efficacy Analyses
The maximum percent change from baseline in tumor measurements is shown in Fig 1A. Of 51 patients, 43 had measurable disease and were evaluable for response, five patients withdrew consent for reasons other than progression before first tumor assessment, and three patients were removed as a result of DLTs in cycle one. Nine patients (21%) achieved objective tumor response: RCC (n = 6), thyroid cancer (n = 2), and mesothelioma (n = 1). Among the 10 patients who had experienced disease progression on prior pazopanib monotherapy, seven (70%) had tumor regressions while participating in the study. Of 28 patients who were evaluable for response with prior progression on one or more VEGF-targeting therapies, 19 (68%) experienced tumor regressions on combination study treatment, including six patients (21%) with an objective tumor response. Responses were quite durable, with a median duration of response of 9.1 months (1.2 to ≥ 44 months) and clinical benefit rate (partial response plus stable disease > 6 months) in 16 (37%) of 43 patients (Fig 1B). Overall, eight patients experienced disease stabilization or durable tumor response for > 12 months, including five patients who remain on study treatment. The majority of patients with durable response had experienced progression on prior VEGF-targeting therapy.
Fig 1.
Summary of efficacy in dose escalation and expansion cohorts. (A) Maximal percent change from baseline in tumor-measurement-evaluable patients (n = 43). Confirmed objective responses were observed in 9 (21%) of 43 evaluable patients, including six patients with prior progresssion receiving one or more VEGF-targeting therapies. (B) Duration of response in the dose escalation and renal cell expansion cohorts. Median duration of response was 9.1 months (range, 1.2 to ≥ 44 months) and clinical benefit rate (partial response plus stable disease > 6 months) was observed in 16 (37%) of 43 patients. Eight patients achieved exceptional response defined as response duration of > 12 months. Adr CC, adrenocortical carcinoma; HNSCC, head and neck squamous cell carcinoma; Meso, mesothelioma; NET, neuroendocrine tumor; RCC, renal cell carcinoma; TNBC, triple-negative breast cancer; VEGF, vascular endothelial growth factor.
The subset of patients with RCC (n = 22) had received an average of 2.5 lines of prior therapy and 1.6 lines of prior VEGF-targeting treatment, including 10 patients (45%) with prior progression on pazopanib monotherapy. In this heavily pretreated cohort, objective response rate was 6 (27%) of 22 patients, and median duration of response was 10.5 months (range, 3.1 to ≥ 44 months). Three patients with prior primary refractory disease to pazopanib monotherapy achieved durable minor or partial responses > 12 months with pazopanib plus abexinostat. Response rate by histologic subtype was 5 (31%) of 16 patients with tumors with a clear cell component and 1 (17%) of 6 in those patients who were classified as having papillary histology.
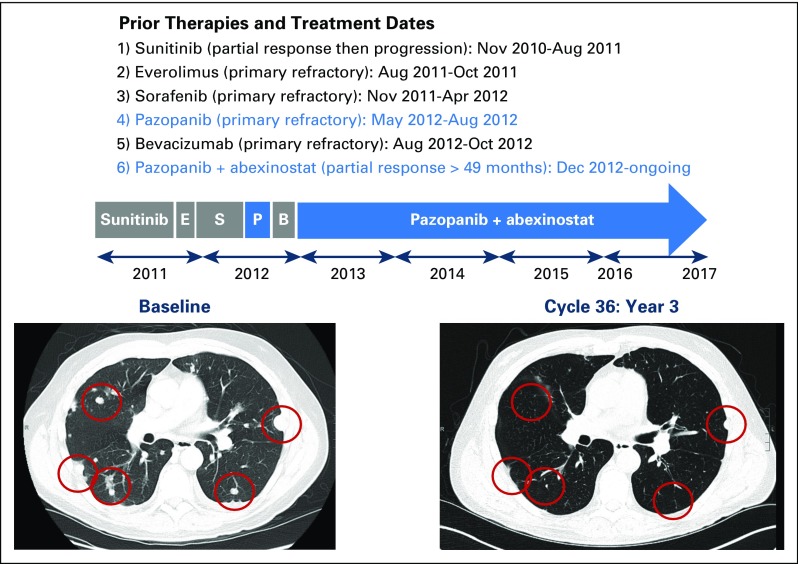
Eight patients were classified as achieving durable response to treatment (response > 12 months). Five patients (63%) had experienced prior progression on at least one line of VEGF-directed therapy. Figure 2 highlights one patient with clear cell RCC who experienced initial response, then progression on first-line sunitinib, then had primary refractory disease to the next three lines of VEGF-targeting systemic therapy, including sorafenib, pazopanib, and bevacizumab. Upon treatment with the combination of pazopanib plus abexinostat, there was an immediate tumor response and the patient remains on study with ongoing partial response for > 3.5 years’ duration.
Fig 2.
Durable response in patient with treatment-refractory clear cell renal cell carcinoma. Patient had previously experienced progression receiving five systemic lines of therapy for kidney cancer, including four prior VEGF-targeting therapies. His cancer was primary refractory to prior pazopanib monotherapy. Upon initiation of study treatment with pazopanib in combination with abexinostat in December 2012, the patient experienced a rapid and deep response to treatment with ongoing response for > 3.5 years. B, bevacizumab; E, everolimus; P, pazopanib monotherapy; S, sorafenib.
Pharmacokinetic Analyses
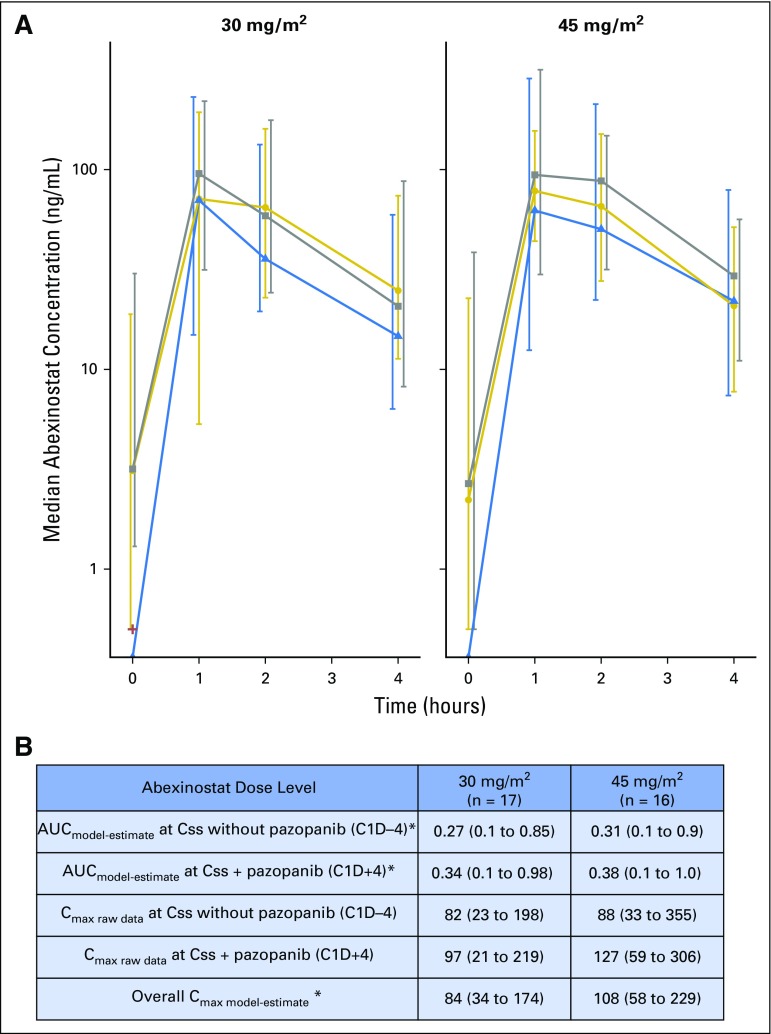
Thirty-three patients were evaluable for pharmacokinetic assessment. Abexinostat plasma concentrations are shown in Fig 3A. Abexinostat exposures were similar to previously published data.11 No significant change in abexinostat exposure in combination with pazopanib (cycle one, day 4) was observed compared with abexinostat alone (cycle one, day −7 to −4; Fig 3A). Pharmacokinetic modeling to estimate abexinostat maximum serum concentration and AUC suggests no significant difference in exposure by dose level adjusted for body surface area (Fig 3B).
Fig 3.
Summary of abexinostat pharmacokinetics. (A) Raw plasma concentrations of abexinostat by dose level. Median and interquartile range of observed concentrations presented by occasion and dose level is shown. Abexinostat concentrations at first dose without pazopanib (C1D-7, blue triangles, dotted blue line); at steady state concentration (Css) without pazopanib (C1D-4, gold circles, gold line); at Css with pazopanib (C1D + 4 gray squares, dashed gray line). (B) Estimated pharmacokinetic parameters of abexinostat. Median area under the concentration-time curve (AUC; ng*h/L) and median maximum serum concentration (Cmax; ng/mL) and 95% CIs are presented. (*)Individual pharmacokinetic parameters AUC and Cmax were estimated by using the population pharmacokinetic parameters of abexinostat as previously described.11 Cmax, maximum serum concentration.
Pharmacodynamic Analyses and Association With Pharmacokinetic Parameters and Clinical Outcomes
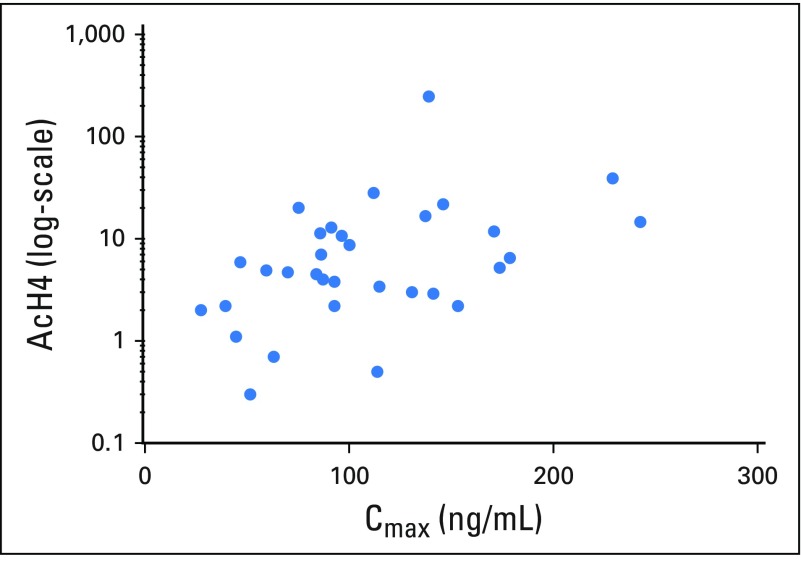
Of 45 evaluable patients with PBMC histone H4 acetylation assessment at baseline and after initiation of abexinostat administration, 38 (84%) had a two-fold or greater increase from baseline in PBMC histone acetylation, which supports an on-target drug effect at dosing levels evaluated in the current study. The assigned abexinostat dose level did not significantly impact acetylation; however, change from baseline in PBMC histone acetylation was significantly correlated with abexinostat concentration at 4 hours postdose on day −4 (r2 = 0.45; P = .0094; Appendix Fig A1, online only).
In patients with RCC, change from baseline in PBMC histone acetylation was significantly inversely correlated with change in plasma VEGF levels after 4 days of lead-in treatment with abexinostat monotherapy (r2 = 0.35; P = .01; Appendix Fig A2, online only). This effect persisted on day +4 after addition of pazopanib.
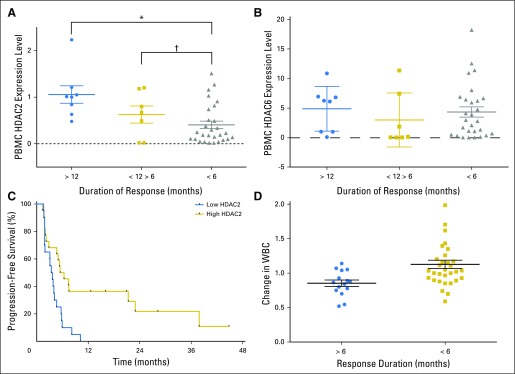
Multiple exploratory analyses were performed to identify potential factors associated with exceptional response (response > 12 months), including pharmacokinetic parameters, abexinostat dose level, and pharmacodynamic markers. Baseline PBMC HDAC2 gene expression was the strongest predictive factor, which was highest in those who experienced response for > 12 months, intermediate in those with 6 to 12 month duration of response, and lowest among those with no response or response < 6 months (mean HDAC2 expression: response > 12 months, 1.07 ± 0.19; response duration 6 to 12 months, 0.62 ± 0.18; response < 6 months or no response, 0.41 ± 0.08; Pfor interaction = .0025; Fig 4A). This pattern was not observed with baseline PBMC HDAC6 expression (P = .898; Fig 4B). Time to disease progression was significantly correlated with both PBMC HDAC2 expression (P = .0019) and fold change in PBMC histone H4 acetylation (P = .003; data not shown). Progression-free survival in patients with high PBMC HDAC2 expression (> 0.4) was significantly longer than in those with low expression (median, 5.9 months v 3.5 months; hazard ratio, 0.41; 95% CI, 0.17 to 0.67; P = .0034; Fig 4C). Similarly, decrease in total WBCs on day 8 compared with baseline was only observed in patients with response duration > 6 months, but not in those with no response or response < 6 months (P = .0029; Fig 4D). There was no association between abexinostat exposure or dose level with response or progression-free survival (data not shown).
Fig 4.
Biomarkers predictive of response to therapy. (A) Peripheral blood mononuclear cell (PBMC) histone deacetylase 2 (HDAC2) expression levels by subgroups of patients categorized by duration of response. Kruskal-Wallis test for comparison across three groups: P = .0025. Mean (± standard error of the mean [SEM]) values are as follows: > 12 months, 1.1 (± 0.19); 6 to 12 months, 0.63 (± 0.18); and < 6 months, 0.41 (± 0.08). (*)Mann-Whitney test to compare > 12 months versus < 6 months: P = .0019. (†)Mann-Whitney test to compare 6 to 12 month versus < 6 months: P = .0129. (B) PBMC HDAC6 expression levels and duration of response. Kruskal-Wallis test for three-way comparison, P = .39. Mann-Whitney test for pairwise comparisons: < 6 months v > 6 to < 12 months, P = .23; < 6 months and > 12 months, P = .59. (C) Progression-free survival according to HDAC2 expression in PBMCs. Kaplan-Meier curve for patients (n = 43) according to HDAC2 expression in PBMCs: low HDAC2 expression (< 0.4; 3.5 months) versus high HDAC2 expression (> 0.4; 5.9 months); hazard ratio (95% CI) by log rank: 0.41 (0.17 to 0.67); P = .0034. (D) Change in WBC count and duration of response. Effects of abexinostat without effects of pazopanib on patients’ WBC count on cycle one, day 8 (depicted as ratio of day 1 to day 8) by response duration: > 6 versus < 6 months. Means (± standard error of the mean), 0.84 (± 0.04) versus 1.13 (± 0.06); P = .0029.
DISCUSSION
Pazopanib and other VEGF-targeting agents have shown significant clinical activity in multiple tumor types, yet resistance to treatment is universal. In the current study, we performed the first clinical trial, to our knowledge, to test the hypothesis that epigenetic modification with HDAC inhibition may provide the means to recapture response and reverse resistance to pazopanib in RCC and other solid tumor malignancies. The approved single-agent dose of pazopanib was safely combined with abexinostat at its recommended phase II single-agent dose. Prolonged dose administration was feasible with maintenance of weekly cumulative exposure upon adoption of a 4 day per week abexinostat dose administration schedule. Sustained responses and stable disease ≥ 6 months were observed in a significant subset of patients with VEGF- and pazopanib-refractory disease, which provides strong support for the ability of HDAC inhibition to restore and enhance response to VEGF-targeting therapy.
Treatment rechallenge and continued blockade of the VEGF signaling pathway has been observed in RCC in 10% to 15% of patients who have been previously exposed to VEGF inhibitors; however, responses are typically short.12,13 In the current study, durability of the observed tumor regressions in 70% of patients with documented acquired and primary pazopanib resistance suggests a treatment effect that extends beyond continued blockade of the VEGF pathway with pazopanib and supports the potential of HDAC inhibition as a means to reverse resistance to VEGF-directed therapy in a subset of patients. Conversely, although the possibility exists that single-agent abexinostat activity solely drove responses observed in treatment-refractory tumors, the notable lack of durable responses in previous single-agent HDAC inhibitor studies in advanced solid tumor malignancies suggests that it is more likely that the combination of HDAC inhibition plus continued VEGF pathway blockade is required to recapture treatment response.14-16 Whether HDAC inhibition can enhance response to pazopanib or reverse primary and acquired resistance will be further studied in a planned randomized study with crossover design in RCC.
In determining the predictive factors associated with sustained tumor response, this study provides further evidence that host factors predispose patients to increased sensitivity to HDAC inhibitor treatment. In a prior study of panobinostat in combination with chemotherapy, greater fold induction of histone acetylation in PBMCs—and not panobinostat dose level or exposure—was predictive of clinical benefit.17 Likewise, in the current study, host factors and, in particular, baseline PBMC HDAC2 expression—rather than abexinostat dose level or drug exposure—were strongly associated with durable response. Review of the multiple data sets in the Cancer Genome Atlas (TCGA) suggests that somatic HDAC2 mutations and amplification are rare in RCC and other solid tumor malignancies.18 Hence, host factors linked to HDAC expression and acetylation status, rather than somatic genomic alteration of HDAC2 in tumors, seem to explain the clinical benefit.
The putative mechanism of action via epigenetically mediated downregulation of HIF-1α and VEGF expression is supported by pharmacodynamic analyses. Downregulation of plasma VEGF levels—a direct transcriptional target of HIF-1α—was significantly correlated with induction of PBMC histone acetylation, a validated biomarker of HDAC inhibition.19 HDAC2 is the central HDAC enzyme that directly regulates VEGF expression via binding to its promoter; inhibition of HDAC2 suppresses VEGF expression and angiogenesis.20,21 In contrast, HDAC6 is a microtubule-associated cytoplasmic HDAC that does not affect VEGF or HIF-1α expression.22 The differential mechanism of cellular localization and function may explain why expression of HDAC2, and not HDAC6, was strongly associated with durable treatment responses in the current study. Tissue-based analyses of HIF-1a and VEGF expression are planned in the upcoming randomized phase II study.
The safety profile of the treatment combination was consistent with prior single-agent studies of the two agents.1,9 The dosing schedule had a clear impact on treatment tolerability; shortening the treatment interval from 5 to 4 days per week, with maintenance of weekly cumulative dose exposure, led to substantially lower toxicities while maintaining response.11,23
The main limitations of the study pertain to the lack of a control group and absence of tissue-based correlative assays precluding the ability to understand changes in patterns of gene expression that may be associated with durable response to treatment. These limitations will be addressed in planned randomized patient studies.
In conclusion, addition of HDAC inhibitor abexinostat to pazopanib is well tolerated and resulted in strikingly durable responses in patients who experienced prior progression during treatment with VEGF inhibitors, including pazopanib-refractory RCC. PBMC HDAC2 expression was strongly associated with response, which suggests that host factors are most predictive of sensitivity to HDAC inhibition. Randomized studies are planned in clear cell RCC and other solid tumors.
ACKNOWLEDGMENT
We thank Sriram Balasubramanian, MD, for his scientific input on the study design.
Appendix
Fig A1.
Correlation between induction in peripheral blood mononuclear cell (PBMC) histone acetylation with exposure to abexinostat on day −4. Correlation between induction of PBMC histone acetylation at 4 hours postdose and abexinostat concentration on day −4 (R2 = 0.45; P = .0094). AcH4, acetylated H4 expression relative to pan H3 expression; Cmax, serum concentration of abexinostat 4 hours postdose. Cmax, maximum serum concentration.
Fig A2.
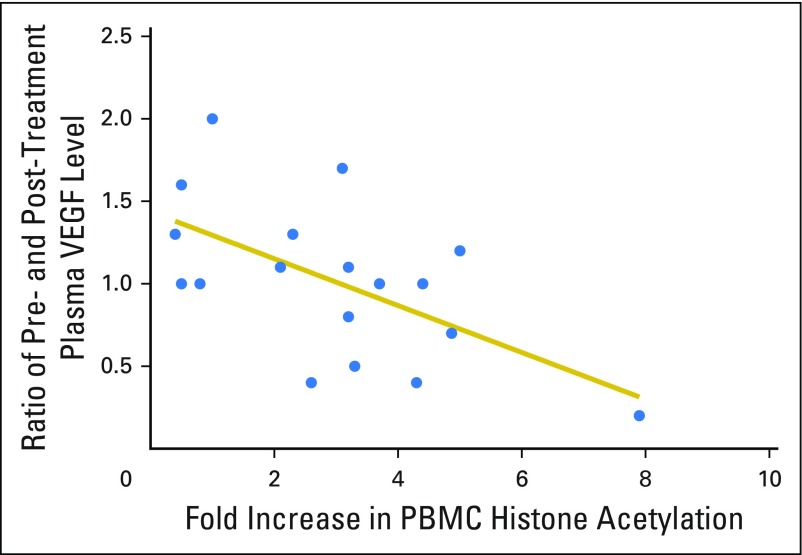
Correlation between induction in peripheral blood mononuclear cell (PBMC) histone acetylation with plasma vascular endothelial growth factor (VEGF) levels in patients with renal cell carcinoma. Correlation between fold increase in PBMC histone acetylation and ratio of post-treatment to pretreatment plasma VEGF levels among patients with renal cell carcinoma (Pearson’s r = −0.59; P = .01).
Table A1.
Summary of Related Adverse Events (any grade) by Dose Level and Schedule
Footnotes
Supported by GlaxoSmithKline. Study drug supply for this trial was provided by GlaxoSmithKline and drug supply by Pharmacyclics.
Presented in part at the 2016 Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2016, and the 2016 American Association for Cancer Research Annual Meeting, New Orleans, LA, April 16-20, 2016.
The funding sources were not involved in data monitoring, data analysis, or manuscript preparation.
Clinical trial information: NCT01543763.
AUTHOR CONTRIBUTIONS
Conception and design: Rahul Aggarwal, Jennifer Grabowsky, Thierry Jahan, Amy Cripps, Thach-Giao Truong, Pamela N. Munster
Collection and assembly of data: All authors
Data analysis and interpretation: Rahul Aggarwal, Scott Thomas, Nela Pawlowska, Imke Bartelink, Jennifer Grabowsky, Thierry Jahan, Thach-Giao Truong, Pamela N. Munster
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Inhibiting Histone Deacetylase as a Means to Reverse Resistance to Angiogenesis Inhibitors: Phase I Study of Abexinostat Plus Pazopanib in Advanced Solid Tumor Malignancies
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Rahul Aggarwal
Research Funding: Novartis, Sanofi
Scott Thomas
No relationship to disclose
Nela Pawlowska
No relationship to disclose
Imke Bartelink
No relationship to disclose
Jennifer Grabowsky
No relationship to disclose
Thierry Jahan
Consulting or Advisory Role: Turnstone Bio
Research Funding: Aduro Biotech (Inst), Acerta Pharma (Inst), AstraZeneca (Inst), MedImmune (Inst), Eli Lilly (Inst), BIND Therapeutics (Inst), Golden Biotechnology (Inst), Verastem (Inst), Boehringer Ingelheim (Inst)
Travel, Accommodations, Expenses: Aduro Biotech, Eli Lilly, Novartis, AstraZeneca
Amy Cripps
No relationship to disclose
Armand Harb
No relationship to disclose
Jim Leng
No relationship to disclose
Anne Reinert
No relationship to disclose
Ilaria Mastroserio
No relationship to disclose
Thach-Giao Truong
Employment: The Permanente Medical Group
Charles J. Ryan
No relationship to disclose
Pamela N. Munster
Stock or Other Ownership: OncoSec (I)
Honoraria: Threshold Pharmaceuticals, Amgen (I), Prometheus (I)
Consulting or Advisory Role: HUYA Bioscience
Research Funding: Merck (Inst), Pfizer (Inst), Novartis (Inst), GlaxoSmithKline (Inst), OncoMed (Inst), Celgene (Inst), Intellikine (Inst), Onconova Therapeutics (Inst), Nektar (Inst), Sanofi (Inst), Merrimack Pharmaceuticals (Inst), Genentech (Inst), OncoSec (Inst), Bristol-Myers Squibb (Inst), Plexxikon (Inst), Piramal Life Science (Inst), Andes Biotechnologies (Inst), Immune Design (Inst), Biomain (Inst)
REFERENCES
- 1.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 2.van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 4.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 5.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma. N Engl J Med. 2015;373:1814–1823. doi: 10.1056/NEJMoa1510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellis L, Hammers H, Pili R. Targeting tumor angiogenesis with histone deacetylase inhibitors. Cancer Lett. 2009;280:145–153. doi: 10.1016/j.canlet.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tavallai S, Hamed HA, Grant S, et al. Pazopanib and HDAC inhibitors interact to kill sarcoma cells. Cancer Biol Ther. 2014;15:578–585. doi: 10.4161/cbt.28163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera-Del VN, Gao S, Miller CP, et al. PCI-24781, a novel hydroxamic acid HDAC inhibitor, exerts cytotoxicity and histone alterations via caspase-8 and FADD in leukemia cells. Int J Cell Biol. 2010;2010:207420. doi: 10.1155/2010/207420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morschhauser F, Terriou L, Coiffier B, et al. Phase 1 study of the oral histone deacetylase inhibitor abexinostat in patients with Hodgkin lymphoma, non-Hodgkin lymphoma, or chronic lymphocytic leukaemia. Invest New Drugs. 2015;33:423–431. doi: 10.1007/s10637-015-0206-x. [DOI] [PubMed] [Google Scholar]
- 10.Munster P, Marchion D, Bicaku E, et al. Clinical and biological effects of valproic acid as a histone deacetylase inhibitor on tumor and surrogate tissues: Phase I/II trial of valproic acid and epirubicin/FEC. Clin Cancer Res. 2009;15:2488–2496. doi: 10.1158/1078-0432.CCR-08-1930. [DOI] [PubMed] [Google Scholar]
- 11.Chalret du Rieu Q, Fouliard S, Jacquet-Bescond A, et al. Application of hematological toxicity modeling in clinical development of abexinostat (S-78454, PCI-24781), a new histone deacetylase inhibitor. Pharm Res. 2013;30:2640–2653. doi: 10.1007/s11095-013-1089-1. [DOI] [PubMed] [Google Scholar]
- 12.Zama IN, Hutson TE, Elson P, et al. Sunitinib rechallenge in metastatic renal cell carcinoma patients. Cancer. 2010;116:5400–5406. doi: 10.1002/cncr.25583. [DOI] [PubMed] [Google Scholar]
- 13.Nozawa M, Yamamoto Y, Minami T, et al. Sorafenib rechallenge in patients with metastatic renal cell carcinoma. BJU Int. 2012;110:E228–E234. doi: 10.1111/j.1464-410X.2011.10905.x. [DOI] [PubMed] [Google Scholar]
- 14.Rathkopf D, Wong BY, Ross RW, et al. A phase I study of oral panobinostat alone and in combination with docetaxel in patients with castration-resistant prostate cancer. Cancer Chemother Pharmacol. 2010;66:181–189. doi: 10.1007/s00280-010-1289-x. [DOI] [PubMed] [Google Scholar]
- 15.Luu TH, Morgan RJ, Leong L, et al. A phase II trial of vorinostat (suberoylanilide hydroxamic acid) in metastatic breast cancer: A California Cancer Consortium study. Clin Cancer Res. 2008;14:7138–7142. doi: 10.1158/1078-0432.CCR-08-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan QC, Headlee D, Acharya M, et al. Phase I and pharmacokinetic study of MS-275, a histone deacetylase inhibitor, in patients with advanced and refractory solid tumors or lymphoma. J Clin Oncol. 2005;23:3912–3922. doi: 10.1200/JCO.2005.02.188. [DOI] [PubMed] [Google Scholar]
- 17.Thomas S, Aggarwal R, Jahan T, et al. A phase I trial of panobinostat and epirubicin in solid tumors with a dose expansion in patients with sarcoma. Ann Oncol. 2016;27:947–952. doi: 10.1093/annonc/mdw044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer Genome Atlas Research Network Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–49. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prince HM, Bishton MJ, Harrison SJ. Clinical studies of histone deacetylase inhibitors. Clin Cancer Res. 2009;15:3958–3969. doi: 10.1158/1078-0432.CCR-08-2785. [DOI] [PubMed] [Google Scholar]
- 20.Karadedou CT, Gomes AR, Chen J, et al. FOXO3a represses VEGF expression through FOXM1-dependent and -independent mechanisms in breast cancer. Oncogene. 2012;31:1845–1858. doi: 10.1038/onc.2011.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian DZ, Kato Y, Shabbeer S, et al. Targeting tumor angiogenesis with histone deacetylase inhibitors: The hydroxamic acid derivative LBH589. Clin Cancer Res. 2006;12:634–642. doi: 10.1158/1078-0432.CCR-05-1132. [DOI] [PubMed] [Google Scholar]
- 22.Haggarty SJ, Koeller KM, Wong JC, et al. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci USA. 2003;100:4389–4394. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chalret du Rieu Q, Fouliard S, White-Koning M, et al. Pharmacokinetic/pharmacodynamic modeling of abexinostat-induced thrombocytopenia across different patient populations: Application for the determination of the maximum tolerated doses in both lymphoma and solid tumour patients. Invest New Drugs. 2014;32:985–994. doi: 10.1007/s10637-014-0118-1. [DOI] [PubMed] [Google Scholar]