Abstract
Purpose
The role of adjuvant chemotherapy (AC) or induction chemotherapy (IC) in the treatment of locally advanced nasopharyngeal carcinoma is controversial. The individual patient data from the Meta-Analysis of Chemotherapy in Nasopharynx Carcinoma database were used to compare all available treatments.
Methods
All randomized trials of radiotherapy (RT) with or without chemotherapy in nonmetastatic nasopharyngeal carcinoma were considered. Overall, 20 trials and 5,144 patients were included. Treatments were grouped into seven categories: RT alone (RT), IC followed by RT (IC-RT), RT followed by AC (RT-AC), IC followed by RT followed by AC (IC-RT-AC), concomitant chemoradiotherapy (CRT), IC followed by CRT (IC-CRT), and CRT followed by AC (CRT-AC). P-score was used to rank the treatments. Fixed- and random-effects frequentist network meta-analysis models were applied.
Results
The three treatments with the highest probability of benefit on overall survival (OS) were CRT-AC, followed by CRT and IC-CRT, with respective hazard ratios (HRs [95% CIs]) compared with RT alone of 0.65 (0.56 to 0.75), 0.77 (0.64 to 0.92), and 0.81 (0.63 to 1.04). HRs (95% CIs) of CRT-AC compared with CRT for OS, progression-free survival (PFS), locoregional control, and distant control (DC) were, respectively, 0.85 (0.68 to 1.05), 0.81 (0.66 to 0.98), 0.70 (0.48 to 1.02), and 0.87 (0.61 to 1.25). IC-CRT ranked second for PFS and the best for DC. CRT never ranked first. HRs of CRT compared with IC-CRT for OS, PFS, locoregional control, and DC were, respectively, 0.95 (0.72 to 1.25), 1.13 (0.88 to 1.46), 1.05 (0.70 to 1.59), and 1.55 (0.94 to 2.56). Regimens with more chemotherapy were associated with increased risk of acute toxicity.
Conclusion
The addition of AC to CRT achieved the highest survival benefit and consistent improvement for all end points. The addition of IC to CRT achieved the highest effect on DC.
INTRODUCTION
During the past decades, advances in the treatment of locally advanced nasopharyngeal carcinoma (NPC) have led to higher cure rates. The individual patient data (IPD) Meta-Analysis of Chemotherapy (MAC) in NPC (MAC-NPC) has clearly demonstrated that the addition of concomitant chemotherapy (CT) to radiotherapy (RT) improves overall survival (OS), progression-free survival (PFS), locoregional control, and distant control.1 Controversy remains regarding the additional benefit of induction CT (IC) or adjuvant CT (AC) to concomitant chemoradiotherapy (CRT). Treatment guidelines, therefore, allow multiple treatment options.2,3 In the MAC-NPC analysis, locoregional and distant failure rates at 5 years were both in the range of 20% in patients receiving CT.1 Although the use of concomitant CT and intensity-modulated RT (IMRT) has reduced the occurrence of locoregional relapses,4 distant recurrences remain a major concern. This underlines the potential role for additional systemic therapy.
The MAC-NPC meta-analysis mostly evaluated the addition of CT to RT compared with RT alone, but did not formally perform direct comparisons between the different timings of CT. Network meta-analysis (NMA) is a way to determine whether additional CT is beneficial in the management of NPC. NMA has been applied to head and neck squamous cell carcinomas5 and was able to predict the results of randomized trials published afterward.6 NMA was planned in the MAC-NPC protocol, and the presentation of its results is the purpose of this article.
METHODS
MAC-NPC Database and End Point Definitions
The MAC-NPC is an IPD meta-analysis that comprises most randomized trials conducted up to December 31, 2010, evaluating the benefit of adding CT to local treatment in patients with nonmetastatic NPC. The inclusion criteria, trial search, trial flowchart, data collection, and checking have been detailed in a previous publication along with the results of the standard meta-analysis.1 The primary end point was OS, defined as the time from randomization until death from any cause. The secondary end points were PFS and locoregional and distant control. PFS was defined as the time from randomization to first progression (locoregional or distant) or death. Locoregional and distant control were defined as the time from randomization to the occurrence of a locoregional or distant failure, respectively. If both a locoregional failure and a distant failure occurred at the same time, patients were considered as having a distant failure only. Patients without locoregional and distant failure were censored at the date of death or last follow-up for those alive. Only severe acute toxicity was studied. End points included in the network toxicity analysis were those with a sufficient number of patients, with a significant interaction with CT timing in the standard meta-analysis, and that were considered clinically relevant. Nausea-vomiting and hematologic toxicities other than neutropenia were not included. The toxicities retained for analysis were therefore acute neutropenia, mucositis, weight loss, and hearing loss.
Statistical Methods
The NMA was planned in the protocol of the MAC-NPC update. A detailed statistical analysis plan was written before NMA analysis. A two-step method was used, the first step being the computation of hazard ratios (HRs) on the basis of the IPD gathered by the MAC-NPC Collaborative Group, using the Peto estimator for OS and PFS,7 and a competing risk model for locoregional and distant control.8 The proportional hazards assumption was checked at each meta-analysis level for OS and PFS.9 The second step was the actual NMA, using as input data for each trial the two treatments compared, the logarithm of the HRs, which is usually normally distributed,5,10,11 and its variance. Therefore, all the analyses were stratified by trial. The first analysis was initially reported12 using Bayesian modeling.13 Because of easier computation and programming, especially for the handling of multiarm trials or inconsistency, the final analysis was performed using a frequentist approach and the R package netmeta,14,15 but both methods gave similar results and provided the same ranking.
Heterogeneity was quantified using the I2, which represents the proportion of total variation in study estimates that is due to heterogeneity.16 To limit the number of tests for both heterogeneity and inconsistency, Rücker14 has proposed a global test, called Q test. This test is a generalization of Cochran’s test that is used to assess heterogeneity in conventional meta-analysis. The Q statistic is the sum of a statistic for heterogeneity and a statistic for inconsistency, which represents the variability of treatment effect between direct and indirect comparisons at the meta-analytic level. The protocol for the NMA stated that a fixed-effects model had to be used first and that in case of significant heterogeneity (P < .1), two solutions would be investigated: the use of random-effects models and the performance of sensitivity analyses after the exclusion of trials that were considered as outliers in the standard meta-analysis.1 The netmeta package allows identifying in which closed loop the inconsistency is located.15 The trials responsible for inconsistency could be determined by comparing direct and indirect estimates and trial forest plots within the inconsistent closed loop; the effect of trial removal on the network consistency and estimation could therefore be investigated.
Within the Bayesian framework, the treatments are ranked using the surface under the cumulative ranking curve.17,18 Rücker and Schwarzer19 have proposed a frequentist analog to surface under the cumulative ranking curve, which is named P-score, that works without resampling and measures the mean extent of certainty that a treatment is better than the competing treatments. P-score would be 100% when a treatment is certain to be the best and 0% when a treatment is certain to be the worst.19 Five-year absolute benefit was computed using the survival rate at 5 years for the RT-only arms in MAC-NPC1 and the HR using the method by Stewart and Parmar.20 One unplanned sensitivity analysis was performed using HR adjusted on patient sex, age, performance status, and stage. This work was performed in accordance with published guidelines.21 P values < .05 were considered significant for the difference between treatments. All analyses were performed using the R software (version 3.0.2; R Foundation, Vienna, Austria).
RESULTS
Description of the Network and Patients
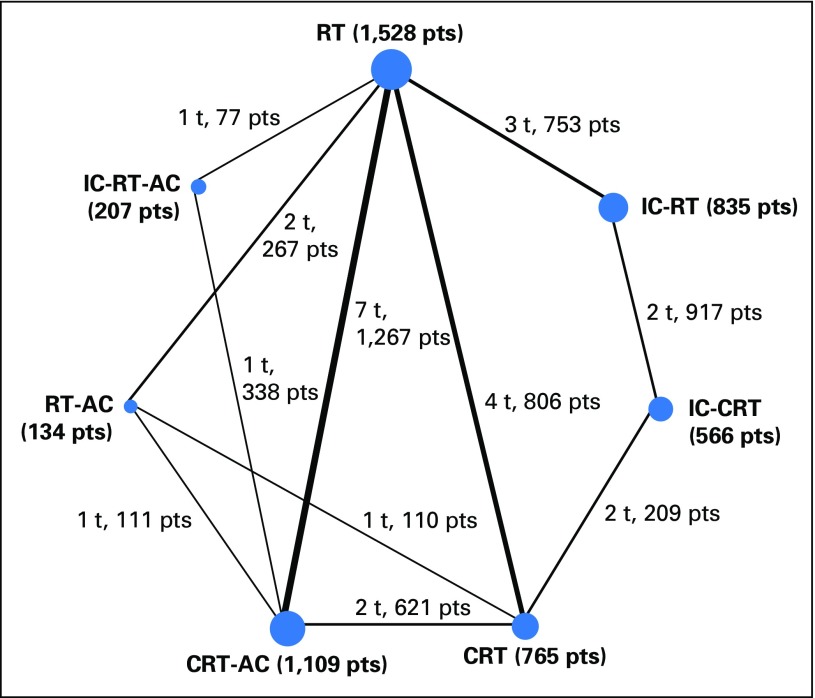
The network consists of 20 trials and 5,144 patients: 19 trials22-39 (including one unpublished trial, VUMCA-95), which were included in the standard meta-analysis (4,806 patients, described in Blanchard et al1), and one trial (338 patients), which compared two timings of CT,40 that was ineligible for the standard meta-analysis. Because of a factorial design in two trials, these 20 trials were split into 26 comparisons. There were seven different treatments: RT alone, which was used as the reference category; IC followed by RT (IC-RT); RT followed by AC (RT-AC); IC followed by RT followed by AC (IC-RT-AC); CRT; IC followed by CRT (IC-CRT); and CRT followed by AC (CRT-AC). Only IC-CRT was not directly compared with RT. The network is represented in Figure 1. The network comprised five independent closed loops for consistency analysis and one 2 × 2 factorial design trial.27
Fig 1.
Graphical representation of the trial network for overall and progression-free survival. The size of the nodes is proportional to the number of patients (pts), which is given in parenthesis in each treatment category. The width of the lines is proportional to the number of comparisons. The number of trials (t) and pts in each comparison are displayed next to each line. The network included 26 comparisons from 20 trials. Six comparisons were counted for the QMH-95 trial (2 × 2 design) and two for the NPC-9902 trial (2 × 2 design; second randomization on radiotherapy [RT] modalities). Because of the duplication of QMH-95 trial arms, some pts are counted multiple times in this figure (in the RT, concomitant chemoradiotherapy [CRT], CRT-adjuvant chemotherapy [AC], and RT-AC groups). However, the statistical analysis takes into account the correlation structure in this design and does not give an excessive weight to duplicated patients. IC, induction chemotherapy.
Median follow-up (interquartile range) was 7.4 years (6.2 to 11.9). Patients (Data Supplement) were mostly male (3,826 patients; 74%), < 50 years of age (3,177 patients; 62%), and had good performance status (0 in 2,743 patients [65%] and 1 in 1,404 patients [33%]). Patients presented most frequently with locally advanced tumor (stage III in 2,519 patients [49%]; stage IVA-B in 2,133 [42%]; and nonkeratinizing histology [WHO grade I in 196 patients; 4%).
Overall Survival
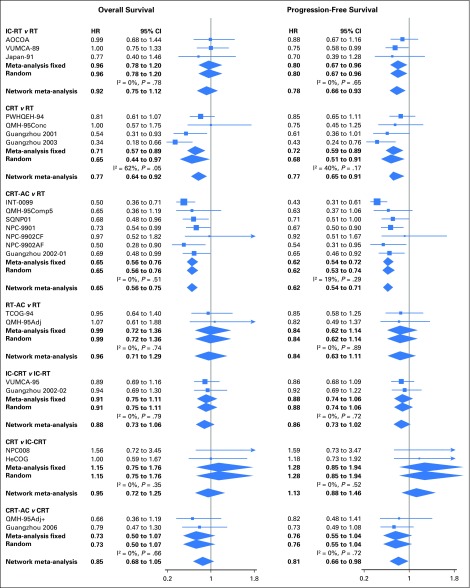
The three treatments that had the highest effect on OS were CRT-AC, CRT, and IC-CRT, with respective P-scores (higher score meaning a higher probability of being the best treatment) of 96%, 70%, and 63%, respectively, and corresponding absolute benefit at 5 years of 12%, 8% and 6% compared with radiotherapy alone (Tables 1 and 2; Data Supplement). There was no significant heterogeneity (I2 = 5.5%; P = .30) or inconsistency (P = .53), and the proportional hazards assumption was valid. The HRs (95% CIs) on the basis of the NMA for each pairwise comparison are presented in the lower left triangle of the league table and instructions for reading are given in the footnote of Table 2 (Data Supplement). Compared with RT alone, the HRs (95% CIs) for OS for CRT-AC, CRT, and IC-CRT, respectively, were 0.65 (0.56 to 0.75), 0.77 (0.64 to 0.92), and 0.81 (0.63 to 1.04). The HRs (95% CIs) of CRT-AC compared with CRT or IC-CRT showed no significant differences, with respective values of 0.85 (0.68 to 1.05) and 0.81 (0.61 to 1.07).
Table 1.
Summary of Network Meta-Analysis Results for the Six Treatments Compared With RT Alone and the Four Efficacy End Points
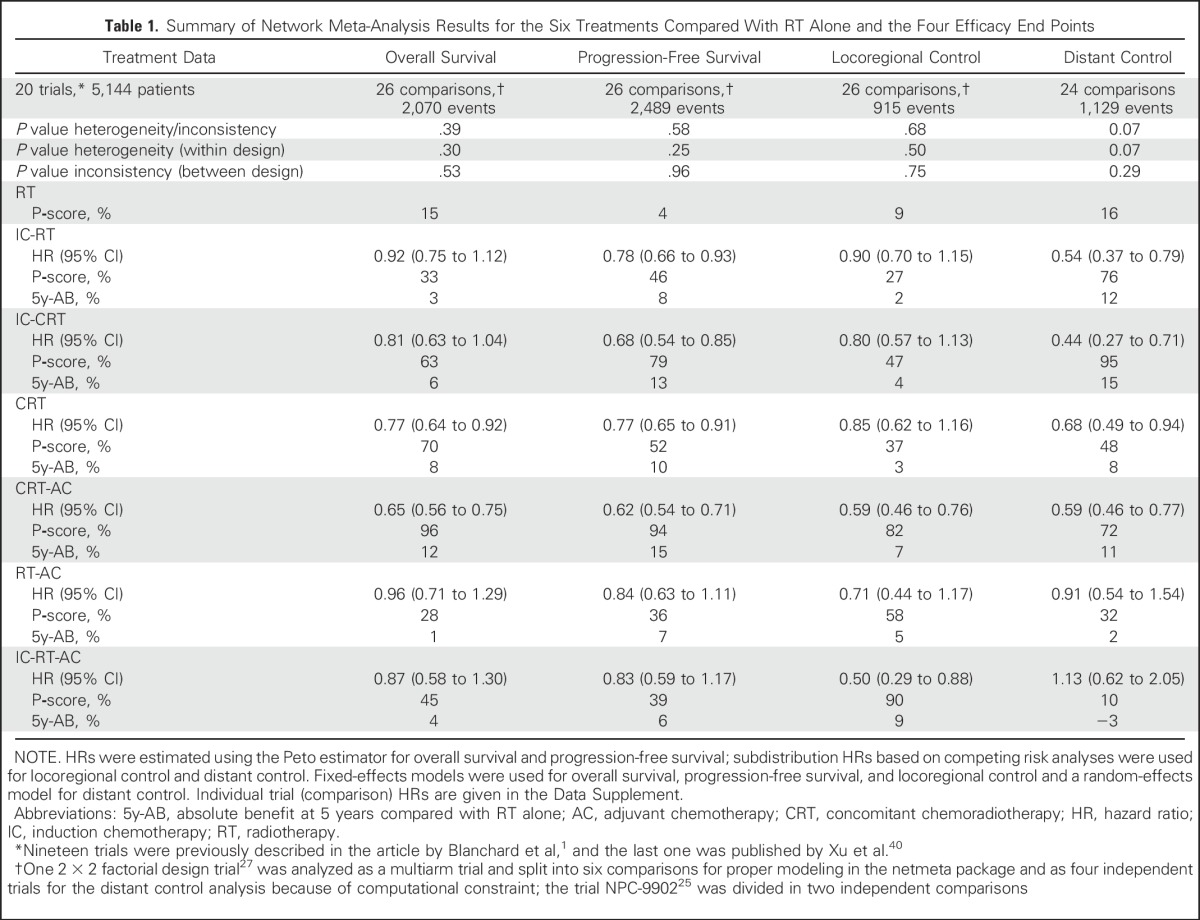
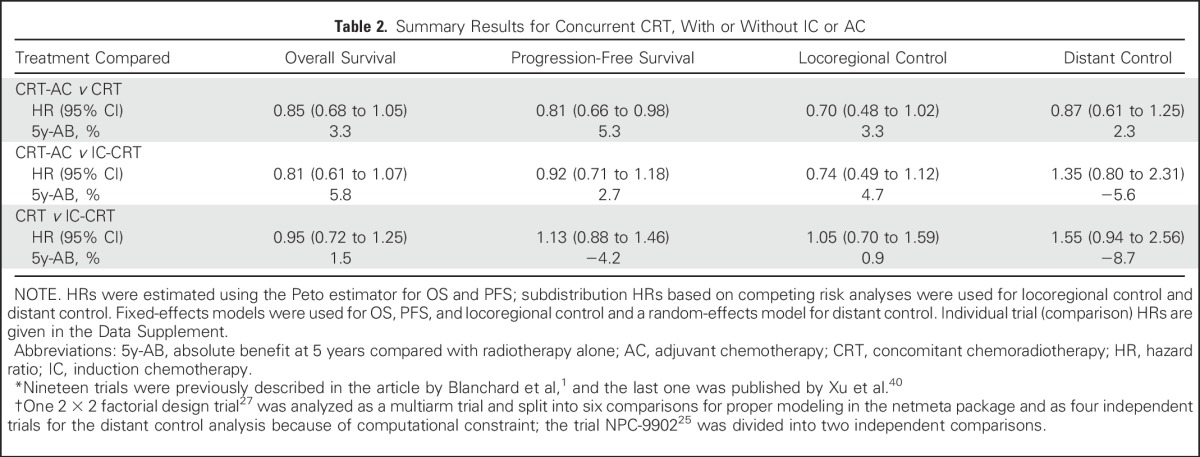
Table 2.
Summary Results for Concurrent CRT, With or Without IC or AC
Secondary End Points
The results of PFS (Tables 1 and 2; Data Supplement) are in agreement with OS. No heterogeneity (I2 = 0%; P = .25) or inconsistency (P = .96) was detected for this end point. The three best treatments were the same as for OS, with CRT-AC being the most effective, with a P-score of 94%; IC-CRT and CRT, with respective P-scores of 79% and 52%, ranked second and third. The HRs (95% CIs) of CRT-AC compared with CRT or IC-CRT were, respectively, 0.81 (0.66 to 0.98) and 0.92 (0.71 to 1.18). A graphical assessment of local heterogeneity and inconsistency for OS and PFS is presented in Figure 2. For PFS, the proportional hazards assumption was not valid for the comparison of RT to CRT-AC because of the absence of proportional hazards in the INT-0099 trial. Results of a planned sensitivity performed after the exclusion of this trial showed the robustness of the results and the validity of the proportional hazards assumption (Data Supplement).
Fig 2.
Forest plot for overall survival (on the left) and progression-free survival (on the right), showing results from direct comparisons and network meta-analysis. HR < 1 is in favor of the first treatment mentioned in the title (ie, IC-RT for the comparison IC-RT v RT). Only comparisons involving two trials or more are presented here. For comparisons with only one trial, the hazard ratios used are reported in the Data Supplement. AC, adjuvant chemotherapy; CRT, concomitant chemoradiotherapy; HR, hazard ratio; IC, induction chemotherapy; RT, radiotherapy;
The three best treatments for locoregional control were IC-RT-AC, CRT-AC, and RT-AC, with respective P-scores of 90%, 82%, and 58% (Tables 1 and 2; Data Supplement). There was no heterogeneity (I2 = 0%, P = .50) or inconsistency (P = 0.75) for this end point. The comparison between CRT-AC and CRT showed a nonsignificant difference in favor of CRT-AC, with an HR (95% CI) of 0.70 (0.48 to 1.02). Regarding distant control (Tables 1 and 2; Data Supplement), the results are presented using a random effects NMA because of the presence of heterogeneity (P = .07). No inconsistency was noticed (P = .29). The three best treatments for distant control were IC-CRT, IC-RT, and CRT-AC, with respective P-scores of 95%, 76%, and 72%. The comparison between CRT-AC and CRT showed the absence of significant difference, with an HR (95% CI) of 0.87 (0.61 to 1.25). CRT was nonsignificantly inferior to IC-CRT, with an HR (95% CI) of 1.55 (0.94 to 2.56).
Sensitivity Analyses
Two sensitivity analyses for OS were planned after the exclusion of the two outliers in the standard meta-analysis (INT-0099,26 Guangzhou 200329) and after excluding the trials that did not include cisplatin as part of the randomized CT. In these two analyses, CRT-AC remained ranked first and IC-CRT was ranked second, although closely followed by CRT (Data Supplement). The unplanned sensitivity analysis on the basis of HR adjusted on covariates instead of unadjusted HR for OS and PFS did not significantly modify the network estimates, the two first treatments being the same in both cases (Data Supplement).
Results for distant control were not entirely robust to sensitivity analyses. The exclusion of the two trials responsible for heterogeneity in the standard meta-analysis for distant control (Int-009926 and QMH-9527) reduced heterogeneity (P = .24) and improved consistency (P = .41) but changed notably the estimates and ranking. IC-CRT remained ranked first (P-score, 88%; Data Supplement), but the three next best treatments were RT-AC, IC-RT, and CRT-AC. When only trials using cisplatin were included (Data Supplement), IC-CRT and CRT-AC were respectively ranked first and second, with P-scores of 99% and 74%. From a statistical standpoint, the analyses of locoregional control and distant control using the Peto estimator led to results similar to the analysis using competing risk (Data Supplement).
Toxicity
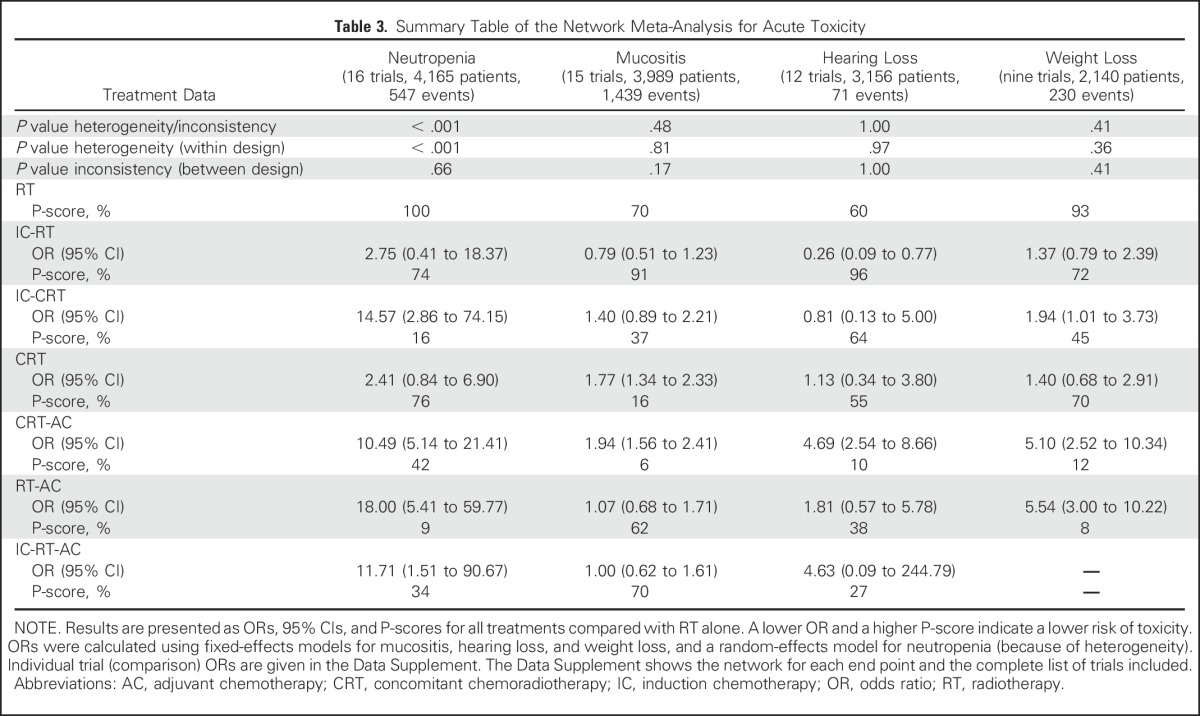
Toxicity analyses were based on slightly different networks (Data Supplement), and their results are presented in Table 3. CRT-AC and RT-AC were the most toxic regimens, as measured by their P-scores, for mucositis/hearing loss and neutropenia/weight loss, respectively, which underlines the potential toxicity of AC, either alone or administered with CRT.
Table 3.
Summary Table of the Network Meta-Analysis for Acute Toxicity
DISCUSSION
The major findings of this IPD NMA of CT in NPC can be summarized as follows. First, schedules containing concomitant CT most often ranked better than schedules without concomitant CT. Second, when focusing on schedules containing concomitant CT, the ones with the addition of AC always ranked better than concomitant CT alone, although the differences in head-to-head comparison were only significant for PFS and locoregional control, whereas IC added to CRT ranked better than CRT for PFS, locoregional control, and distant control. These results were overall consistent between end points and robust to sensitivity analyses. Finally, although toxicity data were available for only a minority of acute toxicities and a subset of trials, the schedules containing more than one timing of CT generally resulted in more toxicity than the use of only one timing.
Four NMAs on the role of CT in NPC on the basis of published data have been reported41-44 in the past year, one as a full network of all treatments43 and the other three as small subnetworks, centered around the comparison between CRT and CRT-AC,41 CRT, and IC-CRT42 or CRT, CRT-AC, and IC-CRT.44 All four reports concluded that CRT was as efficacious as more intensified treatments, such as CRT-AC or IC-CRT, and should be the preferred treatment in locally advanced NPC. The differences between their findings and ours can be explained by the selection of trials, the performance of analyses on secondary/limited networks,41,42,44 the use of published data of unchecked quality, the absence of data updates or use of intent-to-treat analysis, and the potential inaccuracy of some data used. Indeed, when the HRs are not reported in the publications, their estimates on the basis of other parameters, such as survival curves, are known to be imprecise.45 These differences might be diluted and less easy to point out in NMA compared with standard meta-analysis, because the amount of data analyzed and statistical tests produced can be overwhelming for readers. We believe that the high quality of data with updated follow-up, the use of IPD, multiple standardized secondary end points such as PFS and locoregional/distant control, and the rigorous methodology are major strengths of our work. Our work highlights once more that IPD meta-analyses are the gold standard method and probably even more so in the context of NMA. The publication of multiple articles on the same meta-analysis is also typical of the type of research waste46 that is seen more and more often. It does not add much value but produces confusion in the scientific debate. The use of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) extension to network meta-analyses21 and the prospective registration of meta-analyses in a centralized database47 might help select the meta-analyses that are trustworthy in the future.
The present work has limitations. First, patients with stage II disease or WHO grade I histology were included, but they represent a minority of patients with NPC in both clinical practice and trials (Data Supplement). The results of the entire network might not apply to these patients, and their inclusion might also bias its results. However, because there was no interaction between tumor stage and treatment effect in the standard meta-analysis,1 it is unlikely that the exclusion of such patients would lead to anything but a lower power of the NMA and increased confusion due to postrandomization exclusion. Besides, no differences were seen when using adjusted HRs as input data for the NMA. Second, although treatment ranking is an attractive output of NMAs, readers should be aware that the computation of ranking probabilities mostly relies on the point estimates (the hazard and odds ratios here).19 Ranking is also influenced by the network geometry.48 To evaluate the certainty that a treatment is superior to another, attention should be paid to the HR estimates along with their CIs, as well as the consistency of the HR estimates across different end points and not entirely rely on the treatment ranking. Moreover, the ranking uncertainty could not be computed. Third, incomplete and probably heterogeneous data were available on acute toxicity, along with few reliable data on late toxicity, and given the old RT techniques used, even if these data had been recorded correctly, they would have been difficult to interpret in light of the major technical changes in radiotherapy techniques. Fourth, because results on distant control are not entirely robust to sensitivity analyses, they should be considered with caution. Fifth, although we have performed a thorough search on the basis of publications and clinical trial databases, a publication bias cannot be completely ruled out. Finally, although network meta-analyses are now accepted by multiple public health agencies as a way to perform systematic evidence synthesis and guidelines have been published,21,49 readers should keep in mind the limitations associated with the use of indirect comparisons.
Our interpretation is that giving more CT to patients with locally advanced NPC, as induction or adjuvant, provided they receive concomitant CT, achieves a reduction in recurrence rates. This statement is supported by the fact that HR and ranking almost always favor CRT-AC or IC-CRT. The choice of the most suited regimen for a given patient must include a consideration of the risk–benefit ratio. The data on IC before CRT remain conflicting. Indeed, a recent randomized trial evaluating induction gemcitabine, carboplatin, and paclitaxel followed by CRT versus upfront CRT was negative,50 whereas a small randomized trial evaluating induction docetaxel, cisplatin, and fluorouracil before CRT, presented in 2015, was positive for OS.51 Additional trials on IC should be reported in the near future and might help clarify this issue (NCT01245959, NCT01536223, NCT01872962, and NCT02512315). Ongoing research is warranted in the fields of systemic treatment and predictive biomarkers to allow the selection of patients for whom the addition of AC or IC to CRT would be needed, such as is being investigated by NRG Oncology using Epstein-Barr virus quantitative circulating DNA levels (NCT02135042).
Until then, clinical judgment, evaluation of the risk of local and distant relapse, and discussion with the patient about the potential risks and benefits of the different treatment regimens should guide clinical practice. The data presented here should be of major help because they represent the most up to date and least biased analysis of the medical literature. Additional data will help us personalize treatments to each patient’s needs.
ACKNOWLEDGMENT
The investigator meeting was organized with the help of Sanofi. We thank Gerta Rücker for her help in the implementation of the netmeta package, Adam Garden for his comments on the manuscript, and Françoise Delassus for her secretariat assistance.
Meta-Analysis of Chemotherapy in Nasopharynx Carcinoma Collaborative Group: Secretariat—Pierre Blanchard, Jean Bourhis, Anne W.M. Lee, Sophie Marguet, Julie Leclercq, Wai Tong Ng, Jean Pierre Pignon; Steering Committee—Anthony T.C. Chan, Richard J. Chappell, Tai-Xiang Lu, Stefan Michiels, Brian O’Sullivan, Joseph Wee; Investigators—Muhyi Al-Sarraf (Wm. Beaumont Hospital, Royal Oak, MI), Pierre Blanchard (Gustave Roussy, Villejuif, France), Ellen Benhamou (Gustave Roussy, Villejuif, France), Jean Bourhis (Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland), Anthony T.C. Chan (Partner State Key Laboratory of Oncology in South China, Sir Y.K. Pao Centre for Cancer, The Chinese University of Hong Kong, Hong Kong, China), Lai-Kwan Chan (Pamela Youde Nethersole Eastern Hospital, Hong Kong, China), Shie-Lee Cheah (National Cancer Centre Singapore, Singapore), Lei Chen (Sun Yat-Sen University Cancer Center, Guangzhou, China), Qiu-Yan Chen (Sun Yat-Sen University Cancer Center, Guangzhou, China), Yong Chen (Sun Yat-Sen University Cancer Center, Guangzhou, China), Kwan-Hwa Chi (Shin Kong Wu Ho-Su Memorial Hospital, Tapei, Taiwan), Richard J. Chappell (University of Wisconsin, Madison, WI), Daniel T.T. Chua (Hong Kong Sanatorium & Hospital, Hong Kong, China), George Fountzilas (Aristotle University of Thessaloniki School of Medicine, Thessaloniki, Greece), Georgia Gourgioti (Hellenic Cooperative Oncology Group, Athens, Greece), Masuto Hareyama (Sapporo Medical University, Sapporo, Japan), Ming-Huang Hong (Sun Yat-Sen University Cancer Center, Guangzhou, China), Pei-Yu Huang (Sun Yat-Sen University Cancer Center, Guangzhou, China), Edwin Pun Hui (Partner State Key Laboratory of Oncology in South China, Sir Y.K. Pao Centre for Cancer, The Chinese University of Hong Kong, Hong Kong, China), Michael Kam (Partner State Key Laboratory of Oncology in South China, Sir Y.K. Pao Centre for Cancer, The Chinese University of Hong Kong, Hong Kong, China), Dora L.W. Kwong (Queen Mary Hospital, Hong Kong, China), Ka On Lam (Queen Mary Hospital, Hong Kong, China), Julie Leclercq (Gustave Roussy, Villejuif, France), Anne W.M. Lee (Pamela Youde Nethersole Eastern Hospital, Hong Kong, China), Ho Fun Victor Lee (Li Ka Shing Faculty of Medicine, Hong Kong, China), Tai-Xiang Lu (Sun Yat-Sen University Cancer Center, Guangzhou, China), Brigette Ma (Partner State Key Laboratory of Oncology in South China, Sir Y.K. Pao Centre For Cancer, The Chinese University of Hong Kong, China), Jun Ma (Sun Yat-Sen University Cancer Center, Guangzhou, China), Hai-Qiang Mai (Sun Yat-Sen University Cancer Center, Guangzhou, China), Sophie Marguet (Gustave Roussy, Villejuif, France), Stefan Michiels (Gustave Roussy, Villejuif, France), Frankie Mo (Partner State Key Laboratory of Oncology in South China, Sir Y.K. Pao Centre For Cancer, The Chinese University of Hong Kong, China), James Moon (SWOG Statistical Center, Seattle), Wai Tong Ng (Pamela Youde Nethersole Eastern Hospital, Hong Kong, China), Roger Ngan (Queen Elizabeth Hospital, Hong Kong, China), Brian O’Sullivan (Princess Margaret Hospital, Toronto, Canada), Jean Pierre Pignon (Gustave Roussy, Villejuif, France), Jonathan Sham (The University of Hong Kong, Hong Kong, China), Yoke Lim Soong (National Cancer Centre, Singapore, Singapore), Yuk Tung (Tuen Mun Hospital, Hong Kong, China), Joseph Wee (National Cancer Centre, Singapore, Singapore), Xuang Wu (Sun Yat-Sen University Cancer Center, Guangzhou, China), Tingting Xu (Fudan University Shanghai Cancer Center, Shanghai, China), Li Zhang (Sun Yat-Sen University Cancer Center, Guangzhou, China), Guopei Zhu (Fudan University Shanghai Cancer Center, Shanghai, China).
Footnotes
Supported by grants from the French Ministry of Health (Programme d’actions intégrées de recherche VADS: PAIR-VADS 2011-191) and Ligue Nationale Contre le Cancer (PLRC2015.LNCC/JPP). The INT-0099 (SWOG 8892) trial was supported by National Cancer Institute Grant Nos. CA180888 and CA180819. The HeCOG trial was supported by the Hellenic Cooperative Oncology Group research Grant No. HE R_5G.
See accompanying article on page 565
AUTHOR CONTRIBUTIONS
Conception and design: Anne W.M. Lee, Jean Pierre Pignon, Pierre Blanchard
Financial support: Jean Pierre Pignon
Administrative support: Jean Pierre Pignon
Provision of study materials or patients: Anne W.M. Lee, Wai Tong Ng, Jun Ma, Anthony T.C. Chan, Pei-Yu Huang, Guopei Zhu, Daniel T.T. Chua, Yong Chen, Hai-Qiang Mai, Dora L.W. Kwong, Shie-Lee Cheah, James Moon, Yuk Tung, Kwan-Hwa Chi, George Fountzilas, Jean Bourhis
Collection and assembly of data: All authors
Data analysis and interpretation: Laureen Ribassin-Majed, Sophie Marguet, Jean Bourhis, Jean Pierre Pignon, Pierre Blanchard
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
What Is the Best Treatment of Locally Advanced Nasopharyngeal Carcinoma? An Individual Patient Data Network Meta-Analysis
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Laureen Ribassin-Majed
No relationship to disclose
Sophie Marguet
No relationship to disclose
Anne W.M. Lee
No relationship to disclose
Wai Tong Ng
No relationship to disclose
Jun Ma
No relationship to disclose
Anthony T.C. Chan
Research Funding: Bristol-Myers Squibb, Merck, Eli Lilly
Travel, Accommodations, Expenses: Roche, Pfizer, Merck
Pei-Yu Huang
No relationship to disclose
Guopei Zhu
No relationship to disclose
Daniel T.T. Chua
No relationship to disclose
Yong Chen
No relationship to disclose
Hai-Qiang Mai
No relationship to disclose
Dora L.W. Kwong
Honoraria: Merck Sharp & Dohme, Mundipharma
Consulting or Advisory Role: Merck Sharp & Dohme
Shie-Lee Cheah
No relationship to disclose
James Moon
No relationship to disclose
Yuk Tung
No relationship to disclose
Kwan-Hwa Chi
Leadership: Johnpro Biotech
Stock or Other Ownership: Johnpro Biotech, Johnpro Biotech (I)
Honoraria: Johnpro Biotech
Patents, Royalties, Other Intellectual Property: Johnpro Biotech, Johnpro Biotech (I)
George Fountzilas
Stock or Other Ownership: ARIAD Pharmaceuticals (I)
Consulting or Advisory Role: Roche, Sanofi, Astra-Zeneca
Jean Bourhis
No relationship to disclose
Jean Pierre Pignon
Research Funding: Sanofi (Inst)
Pierre Blanchard
Consulting or Advisory Role: Astellas Pharma, Sanofi
Research Funding: Astellas Pharma
Travel, Accommodations, Expenses: Merck Serono, Astellas Pharma
REFERENCES
- 1.Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: An update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16:645–655. doi: 10.1016/S1470-2045(15)70126-9. [DOI] [PubMed] [Google Scholar]
- 2.Chan ATC, Grégoire V, Lefebvre J-L, et al. Nasopharyngeal cancer: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:vii83–vii85. doi: 10.1093/annonc/mds266. (suppl 7) [DOI] [PubMed] [Google Scholar]
- 3. doi: 10.6004/jnccn.2017.0101. National Comprehensive Cancer Network: NCCN Guidelines - Head and Neck Cancers (version 2.2016). Fort Washington, PA, National Comprehensive Cancer Network. [DOI] [PubMed]
- 4.Lee AWM, Ng WT, Chan LLK, et al. Evolution of treatment for nasopharyngeal cancer--success and setback in the intensity-modulated radiotherapy era. Radiother Oncol. 2014;110:377–384. doi: 10.1016/j.radonc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard P, Hill C, Guihenneuc-Jouyaux C, et al. Mixed treatment comparison meta-analysis of altered fractionated radiotherapy and chemotherapy in head and neck cancer. J Clin Epidemiol. 2011;64:985–992. doi: 10.1016/j.jclinepi.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Bourhis J, Sire C, Graff P, et al. Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99-02): An open-label phase 3 randomised trial. Lancet Oncol. 2012;13:145–153. doi: 10.1016/S1470-2045(11)70346-1. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S, Peto R, Lewis J, et al. Beta blockade during and after myocardial infarction: An overview of the randomized trials. Prog Cardiovasc Dis. 1985;27:335–371. doi: 10.1016/s0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
- 8.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 9.Wei Y, Royston P, Tierney JF, et al. Meta-analysis of time-to-event outcomes from randomized trials using restricted mean survival time: Application to individual participant data. Stat Med. 2015;34:2881–2898. doi: 10.1002/sim.6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mauri D, Polyzos NP, Salanti G, et al. Multiple-treatments meta-analysis of chemotherapy and targeted therapies in advanced breast cancer. J Natl Cancer Inst. 2008;100:1780–1791. doi: 10.1093/jnci/djn414. [DOI] [PubMed] [Google Scholar]
- 11. Efthimiou O, Debray TPA, van Valkenhoef G, et al: GetReal in network meta-analysis: A review of the methodology. Res Synth Methods 7:236-263, 2016. [DOI] [PubMed]
- 12. Blanchard P, Lee A, Leclercq J, et al: OC-003: What is the best treatment in nasopharyngeal carcinoma? An individual patient data network meta-analysis. Radiother Oncol 114:6-7, 2015 (suppl 1) [Google Scholar]
- 13.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 14.Rücker G. Network meta-analysis, electrical networks and graph theory. Res Synth Methods. 2012;3:312–324. doi: 10.1002/jrsm.1058. [DOI] [PubMed] [Google Scholar]
- 15. Rücker G, Schwarzer G, Krahn U, et al: Netmeta: Network meta-analysis using frequentist methods. http://cran.r-project.org/web/packages/netmeta/index.html.
- 16.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 17.Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Chaimani A, Higgins JPT, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8:e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58. doi: 10.1186/s12874-015-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart LA, Parmar MK. Meta-analysis of the literature or of individual patient data: Is there a difference? Lancet. 1993;341:418–422. doi: 10.1016/0140-6736(93)93004-k. [DOI] [PubMed] [Google Scholar]
- 21.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 22.Chi K-H, Chang Y-C, Guo W-Y, et al. A phase III study of adjuvant chemotherapy in advanced nasopharyngeal carcinoma patients. Int J Radiat Oncol Biol Phys. 2002;52:1238–1244. doi: 10.1016/s0360-3016(01)02781-x. [DOI] [PubMed] [Google Scholar]
- 23.Chan AT, Teo PM, Leung TW, et al. A prospective randomized study of chemotherapy adjunctive to definitive radiotherapy in advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1995;33:569–577. doi: 10.1016/0360-3016(95)00218-N. [DOI] [PubMed] [Google Scholar]
- 24.Huang P-Y, Cao K-J, Guo X, et al. A randomized trial of induction chemotherapy plus concurrent chemoradiotherapy versus induction chemotherapy plus radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Oral Oncol. 2012;48:1038–1044. doi: 10.1016/j.oraloncology.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Lee AWM, Tung SY, Chan ATC, et al. A randomized trial on addition of concurrent-adjuvant chemotherapy and/or accelerated fractionation for locally-advanced nasopharyngeal carcinoma. Radiother Oncol. 2011;98:15–22. doi: 10.1016/j.radonc.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–1317. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 27.Kwong DLW, Sham JST, Au GKH, et al. Concurrent and adjuvant chemotherapy for nasopharyngeal carcinoma: A factorial study. J Clin Oncol. 2004;22:2643–2653. doi: 10.1200/JCO.2004.05.173. [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Hu C-S, Chen X-Z, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012;13:163–171. doi: 10.1016/S1470-2045(11)70320-5. [DOI] [PubMed] [Google Scholar]
- 29.Chen Q-Y, Wen Y-F, Guo L, et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: Phase III randomized trial. J Natl Cancer Inst. 2011;103:1761–1770. doi: 10.1093/jnci/djr432. [DOI] [PubMed] [Google Scholar]
- 30.Fountzilas G, Ciuleanu E, Bobos M, et al. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: A randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Ann Oncol. 2012;23:427–435. doi: 10.1093/annonc/mdr116. [DOI] [PubMed] [Google Scholar]
- 31.Wu X, Huang PY, Peng PJ, et al. Long-term follow-up of a phase III study comparing radiotherapy with or without weekly oxaliplatin for locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2013;24:2131–2136. doi: 10.1093/annonc/mdt163. [DOI] [PubMed] [Google Scholar]
- 32.Chan ATC, Leung SF, Ngan RKC, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2005;97:536–539. doi: 10.1093/jnci/dji084. [DOI] [PubMed] [Google Scholar]
- 33.Chua DT, Sham JS, Choy D, et al. Preliminary report of the Asian-Oceanian Clinical Oncology Association randomized trial comparing cisplatin and epirubicin followed by radiotherapy versus radiotherapy alone in the treatment of patients with locoregionally advanced nasopharyngeal carcinoma. Cancer. 1998;83:2270–2283. [PubMed] [Google Scholar]
- 34.Chen Y, Sun Y, Liang S-B, et al. Progress report of a randomized trial comparing long-term survival and late toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy versus radiotherapy alone in patients with stage III to IVB nasopharyngeal carcinoma from endemic regions of China. Cancer. 2013;119:2230–2238. doi: 10.1002/cncr.28049. [DOI] [PubMed] [Google Scholar]
- 35.Hui EP, Ma BB, Leung SF, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009;27:242–249. doi: 10.1200/JCO.2008.18.1545. [DOI] [PubMed] [Google Scholar]
- 36.Lee AWM, Tung SY, Chua DTT, et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2010;102:1188–1198. doi: 10.1093/jnci/djq258. [DOI] [PubMed] [Google Scholar]
- 37.Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005;23:6730–6738. doi: 10.1200/JCO.2005.16.790. [DOI] [PubMed] [Google Scholar]
- 38.Hareyama M, Sakata K, Shirato H, et al. A prospective, randomized trial comparing neoadjuvant chemotherapy with radiotherapy alone in patients with advanced nasopharyngeal carcinoma. Cancer. 2002;94:2217–2223. doi: 10.1002/cncr.10473. [DOI] [PubMed] [Google Scholar]
- 39.International Nasopharynx Cancer Study Group; VUMCA I Trial Preliminary results of a randomized trial comparing neoadjuvant chemotherapy (cisplatin, epirubicin, bleomycin) plus radiotherapy vs. radiotherapy alone in stage IV(> or = N2, M0) undifferentiated nasopharyngeal carcinoma: A positive effect on progression-free survival. Int J Radiat Oncol Biol Phys. 1996;35:463–469. doi: 10.1016/s0360-3016(96)80007-1. [DOI] [PubMed] [Google Scholar]
- 40.Xu T, Zhu G, He X, et al. A phase III randomized study comparing neoadjuvant chemotherapy with concurrent chemotherapy combined with radiotherapy for locoregionally advanced nasopharyngeal carcinoma: Updated long-term survival outcomes. Oral Oncol. 2014;50:71–76. doi: 10.1016/j.oraloncology.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Chen YP, Wang ZX, Chen L, et al. A Bayesian network meta-analysis comparing concurrent chemoradiotherapy followed by adjuvant chemotherapy, concurrent chemoradiotherapy alone and radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2015;26:205–211. doi: 10.1093/annonc/mdu507. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y-P, Guo R, Liu N, et al. Efficacy of the additional neoadjuvant chemotherapy to concurrent chemoradiotherapy for patients with locoregionally advanced nasopharyngeal carcinoma: A Bayesian network meta-analysis of randomized controlled trials. J Cancer. 2015;6:883–892. doi: 10.7150/jca.11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan M, Kumachev A, Siu LL, et al. Chemoradiotherapy regimens for locoregionally advanced nasopharyngeal carcinoma: A Bayesian network meta-analysis. Eur J Cancer. 2015;51:1570–1579. doi: 10.1016/j.ejca.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 44.Yu H, Gu D, He X, et al. The role of induction and adjuvant chemotherapy in combination with concurrent chemoradiotherapy for nasopharyngeal cancer: A Bayesian network meta-analysis of published randomized controlled trials. Onco Targets Ther. 2016;9:159–170. doi: 10.2147/OTT.S96983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. doi: 10.1016/j.ejca.2015.10.067. Blanchard P, Ribassin-Majed L, Lee A, et al: Comment on “Chemoradiotherapy regimens for locoregionally advanced nasopharyngeal carcinoma: A Bayesian network meta-analysis”, published in Eur J Cancer 51 (2015), 1570-1579. Eur J Cancer 56:183-185, 2016. [DOI] [PubMed] [Google Scholar]
- 46.Macleod MR, Michie S, Roberts I, et al. Biomedical research: Increasing value, reducing waste. Lancet. 2014;383:101–104. doi: 10.1016/S0140-6736(13)62329-6. [DOI] [PubMed] [Google Scholar]
- 47. PROSPERO: Welcome to PROSPERO: International prospective register of systematic reviews. http://www.crd.york.ac.uk/PROSPERO/
- 48.Kibret T, Richer D, Beyene J. Bias in identification of the best treatment in a Bayesian network meta-analysis for binary outcome: A simulation study. Clin Epidemiol. 2014;6:451–460. doi: 10.2147/CLEP.S69660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. NICE Decision Support Unit: About the evidence synthesis TSD series. http://www.nicedsu.org.uk/evidence-synthesis-tsd-series%282391675%29.htm.
- 50.Tan T, Lim W-T, Fong K-W, et al. Concurrent chemo-radiation with or without induction gemcitabine, carboplatin, and paclitaxel: A randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2015;91:952–960. doi: 10.1016/j.ijrobp.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 51. Daoud J, Aupérin A, Tao YG, et al: OC-004: A randomized trial of concomitant cisplatin-RT +/− induction TPF in locally advanced nasopharyngeal carcinomas. 114:7 (suppl 1) [Google Scholar]