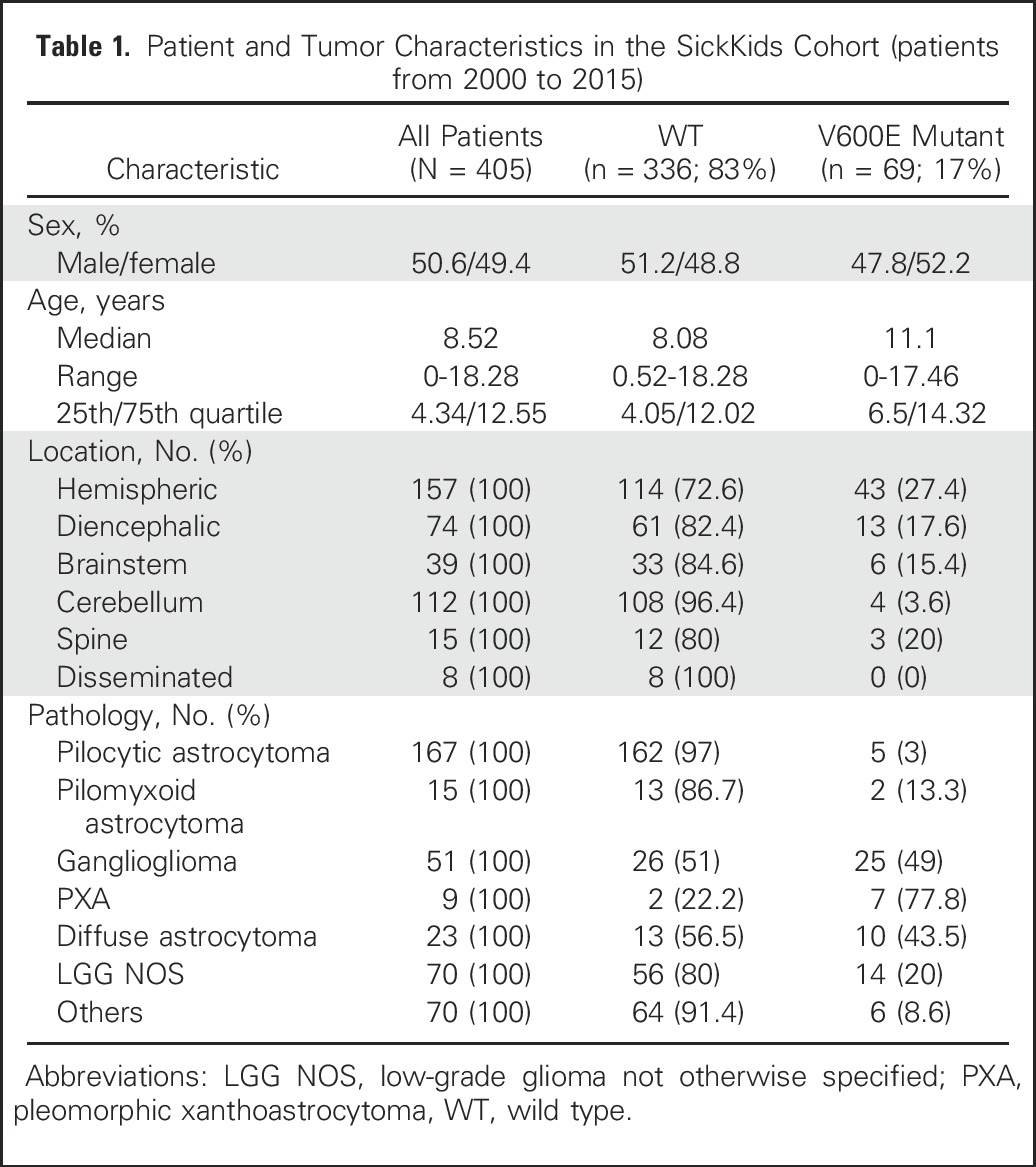
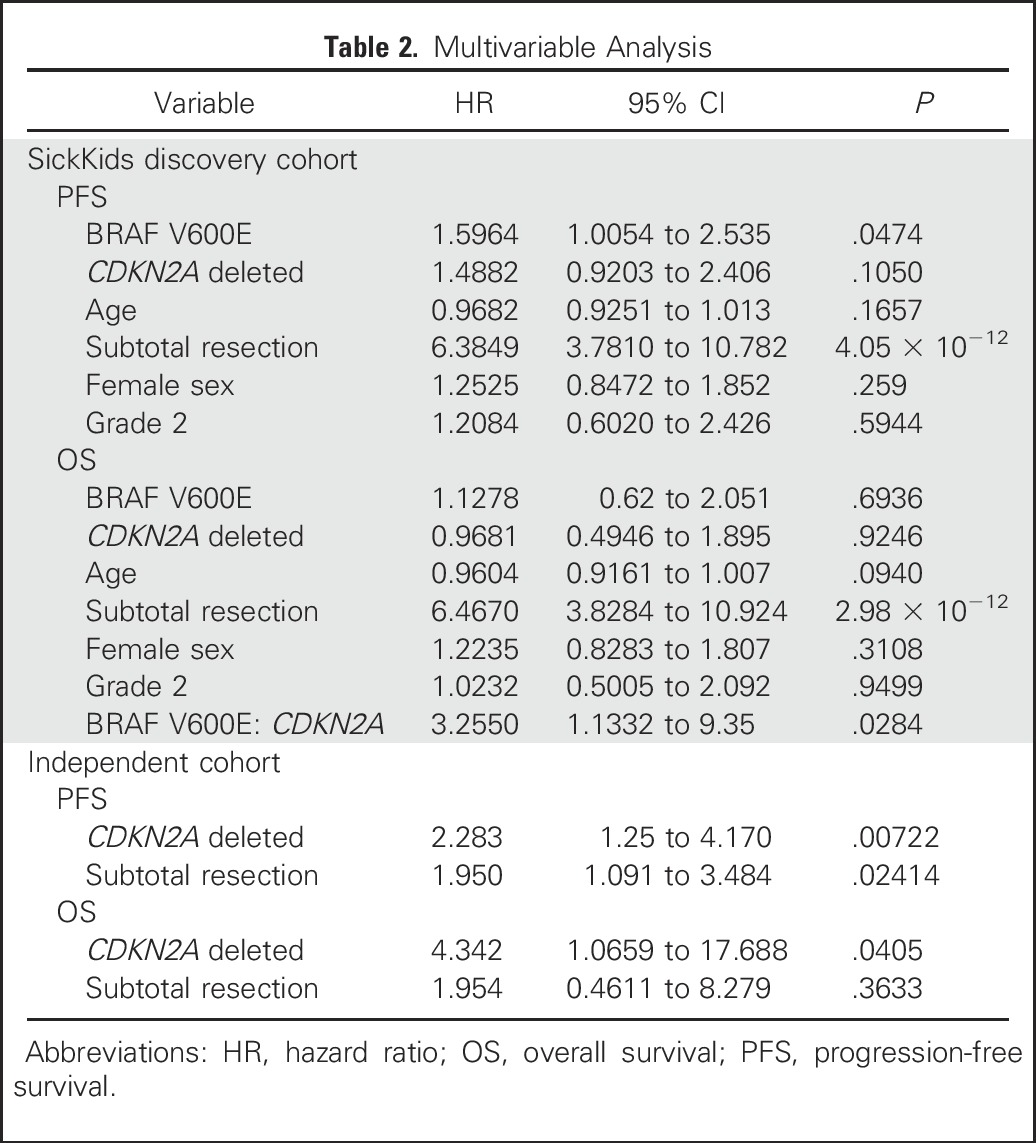
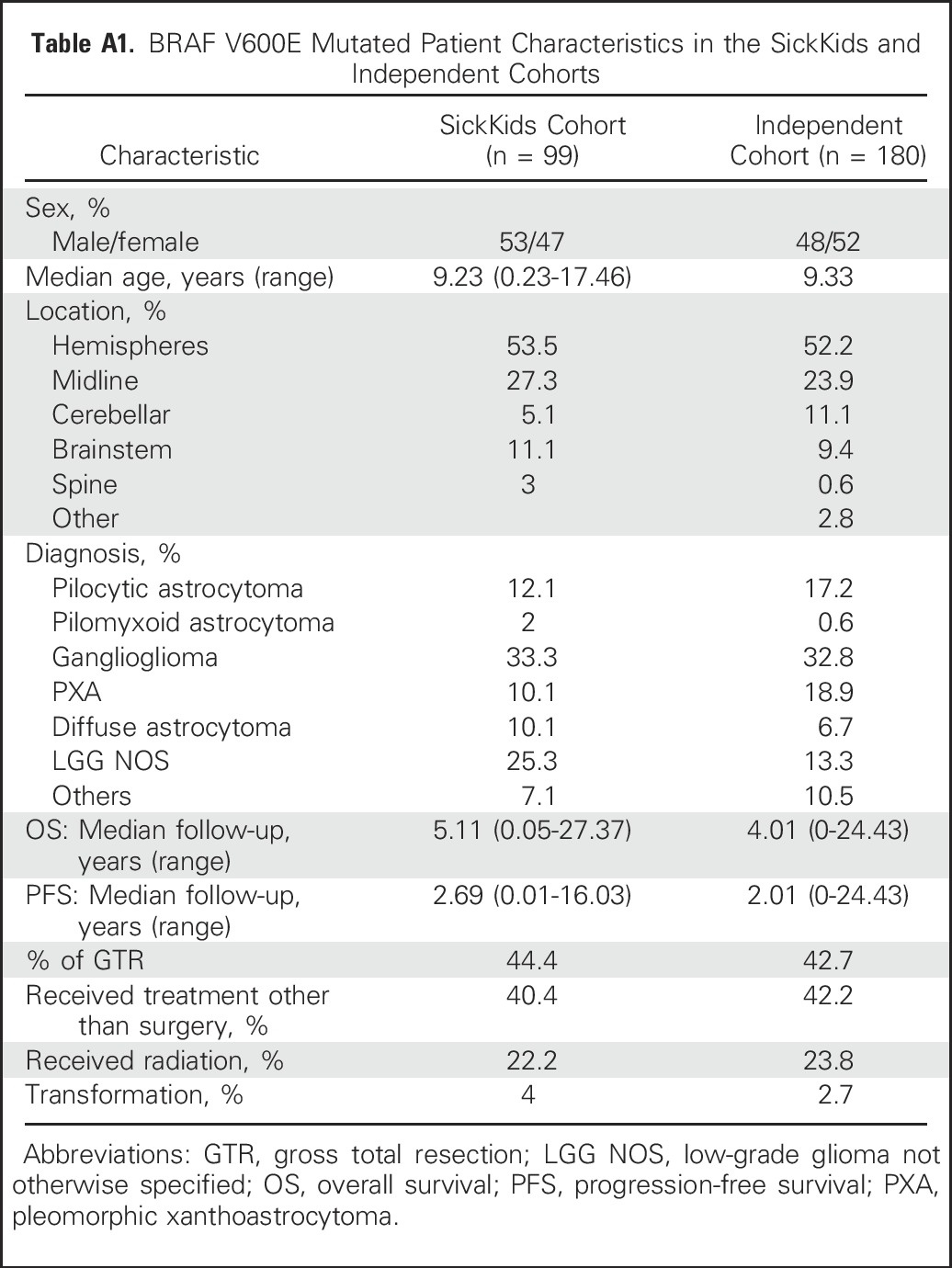
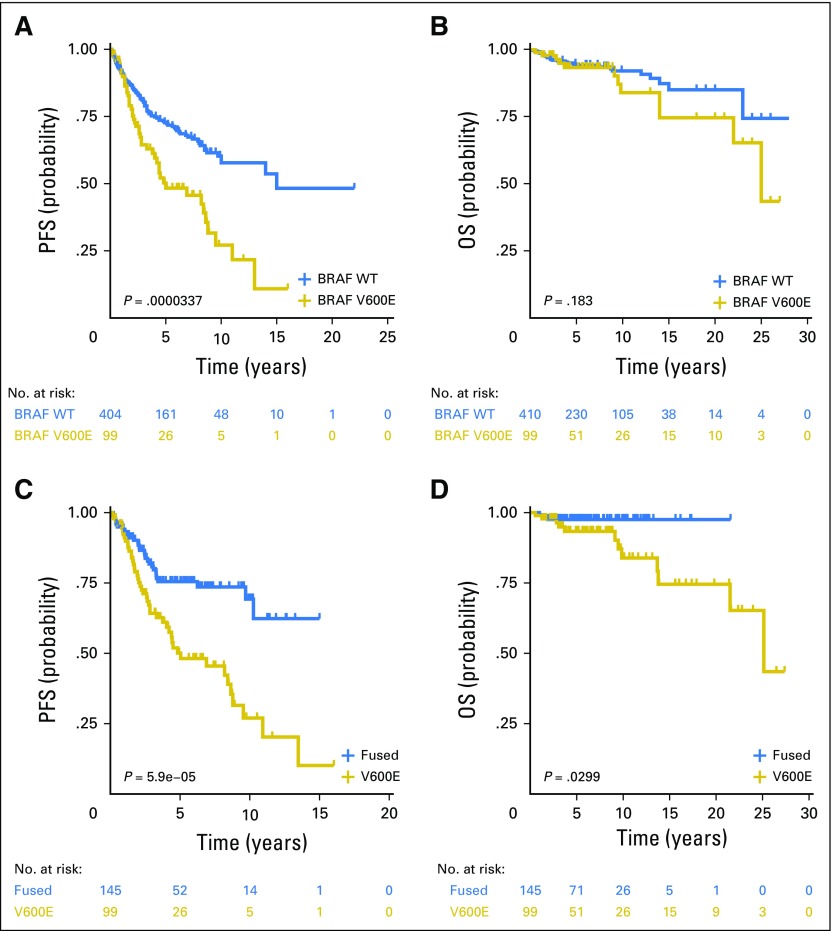
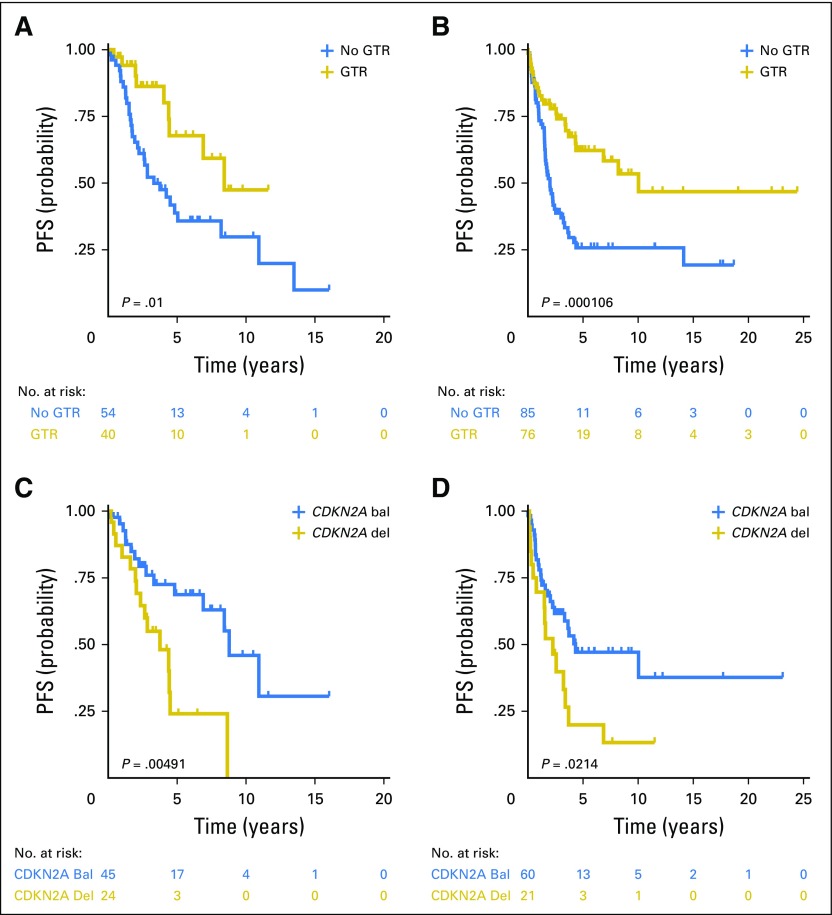
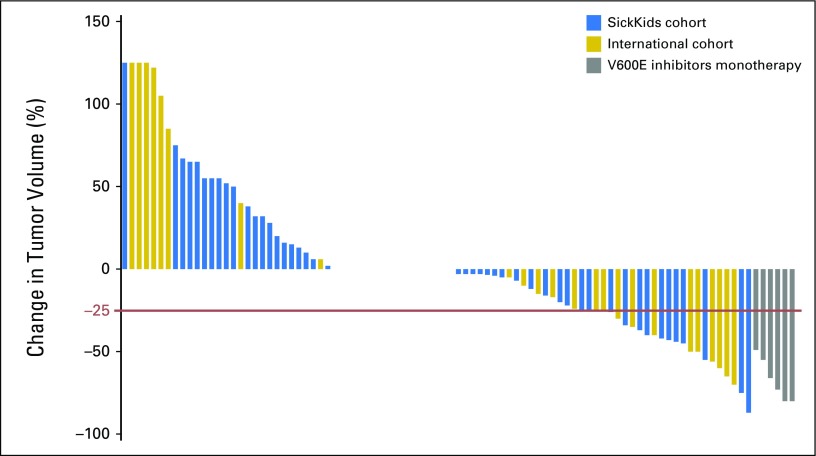
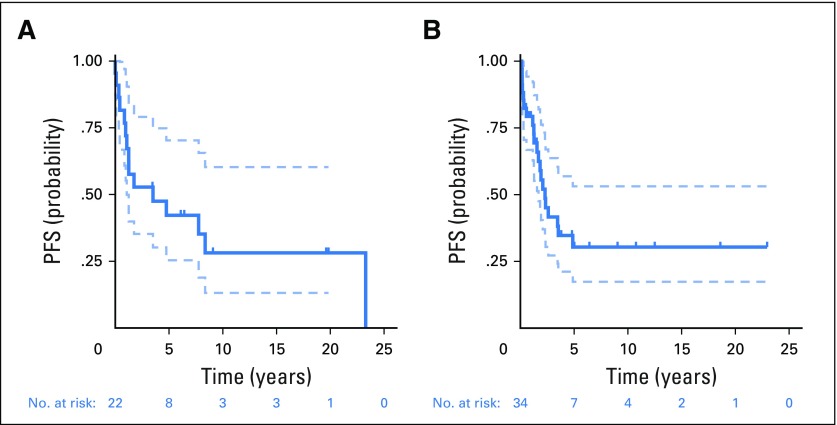
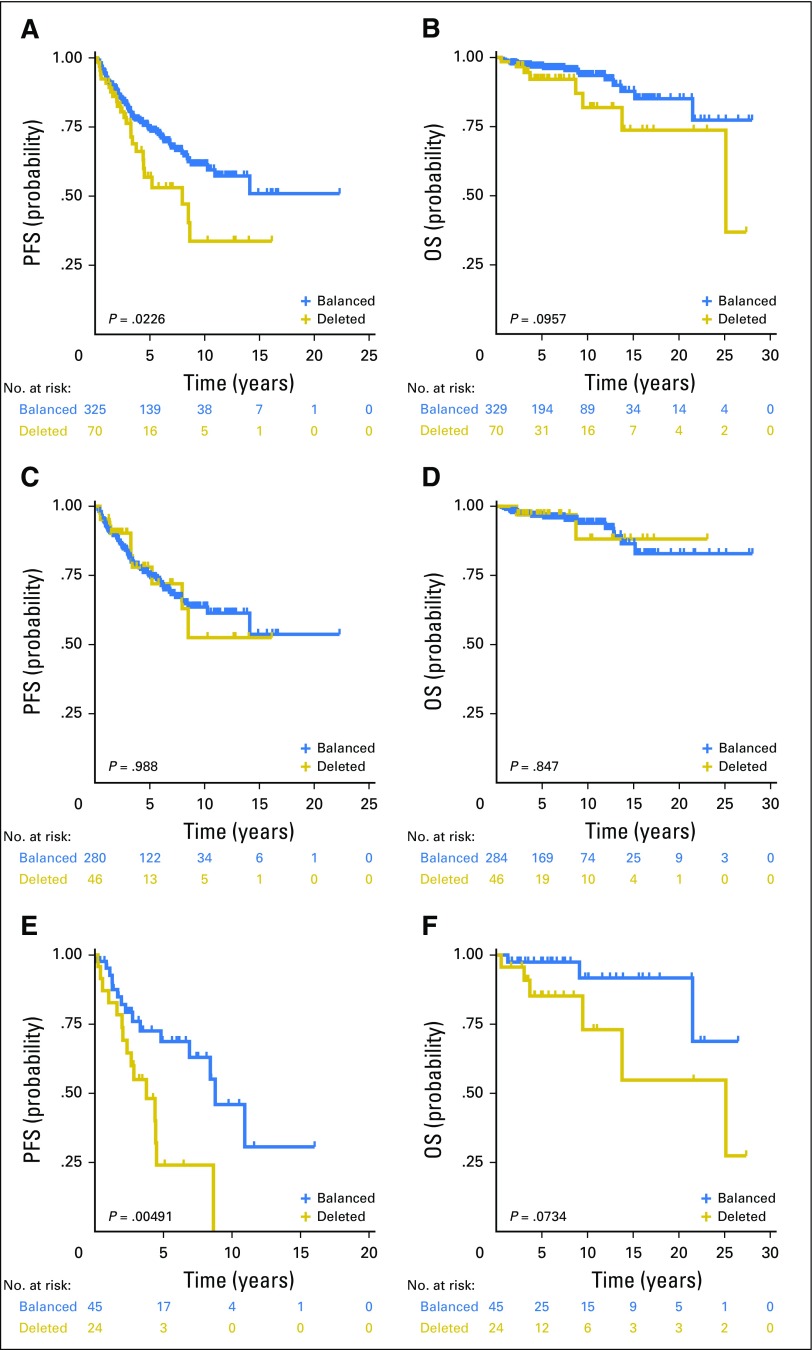
Alvaro Lassaletta
Alvaro Lassaletta
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Michal Zapotocky
Michal Zapotocky
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Matthew Mistry
Matthew Mistry
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Vijay Ramaswamy
Vijay Ramaswamy
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Marion Honnorat
Marion Honnorat
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Rahul Krishnatry
Rahul Krishnatry
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Ana Guerreiro Stucklin
Ana Guerreiro Stucklin
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Nataliya Zhukova
Nataliya Zhukova
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Anthony Arnoldo
Anthony Arnoldo
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Scott Ryall
Scott Ryall
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Catriona Ling
Catriona Ling
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Tara McKeown
Tara McKeown
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Jim Loukides
Jim Loukides
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Ofelia Cruz
Ofelia Cruz
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Carmen de Torres
Carmen de Torres
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Cheng-Ying Ho
Cheng-Ying Ho
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Roger J Packer
Roger J Packer
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Ruth Tatevossian
Ruth Tatevossian
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Ibrahim Qaddoumi
Ibrahim Qaddoumi
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Julie H Harreld
Julie H Harreld
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
James D Dalton
James D Dalton
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Jean Mulcahy-Levy
Jean Mulcahy-Levy
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Nicholas Foreman
Nicholas Foreman
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Matthias A Karajannis
Matthias A Karajannis
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Shiyang Wang
Shiyang Wang
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Matija Snuderl
Matija Snuderl
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Amulya Nageswara Rao
Amulya Nageswara Rao
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Caterina Giannini
Caterina Giannini
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Mark Kieran
Mark Kieran
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Keith L Ligon
Keith L Ligon
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Maria Luisa Garre
Maria Luisa Garre
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Paolo Nozza
Paolo Nozza
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Samantha Mascelli
Samantha Mascelli
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Alessandro Raso
Alessandro Raso
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Sabine Mueller
Sabine Mueller
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Theodore Nicolaides
Theodore Nicolaides
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Karen Silva
Karen Silva
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Romain Perbet
Romain Perbet
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Alexandre Vasiljevic
Alexandre Vasiljevic
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Cécile Faure Conter
Cécile Faure Conter
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Didier Frappaz
Didier Frappaz
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Sarah Leary
Sarah Leary
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Courtney Crane
Courtney Crane
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Aden Chan
Aden Chan
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Ho-Keung Ng
Ho-Keung Ng
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Zhi-Feng Shi
Zhi-Feng Shi
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Ying Mao
Ying Mao
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Elizabeth Finch
Elizabeth Finch
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
David Eisenstat
David Eisenstat
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Bev Wilson
Bev Wilson
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Anne Sophie Carret
Anne Sophie Carret
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Peter Hauser
Peter Hauser
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
David Sumerauer
David Sumerauer
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Lenka Krskova
Lenka Krskova
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Valerie Larouche
Valerie Larouche
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Adam Fleming
Adam Fleming
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Shayna Zelcer
Shayna Zelcer
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Nada Jabado
Nada Jabado
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
James T Rutka
James T Rutka
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Peter Dirks
Peter Dirks
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Michael D Taylor
Michael D Taylor
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Shiyi Chen
Shiyi Chen
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Ute Bartels
Ute Bartels
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Annie Huang
Annie Huang
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
David W Ellison
David W Ellison
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Eric Bouffet
Eric Bouffet
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Cynthia Hawkins
Cynthia Hawkins
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,
Uri Tabori
Uri Tabori
1Alvaro Lassaletta, Michal Zapotocky, Matthew Mistry, Vijay Ramaswamy, Marion Honnorat, Rahul Krishnatry, Ana Guerreiro Stucklin, Nataliya Zhukova, Anthony Arnoldo, Scott Ryall, Catriona Ling, Tara McKeown, Jim Loukides, James T. Rutka, Peter Dirks, Michael D. Taylor, Shiyi Chen, Ute Bartels, Annie Huang, Eric Bouffet, Cynthia Hawkins, and Uri Tabori, The Hospital for Sick Children, Toronto; Adam Fleming, McMaster Children’s Hospital, McMaster University, Hamilton; Shayna Zelcer, Children's Hospital of Western Ontario, London, Ontario; David Eisenstat and Bev Wilson, Stollery Children’s Hospital, University of Alberta, Edmonton, Alberta; Anne Sophie Carret, Hospital Sainte Justine; Nada Jabado, McGill University, Montreal; Valerie Larouche, Centre Hospitalier Universitaire de Québec, Québec City, Quebec, Canada; Ofelia Cruz and Carmen de Torres, Hospital Sant Joan de Déu, Barcelona, Spain; Cheng-Ying Ho, University of Maryland School of Medicine, Baltimore, MD; Roger J. Packer, Children’s National Health System, Washington, DC; Ruth Tatevossian, Ibrahim Qaddoumi, Julie H. Harreld, James D. Dalton, and David W. Ellison, St Jude Children’s Research Hospital, Memphis, TN; Jean Mulcahy-Levy and Nicholas Foreman, Children’s Hospital Colorado, Aurora, CO; Matthias A. Karajannis, Shiyang Wang, and Matija Snuderl, New York University Langone Medical Center, New York, NY; Amulya Nageswara Rao and Caterina Giannini, The Mayo Clinic, Rochester, MN; Mark Kieran and Keith L. Ligon, Dana-Farber Cancer Institute, Boston Children’s Hospital, Boston, MA; Maria Luisa Garre, Paolo Nozza, Samantha Mascelli, and Alessandro Raso, Istituto Giannina Gaslini, Genoa, Italy; Sabine Mueller and Theodore Nicolaides, University of California, San Francisco, San Francisco, CA; Karen Silva and Romain Perbet, Hospices Civils de Lyon; Alexandre Vasiljevic, Cécile Faure Conter, and Didier Frappaz, Institute of Pediatric Hematology and Oncology, Lyon, France; Sarah Leary and Courtney Crane, Seattle Children's Hospital, Seattle, WA; Aden Chan and Ho-Keung Ng, The Chinese University of Hong Kong, Hong Kong; Zhi-Feng Shi and Ying Mao, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; Elizabeth Finch, University of North Carolina, School of Medicine, Chapel Hill, NC; Peter Hauser, Semmelweis University, Budapest, Hungary; and David Sumerauer and Lenka Krskova, University Hospital Motol, Charles University, 2nd Medical School, Prague, Czech Republic.
1,✉