Abstract
Purpose
Classical Hodgkin lymphoma (HL) frequently exhibits genetic alterations leading to overexpression of the programmed death-1 (PD-1) ligands, suggesting a possible vulnerability to PD-1 blockade. The phase Ib study KEYNOTE-013 (NCT01953692) tested the safety and efficacy of the anti–PD-1 antibody pembrolizumab in patients with hematologic malignancies. Based on its genetics, HL was included as an independent cohort.
Methods
We enrolled patients with relapsed or refractory HL whose disease progressed on or after treatment with brentuximab vedotin. Patients received pembrolizumab, 10 mg/kg every 2 weeks, until disease progression occurred. Response to treatment was assessed at week 12 and every 8 weeks thereafter. Principal end points were safety and complete remission (CR) rate.
Results
Thirty-one patients were enrolled; 55% had more than four lines of prior therapy, and 71% had relapsed after autologous stem cell transplantation. Five patients (16%) experienced grade 3 drug-related adverse events (AEs); there were no grade 4 AEs or deaths related to treatment. The CR rate was 16% (90% CI, 7% to 31%). In addition, 48% of patients achieved a partial remission, for an overall response rate of 65% (90% CI, 48% to 79%). Most of the responses (70%) lasted longer than 24 weeks (range, 0.14+ to 74+ weeks), with a median follow-up of 17 months. The progression-free survival rate was 69% at 24 weeks and 46% at 52 weeks. Biomarker analyses demonstrated a high prevalence of PD-L1 and PD-L2 expression, treatment-induced expansion of T cells and natural killer cells, and activation of interferon-γ, T-cell receptor, and expanded immune-related signaling pathways.
Conclusions
Pembrolizumab was associated with a favorable safety profile. Pembrolizumab treatment induced favorable responses in a heavily pretreated patient cohort, justifying further studies.
INTRODUCTION
Classic Hodgkin lymphoma (HL) is unusual among malignancies in that the malignant Hodgkin Reed-Sternberg (HRS) cells are dispersed within an extensive inflammatory/immune cell infiltrate.1 Despite this brisk T-cell–rich infiltrate, there is little evidence of an effective antitumor immune response in HL. Recent studies suggest that HL may rely on the programmed death-1 (PD-1) signaling pathway to evade antitumor immunity. In general, engagement of the immune checkpoint receptor PD-1 on the T-cell surface by its ligands, PD-L1 and PD-L2, triggers the transient downregulation of T-cell function, which normally helps control immune activity in settings of chronic antigen exposure.2,3 Genetic analyses have shown that HRS cells in classic HL frequently exhibit amplification of 9p24.1 and, as a result, overexpress the associated gene products PD-L1 and PD-L2.4 This amplification event also involves the JAK2 locus; in turn, increased activity of the Jak/STAT pathway further drives PD-L1 expression.4 Other mechanisms, in particular, Epstein-Barr virus infection, can also lead to PD-L1 overexpression on the tumor cell surface.5 As a result of those mechanisms, HL tumor cells frequently overexpress PD-L1 and PD-L2 on their surface, which strongly suggests that HL has a unique, genetically determined dependence on PD-1 for survival.
With the clinical availability of monoclonal antibodies targeting PD-1, it is now possible to counter the reliance of tumors on the PD-1 pathway and increase antitumor immunity. This strategy has already achieved successful results in solid tumors, with trials showing significant clinical activity across a range of cancer types.6-9 PD-1 blockade has also shown promising preliminary results in a number of hematologic malignancies.10-13 Pembrolizumab is a humanized, high-affinity, IgG4 monoclonal antibody directed against PD-1. Pembrolizumab has demonstrated clinical activity in several tumor types, including melanoma and non–small-cell lung cancer.7,14 Based on the known genetic deregulation of 9p24.1 in classical HL, this tumor type was included as an independent cohort in a phase Ib study of pembrolizumab in hematologic malignancies (KEYNOTE-013; ClinicalTrials.gov, NCT01953692). Here, we report the results of pembrolizumab treatment in those patients.
METHODS
Patients
The cohort of patients with HL described here was a part of the multicohort, open-label, phase Ib trial KEYNOTE-013, designed to evaluate the safety and antitumor activity of pembrolizumab in patients with select hematologic malignancies. Patients in this cohort were 18 years of age or older with a confirmed diagnosis of classic HL. They had to have relapsed or refractory disease, and to have relapsed after, be ineligible for, or refused autologous stem-cell transplantation (ASCT). In addition, patients were required to have received brentuximab vedotin (BV) treatment. Other inclusion criteria were Eastern Cooperative Oncology Group performance status < 2 with adequate hematologic, renal, hepatic, and coagulation parameters. Principal exclusion criteria were active or past documented autoimmune disease, clinically active CNS involvement, evidence of interstitial lung disease, second malignancy, or HIV infection. Patients who received previous treatment with checkpoint or T-cell costimulatory blockade, systemic immunosuppressive therapy within 7 days, or allogeneic stem cell transplantation within 5 years from the start of study treatment were also excluded. All patients provided written informed consent. The study protocol was approved by the independent institutional review boards or ethics committees at each study site and conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice.
Study Design
Patients were treated with pembrolizumab administered intravenously at a dose of 10 mg/kg every 2 weeks. Response to treatment was assessed by computed tomography and positron emission tomography scan after 12 weeks of treatment and every 8 weeks thereafter. Patients who received pembrolizumab for at least 24 weeks and for at least two treatments beyond confirmed complete remission (CR) could discontinue therapy at the discretion of the investigator. Patients with radiographic progressive disease (PD) at week 12 who were clinically stable could remain on therapy until PD was confirmed by a follow-up scan or longer if they were experiencing clear clinical benefit despite PD. Otherwise, treatment was continued up to 2 years or until confirmed disease progression or unacceptable toxicity. The primary objective of this study was to assess CR rate (CRR) at any time, as determined by investigator review and defined according to the International Harmonization Project (IHP) criteria.15 The main secondary end points were safety, overall response rate (ORR), and duration of response. Adverse events (AEs) were monitored throughout the trial and graded per the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.16
All patients who received at least one dose of pembrolizumab were included in the safety analysis; patients with at least one postbaseline assessment were included in the efficacy analysis. Responses (CR, partial remission [PR], stable disease, or PD) were classified according to IHP criteria.15 ORR was defined as the proportion of patients who achieved either a CR or a PR. The best overall response was defined as the best response during the period between the first dose and the first efficacy assessment showing PD, or, in the absence of PD, the last efficacy assessment before subsequent therapy. Duration of response was defined as the time between the first response and the date of first documented disease progression, or, in the absence of PD, the last efficacy assessment before subsequent therapy. For CRR and ORR, 90% confidence intervals (CIs) were calculated based on the binomial distribution. This design had an approximate power of 80% to detect a 20% improvement in CRR under the null hypothesis that CRR is 10%, with a one-sided type I error rate of 5%.
Biomarker Assessment
Immunohistochemistry.
PD-L1 expression was determined using either fresh or archival formalin-fixed, paraffin-embedded tissue sectioned at 4 to 5 microns, with a proprietary assay developed at QualTek Molecular Laboratories (Newtown, PA) in collaboration with Merck (Kenilworth, NJ). The assay used the 22C3 anti–PD-L1 murine monoclonal antibody (Merck Research Laboratories, Palo Alto, CA) as the primary reagent, and Envision FLEX+ (Dako, Carpinteria, CA) ancillary reagents for antigen retrieval, as the secondary antibody, and for chromogenic development. Staining was automated on the Techmate 500 (no longer commercially available). Scoring was performed by a board-certified pathologist. PD-L1 was considered positive if at least 1% of HL cells (including HRS cells and variants) demonstrated at least partial membrane staining with moderate or strong intensity.
Sections cut from formalin-fixed, paraffin-embedded tissue blocks were deparaffinized and rehydrated with serial passage through changes of xylene and graded ethanols for PD-L2 immunohistochemistry (IHC). All slides were subjected to heat-induced epitope retrieval in Envision FLEX Target Retrieval Solution, High pH (K8012; Dako). Endogenous peroxidase in tissues was blocked by incubation of slides in 3% hydrogen peroxide solution prior to incubation with primary antibody (anti–PD-L2 clone 3G2; Merck Research Laboratories) for 60 minutes. Antigen-antibody binding was visualized via application of the FLEX+ polymer system (K8012; Dako) and application of 3,3′ diaminobenzidine chromogen (K4368, Dako). Stained slides were counterstained with hematoxylin, cover slips were placed, and the slides were reviewed. For PD-L2 expression, a semiquantitative 0 to 5 scoring system (0 = negative; 1 = rare; 2 = low; 3 = moderate; 4 = high; 5 = very high), incorporating combined prevalence of tumor and nontumor cell staining, was applied.
Flow cytometry.
The pharmacodynamic effect of pembrolizumab on lymphocytes was evaluated in peripheral blood specimens collected from patients with HL at pretreatment, cycle 7 predose, or discontinuation time points, using an analytically validated flow cytometric assay. Briefly, peripheral blood was collected in sodium heparin anticoagulant vacutainer tubes and maintained at ambient temperature. Then, 100 µL of sodium-heparin–anticoagulated peripheral blood was incubated in the dark at ambient temperature with a cocktail of fluorochrome-conjugated monoclonal antibodies specific to cell-surface antigens differentially expressed on lymphocytes. After RBC lysis and washing, the cells were fixed in 2% paraformaldehyde buffer and acquired on a BD FACSCanto II flow cytometer (BD Biosciences, San Jose, CA) to collect a minimum of 30,000 CD3+ lymphocyte events. Listmode files were analyzed offline using WinList software 7.0 (Verity Software House, Topsham, ME). CD3, CD4, CD7, CD8, CD16, CD34, CD45, and CD56 were used to assess the frequency of T-cell subsets and NK cells. A WBC differential was used to calculate absolute T- and NK-cell counts. A signed rank test was used to evaluate the significance of the changes from pretreatment to cycle 7 in lymphocyte subsets. No adjustment was made for multiple testing.
NanoString analysis.
Interferon gamma (IFN-γ) is involved in the regulation and differentiation of immune cells and in immune activation,17-20 so we also performed RNA profiling in blood from patients pre- and posttreatment to detect whether IFN-γ pathways were induced by treatment with pembrolizumab. Other previously identified immune-related gene signatures were also evaluated.21 Per blood sample at baseline and cycle 7, 50 ng of total RNA, in a final volume of 5 µL, was mixed with a 3′ biotinylated capture probe and a 5′ reporter probe tagged with a fluorescent barcode from the desired gene expression code set. Probes and target transcripts were hybridized overnight at 65°C for 12 to 16 hours per manufacturers’ recommendations. Hybridized samples were run on the NanoString nCounter preparation station (NanoString Technologies, Seattle, WA) using the high-sensitivity protocol, in which excess capture and reporter probes were removed and transcript-specific ternary complexes were immobilized on a streptavidin-coated cartridge. The samples were scanned at maximum scan resolution using the nCounter Digital Analyzer (NanoString Technologies). NanoString data for each individual sample were normalized by quantile normalization. Gene counts reported by NanoString were used as input variables with a reference distribution generated by using a pool of counts from all samples and 794 genes (excluding data from positive and negative control probes). After performing quantile normalization, a log10 transformation was applied. A paired t test was applied to pre- and postdose patient blood samples to test for significance and P values were adjusted for multiple testing by the Benjamini and Hochberg method to control the false discovery rate.
RESULTS
Patients
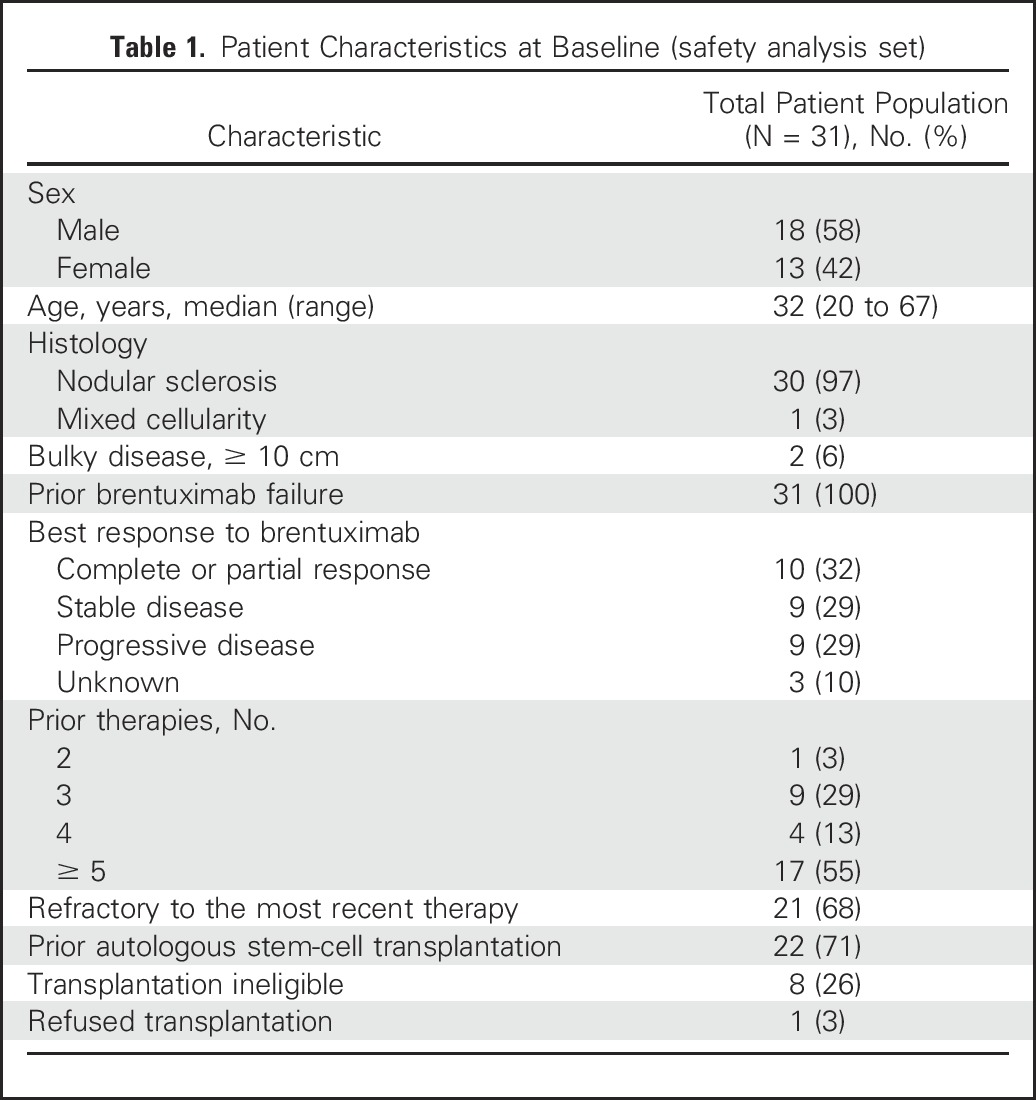
Thirty-one patients with relapsed or refractory classic HL were enrolled in this cohort between December 2013 and September 2014. All had at least one postbaseline scan and were included in the efficacy and safety analyses. The median age of the patients was 32 years (range, 20 to 67 years). All patients previously received BV therapy; 22 (71%) had previously undergone ASCT, eight (26%) were ineligible for transplantation because of chemoresistant disease, and one (3%) had declined transplantation (Table 1). Fifty-five percent of patients had received five or more lines of prior therapy (range, two to 15 lines).
Table 1.
Patient Characteristics at Baseline (safety analysis set)
Safety
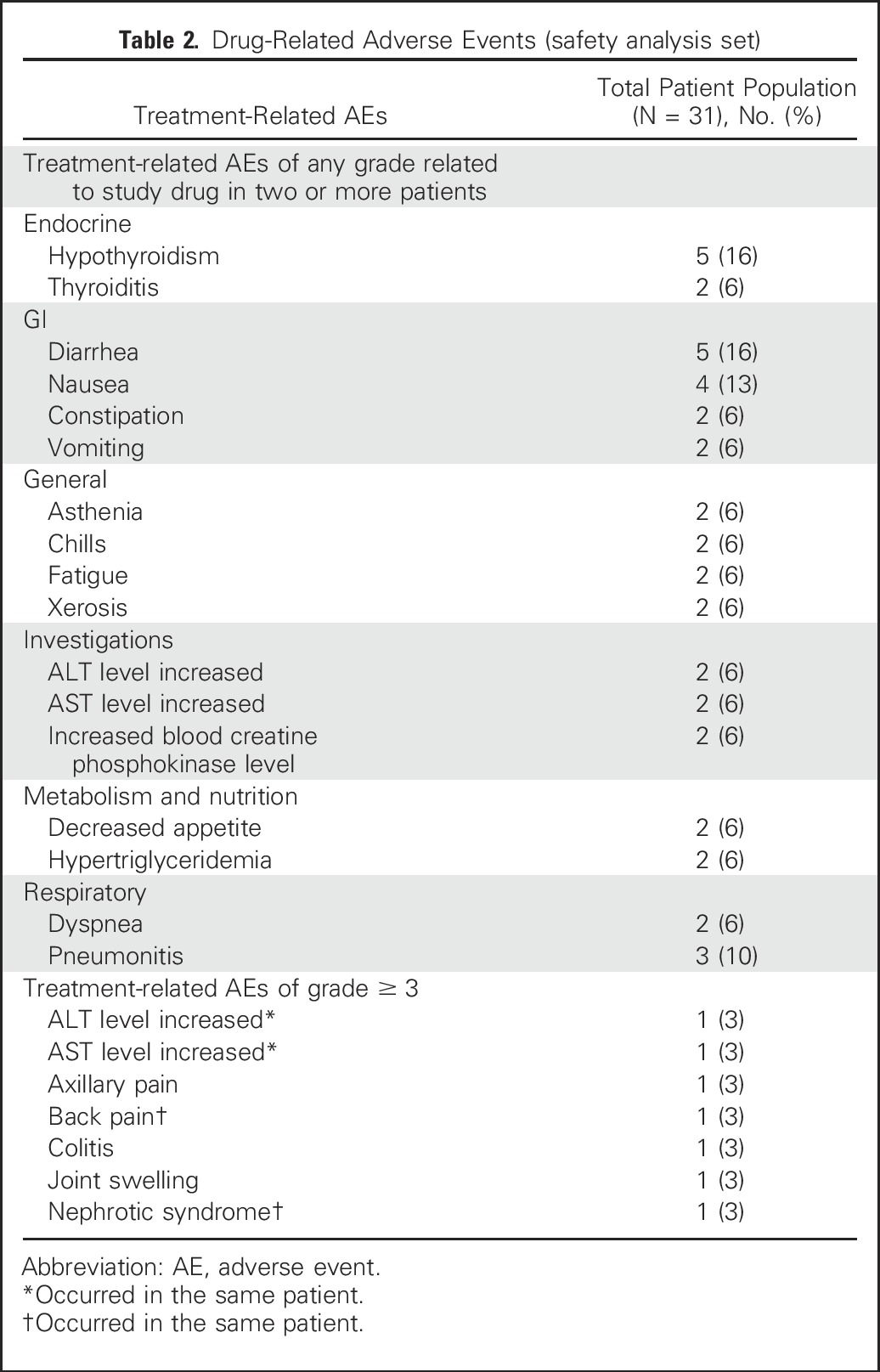
AEs of any grade and attribution were reported in 30 of the 31 patients (97%). Overall, 68% of patients experienced one or more AEs that were deemed related to study treatment. The most common treatment-related AEs (Table 2) were hypothyroidism (16%), diarrhea (16%), nausea (13%), and pneumonitis (10%). Five patients (16%) experienced the following seven treatment-related severe (grade 3) AEs: colitis, increased ALT and AST levels, nephrotic syndrome, joint swelling, back pain, and axillary pain (Table 2). Two patients discontinued treatment because of an AE (grade 2 pneumonitis and grade 3 nephrotic syndrome), and both of these patients received steroids for treatment of the AE. There were no grade 4 treatment-related AEs and no deaths related to study treatment. No instances of treatment-related hepatitis, hypophysitis, or uveitis were reported.
Table 2.
Drug-Related Adverse Events (safety analysis set)
Efficacy
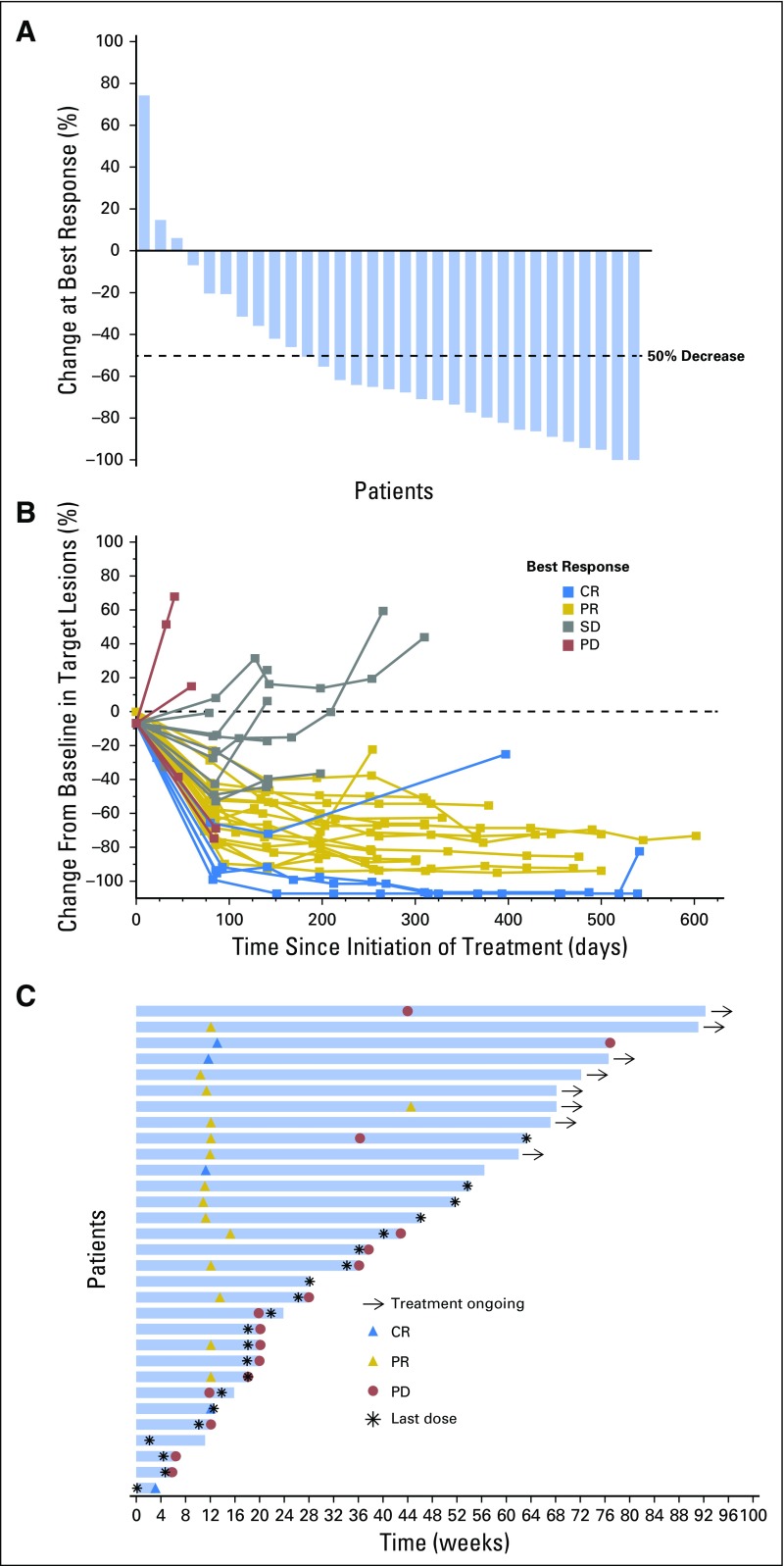
All 31 patients enrolled had at least one postbaseline evaluation and, therefore, were evaluable for efficacy. Five of the 31 patients (16%; 90% CI, 7% to 31%) achieved a CR as their best response (Table 3). In addition, 15 patients (48%) achieved a PR, for an ORR of 65% (90% CI, 48% to 79%). Seven patients (23%) had stable disease, and four (13%) had PD. Overall, 28 patients (90%) experienced some reduction in tumor burden from baseline as best response (Fig 1A). Tumor size changes from baseline demonstrated, in general, reduced tumor burden over time (Fig 1B). Among the 22 patients who had received a prior ASCT, three (14%) achieved CR and 13 (59%) achieved PR, for an ORR of 73%; among the remaining nine patients, two (22%) achieved CR and two (22%) achieved PR, for an ORR of 44% (Table 3).
Table 3.
Antitumor Activity of Pembrolizumab (efficacy analysis set)
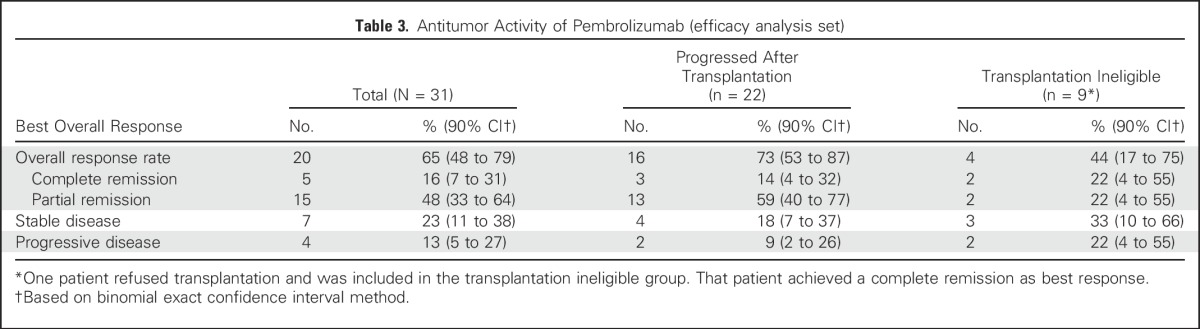
Fig 1.
Response to treatment. (A) Maximum percentage change from baseline in target lesions. (B) Change from baseline in target lesions. (C) Treatment exposure and response duration. Three patients had a formal response assessment before the protocol-required time point of 12 weeks. One patient only received one dose of pembrolizumab, discontinued treatment because of toxicity at 4 weeks, and had nonprotocol scans to assess response, which showed CR. The other two patients had nonprotocol scans to confirm the clinical impression of progressive disease before the 12-week time point. Abbreviations: CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
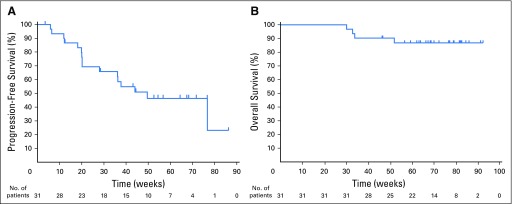
At the time of data cutoff on October 27, 2015, the median follow-up for surviving patients was 17.6 months (range, 10.6 to 22.5 months). Twenty-three patients discontinued pembrolizumab treatment as of the data lock date: 15 because of PD, two because of an AE (pneumonitis and nephrotic syndrome), three to undergo allogeneic stem cell transplantation, one because of therapy switching (to pegylated liposomal doxorubicin), one in CR (allowed by protocol), and one because of consent withdrawal. Among the 20 responding patients, 70% had a duration of response ≥ 24 weeks (range, 0.14+ to 74+ weeks), and the majority (80%) of these patients achieved their best response around the time of the first assessment at 12 weeks (Fig 1C). The progression-free survival and overall survival rates at 24 weeks were 69% and 100%, respectively (Fig 2). Progression-free survival at 52 weeks was 46%.
Fig 2.
Patient survival data. (A) Progression-free survival. (B) Overall survival.
Correlative Studies
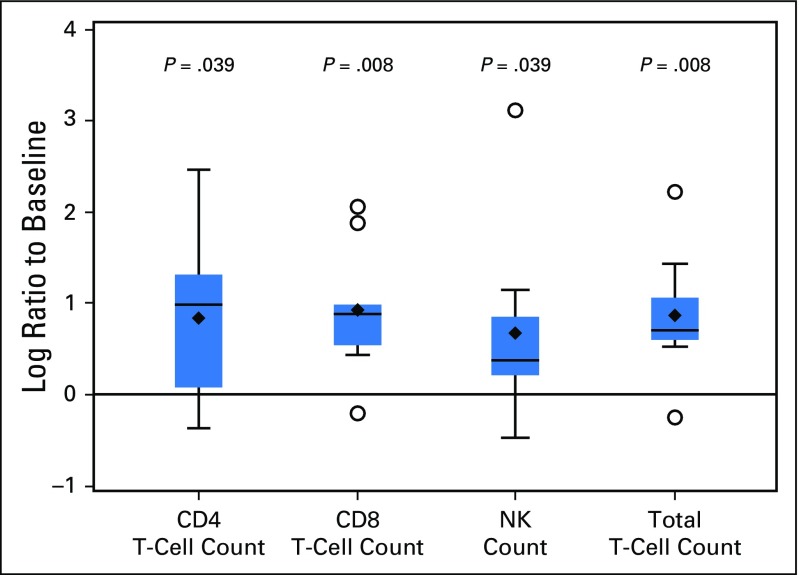
There were 28 tumor samples available from screening from 25 patients. Of those, 16 samples were evaluable from 16 distinct patients (nonevaluable samples [n = 9] were due to inadequate sample or non–tumor-containing tissue); 15 (94%) were PD-L1 positive in the tumor cells. Of 10 available samples from 10 patients at cycle 7, five were evaluable (three of which were also evaluable at screening), and four of those (80%) were PD-L1 positive. Additionally, among 10 patients assessed at baseline for PD-L2 expression by IHC, nine (90%) showed high levels of PD-L2 staining. Among the nine patients with evaluable baseline and on-treatment samples for flow cytometry analysis, we detected a significant increase in the absolute number of total T cells, CD4 and CD8 T subsets, as well as NK cells in the peripheral blood between baseline and cycle 7 (or the time of discontinuation if before cycle 7) (Fig 3). Gene signatures that were previously found to be associated with response to PD-1 blockade21 were evaluated using the NanoString platform. The IFN-γ–induced signature was calculated as the average of the normalized expression levels of CXCL9, CXCL10, HLA-DRA, IDO1, IFN-γ, and STAT1. Among 19 patients with available paired samples, the IFN-γ–induced signature was significantly upregulated after pembrolizumab treatment (adjusted P = .017). In addition, significant increases in gene signatures encompassing T-cell receptor signaling (adjusted P = .004) and expanded immune-related genes (adjusted P = .005) were also observed. However, neither the increase in circulating immune cell subsets nor any of the gene signatures or single-gene expressions at baseline appeared to predict response in this small cohort.
Fig 3.
Distribution of the changes from baseline to cycle 7 (N = 9) on the natural log scale in the absolute count of CD4 and CD8 T cells, total T cells, and natural killer (NK) cells.
DISCUSSION
This study enrolled heavily pretreated patients, all of whom had previously received BV. Despite this, PD-1 blockade with pembrolizumab was associated with a high overall response rate of 65% at 12 weeks with a favorable safety profile. Moreover, 90% of the patients had some evidence of tumor shrinkage on treatment. Although the follow-up is still relatively short, many responses were ongoing. The safety profile was acceptable and concordant with that of pembrolizumab in solid tumors. There were no life-threatening or fatal treatment-related events in this study. Our results overall are consistent with those using another PD-1 antibody (nivolumab) in a similar patient population.10 In the present work, the response rate of pembrolizumab appeared lower in transplantation-naive patients, who composed 30% of the evaluable patients compared with patients who relapsed after ASCT (44% v 73%). This is consistent with prior studies documenting, in general, a lower response rate and worse prognosis for patients ineligible for ASCT because of lack of response to standard-dose salvage regimens.22-24 Larger studies are needed to confirm whether this patient population is, indeed, also less likely to respond to PD-1 blockade.
The CR rate in our cohort, however, was lower than expected when the study was initially designed. In fact, the primary hypothesis of the study was not met because the lower bound of the 90% CI for CRR did not exclude the 10% null hypothesis. Since the time of study design, it has become apparent that CRs are not commonly achieved with checkpoint blockade in solid tumors or hematologic malignancies.7,10,14 Yet PRs can be durable, suggesting that the achievement of CR with checkpoint blockade is not necessary to derive significant clinical benefit. This was the case in the current study, as well, in which many of the PRs were ongoing at the time of analysis despite patients’ extensive histories of prior treatments.
The high response rate to PD-1 blockade seen in this study supports the hypothesis that classic HL is highly dependent on the PD-1 pathway for survival. The genetically defined sensitivity to PD-1 blockade in HL distinguishes this tumor from most other solid and hematologic malignancies, and could explain why the therapeutic response to PD-1 blockade in HL is higher than in any other tumor type studied to date. In HL, the frequent coamplification of PD-L1 and PD-L2 at the 9p24.1 locus suggests that PD-1 receptor blockade may be preferable to selectively targeting the PD-L1 ligand. Our results also suggest that treatment with pembrolizumab promotes expansion of T-cell and NK-cell populations in the peripheral blood and upregulates IFN-γ–activated pathways, suggesting that modulation of the PD-1/PD-L1 axis activates T-cell/IFN-γ signaling pathways. Those analyses were exploratory, and larger studies are needed to confirm these findings. Overall, the results presented here, together with their strong scientific underpinning, provide a compelling rationale for the further development of PD-1 blockade in HL.
ACKNOWLEDGMENT
We thank Karen Giallella, Jennifer Weimer Anderson, Martha Derosier, Kenneth Emancipator, Ziwen Wei, Guadalupe De Maeyer, Heather Hirsch, Rhada Railkar, Michael Nebhozyn, Erin Murphy, Jonathan Juco, Diana Wu, and Richard Wnek, all employees of Merck, and Alexandra G. Jacob who is employed at Memorial Sloan Kettering, for their contributions to the development of the study. We thank Matthew Grzywacz and the ApotheCom Merck oncology team for assistance with manuscript editing.
Footnotes
Processed as a Rapid Communication manuscript.
C.H.M. received an MSK Cancer Center Support Grant/Core Grant (P30 CA008748).
Funded by Merck.
Presented at the American Society of Hematology 56th Annual Meeting and Exposition, San Francisco, CA, December 6-9, 2014; and American Society of Hematology 57th Annual Meeting and Exposition, Orlando, FL, December 5-8, 2015.
Data were obtained by Merck and analyzed in collaboration with the academic authors. A medical writer contracted by the sponsor provided assistance in preparing the manuscript. The funder of the study had no role in the writing of the report.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01953692.
AUTHOR CONTRIBUTIONS
Conception and design: Philippe Armand, Margaret A. Shipp, Shelonitda Rose, Jean-Marie Michot, Craig H. Moskowitz
Provision of study materials or patients: John Kuruvilla, Alejandro D. Ricart
Collection and assembly of data: Philippe Armand, Jean-Marie Michot, Pier Luigi Zinzani, Alejandro D. Ricart, Arun Balakumaran, Shelonitda Rose, Craig H. Moskowitz
Data analysis and interpretation: Philippe Armand, Margaret A. Shipp, Vincent Ribrag, Jean-Marie Michot, Pier Luigi Zinzani, John Kuruvilla, Alejandro D. Ricart, Arun Balakumaran, Shelonitda Rose, Ellen S. Snyder, Craig H. Moskowitz
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Programmed Death-1 Blockade With Pembrolizumab in Patients With Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Philippe Armand
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Infinity Pharmaceuticals
Research Funding: Bristol-Myers Squibb, Merck, Sequenta, Tensha Therapeutics, Sigma-Tau
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Margaret A. Shipp
Honoraria: Bristol-Myers Squibb, Merck, Gilead Sciences, Takeda
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Gilead Sciences, Takeda
Research Funding: Bristol-Myers Squibb (Inst), Bayer (Inst)
Vincent Ribrag
Honoraria: Infinity Pharmaceuticals, Bristol-Myers Squibb, Eisai, PharmaMar, Gilead Sciences
Consulting or Advisory Role: Infinity Pharmaceuticals, Bristol-Myers Squibb, Eisai, PharmaMar, Gilead Sciences
Research Funding: arGEN-X BVBA
Patents, Royalties, Other Intellectual Property: BAY1000394 studies on mantle-cell lymphoma
Expert Testimony: Servier
Travel, Accommodations, Expenses: Roche, Bristol-Myers Squibb
Jean-Marie Michot
No relationship to disclose
Pier Luigi Zinzani
Honoraria: Pfizer, Roche, Gilead, Janssen, Amgen, Celgene, Infinity
Consulting or Advisory Role: Pfizer, AbbVie, Celgene, Gilead, Janssen, Amgen
John Kuruvilla
Honoraria: Merck, Bristol-Myers Squibb, Seattle Genetics, Roche, Janssen, Celgene, Gilead Sciences, Abbvie
Consulting or Advisory Role: Bristol-Myers Squibb, Seattle Genetics, Gilead Sciences, Abbvie, Janssen
Research Funding: Roche
Ellen S. Snyder
Employment: Merck
Stock or Other Ownership: Merck
Alejandro D. Ricart
Employment: Merck
Stock or Other Ownership: Pfizer
Arun Balakumaran
Employment: Merck, Merck (I)
Stock or Other Ownership: Merck, Amgen (I)
Travel, Accommodations, Expenses: Merck
Shelonitda Rose
Employment: Merck
Stock or Other Ownership: Merck
Travel, Accommodations, Expenses: Merck
Craig H. Moskowitz
Consulting or Advisory Role: Merck, Seattle Genetics, Takeda, Celgene, Bristol-Myers Squibb
Research Funding: Merck, Seattle Genetics
REFERENCES
- 1. National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology. Hodgkin Lymphoma. Version 2.2015. Fort Washington, PA, National Comprehensive Cancer Network Inc., 2015. [DOI] [PubMed]
- 2.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: Implications for targeted therapy. Clin Cancer Res. 2012;18:1611–1618. doi: 10.1158/1078-0432.CCR-11-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 8.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 10.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armand P, Nagler A, Weller EA, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: Results of an international phase II trial. J Clin Oncol. 2013;31:4199–4206. doi: 10.1200/JCO.2012.48.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 13.Westin JR, Chu F, Zhang M, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: A single group, open-label, phase 2 trial. Lancet Oncol. 2014;15:69–77. doi: 10.1016/S1470-2045(13)70551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 16. US Department of Health and Human Services: Common Terminology Criteria for Adverse Events, version 4.0. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 17.Seliger B, Ruiz-Cabello F, Garrido F. IFN inducibility of major histocompatibility antigens in tumors. Adv Cancer Res. 2008;101:249–276. doi: 10.1016/S0065-230X(08)00407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agnello D, Lankford CS, Bream J, et al. Cytokines and transcription factors that regulate T helper cell differentiation: New players and new insights. J Clin Immunol. 2003;23:147–161. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- 19.Hu X, Chakravarty SD, Ivashkiv LB. Regulation of interferon and Toll-like receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol Rev. 2008;226:41–56. doi: 10.1111/j.1600-065X.2008.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaidi MR, Merlino G. The two faces of interferon-γ in cancer. Clin Cancer Res. 2011;17:6118–6124. doi: 10.1158/1078-0432.CCR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ribas A, Robert C, Hodi FS, et al. Association of response to programmed death receptor 1 (PD-1) blockade with pembrolizumab (MK-3475) with an interferon-inflammatory immune gene signature. J Clin Oncol 33, 2015 (suppl; abstr 3001)
- 22.Puig N, Pintilie M, Seshadri T, et al. Different response to salvage chemotherapy but similar post-transplant outcomes in patients with relapsed and refractory Hodgkin’s lymphoma. Haematologica. 2010;95:1496–1502. doi: 10.3324/haematol.2009.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seftel M, Rubinger M. The role of hematopoietic stem cell transplantation in advanced Hodgkin lymphoma. Transfus Apheresis Sci. 2007;37:49–56. doi: 10.1016/j.transci.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Josting A, Engert A, Diehl V, et al. Prognostic factors and treatment outcome in patients with primary progressive and relapsed Hodgkin’s disease. Ann Oncol. doi: 10.1093/annonc/13.s1.112. 13(suppl 1)112-116, 2002. [DOI] [PubMed] [Google Scholar]