Abstract
Purpose
Physicians sometimes make management recommendations on the basis of early results from randomized controlled trials (RCTs) relating to reduced prostate-specific antigen (PSA) failure, yet whether this early end point is associated with all-cause mortality (ACM), particularly in men with competing risks, is unknown. Using a validated metric in men treated within the context of an RCT, we aimed to determine whether PSA failure is associated with the risk of ACM stratified by comorbidity score.
Patients and Methods
Between 1995 and 2001, 206 men with localized (T1b to 2b) intermediate- and high-risk prostate cancer (PC) were randomly assigned to receive radiation therapy or radiation therapy and 6 months of ADT. Cox regression analyses were performed to evaluate whether PSA failure modeled as a time-dependent covariate was associated with an increased risk of ACM among men with Adult Comorbidity Evaluation-27–defined no or minimal versus moderate-to-severe comorbidity adjusting for age, PC prognostic factors, and treatment.
Results
After a median follow-up of 16.62 years, 156 men (76%) died, 29 of whom (19%) died as a result of PC. PSA failure was associated with an increased ACM risk among men with no or minimal (adjusted hazard ratio, 1.59; 95% CI, 1.03 to 2.46; P = .04), but not moderate or severe comorbidity (adjusted hazard ratio, 1.75; 95% CI, 0.76 to 3.99; P = .19).
Conclusion
Recommending treatment on the basis of reduced PSA failure observed from early results of RCTs is unlikely to prolong survival in men with moderate-to-severe comorbidity but may prolong survival in men with no or minimal comorbidity, providing evidence to support discussing the early results with these men.
INTRODUCTION
The combination of external-beam radiation therapy (EBRT) and androgen deprivation therapy (ADT) is a standard of care for patients with intermediate- and high-risk prostate cancer (PC) on the basis of the results of several prospective randomized controlled trials (RCTs).1-9 Recently, a post–random assignment analysis stratified by comorbidity using a validated metric (Adult Comorbidity Evaluation-27 [ACE-27])10 found that the addition of 6 months of ADT to EBRT was associated with a survival benefit only in men with no or minimal comorbidity, whereas an association with shortened survival was observed among men with moderate-to-severe comorbidity.2
This raises the question as to whether time to prostate-specific antigen (PSA) failure, an end point that often occurs many years before metastasis and death from PC,11,12 is a clinically relevant end point only in men who have no or minimal comorbidity. This is a clinically relevant question because multiple contemporary RCTs, including the Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy trial,13 the GETUG-AFU 16 trial,14 and EORTC 22991,15 have recently demonstrated significant improvements in relapse-free survival predominately driven by PSA failure–free survival but no significant difference in overall survival (OS), when a brachytherapy boost (Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy) or 6 months of ADT (GETUG-AFU 16 and EORTC) is added to EBRT in the setting of newly diagnosed PC or postoperative PSA failure, respectively. Given that physicians sometimes apply these treatments broadly, despite no evidence to date of a survival benefit or a reduction in death due to PC, the question remains as to whether comorbidity should influence who should be offered and/or receive these treatments only on the basis of an relapse-free survival benefit, particularly given that both late grade 2 or higher GI and genitourinary toxicity was noted to be significantly higher in patients randomly assigned to dose escalation with a brachytherapy as compared with a radiation therapy (RT) boost,13 and significant adverse effects related to the ADT were observed in patients randomly assigned to RT and ADT in the GETUG-AFU 16 and EORTC 22991 studies.14,15
In principle, fewer competing risks for mortality should provide more opportunity for PSA failure to translate into an increased risk of all-cause mortality (ACM). Therefore, the purpose of this study was to use the results from a mature RCT2 (median follow-up, 16.62 years) to evaluate whether the time-dependent covariate of PSA failure was associated with the risk of ACM after adjusting for age, PC risk group, and randomly assigned treatment arm within comorbidity subgroups defined using the validated ACE-27 metric10 at random assignment.
PATIENTS AND METHODS
Patient Population and Treatment
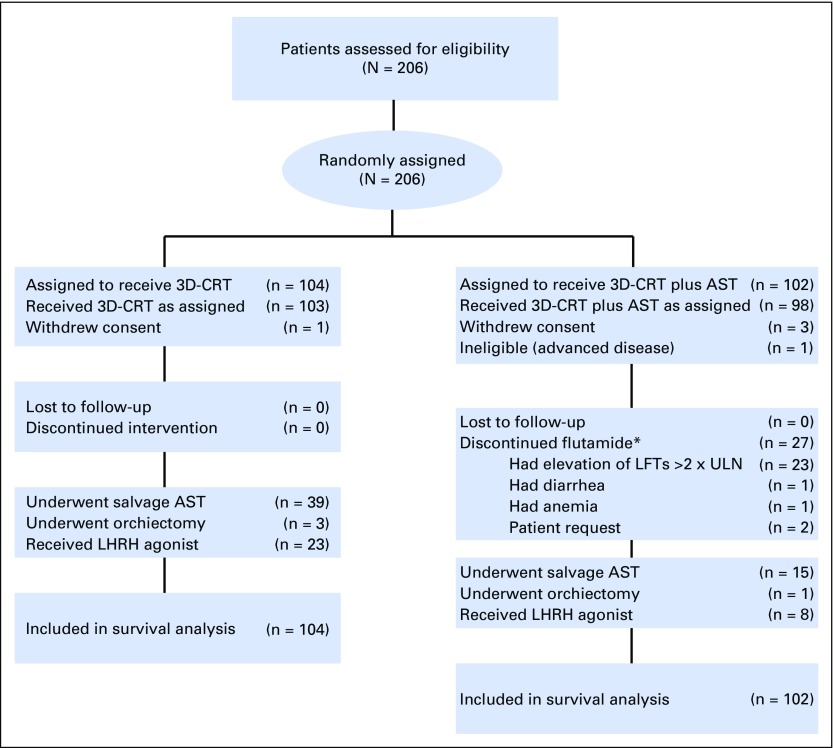
Between December 1, 1995, and April 15, 2001, 206 men with localized (1992 American Joint Commission on Cancer tumor category16 1b to 2b) intermediate- and high-risk PC17 were randomly assigned to receive RT or RT and 6 months of ADT (Fig 1; Data Supplement, online only). A complete listing of eligibility criteria, treatment specifications, and patient characteristics stratified by randomly assigned treatment arm has been published previously.1 This study was approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board.
Fig 1.
CONSORT diagram. AST indicates androgen suppression therapy; 3D-CRT, 3-dimensionalconformal radiation therapy; LFTs, liver function tests; LHRH, luteinizing hormone-releasing hormone; ULN, upper limit of normal. (*) Flutamide is the minor component of AST.
ACE-27 Metric
All medical records, including data on comorbidities, were available for all the patients enrolled in this study. Using detailed information on pre-existing comorbidities, a comorbidity score was assigned independently by the principal investigator (AVD) at the time of random assignment using the ACE-27, a 27-item comorbidity index validated in patients with cancer.10 The metric assigned a comorbidity value of no, minimal, moderate, or severe comorbidity. For the purpose of the current analysis, we grouped men with no or minimal versus moderate-to-severe comorbidity because these groupings have been shown to demonstrate clinical relevance on the ability of ADT to prolong or shorten the end point of OS within these two grouped comorbidity subgroups.1,2 In addition, the RTOG 0815 (NCT00936390) used the same grouping scheme as a pre–random assignment stratification factor.
Follow-Up and Determination of the Cause of Death
Follow-up started on the day of random assignment and concluded on the date the patient was last observed or on the date of death through February 21, 2015, whichever came first; no patient was lost to follow-up. The follow-up protocol has been described previously.1 Lifelong ADT administration for PSA failure was recommended when the PSA level reached 10 ng/mL. However, the treatment decision for salvage ADT was left to the discretion of the treating physician, and this decision was dependent on how quickly the PSA was rising and whether it exceeded 10 ng/mL. Specifically, the number of men with PSA failure who received salvage ADT was 43 (51%) of 85 men with no or minimal comorbidity and 11 (48%) of 23 men with moderate-to-severe comorbidity, which was not significantly different between the two groups (P = .82). The lack of difference results from the fact that the absolute PSA value and the PSA doubling time (DT), and not the comorbidity status, was used as a reason to initiate salvage ADT, in that the association of ADT use and the risk of fatal and nonfatal cardiovascular events2,18 was not appreciated during the period in which most men experienced PSA failure in our study. In addition, only approximately 50% of men who experienced PSA failure (nadir + 2 ng/mL) initiated salvage ADT because the remaining patients had slow increases in PSA with long DTs, and their PSA levels had not yet reached 10 ng/mL at the time of this analysis. Once a patient became refractory to first-line salvage ADT (a luteinizing hormone–releasing hormone agonist), this was followed by second-and third-line hormonal maneuvers, and then usually by cytotoxic chemotherapy. Because all the patients were treated by Harvard-affiliated medical oncology teams in Massachusetts, the practice patterns across patients were similar after PSA failure. The attending oncologist who observed the patient until death determined the cause of death. To record a death as being caused by PC, there had to be documented castration-resistant metastatic PC and evidence that the PSA level was increasing at the time of the last follow-up visit despite the use of second-line hormonal maneuvers and, in most cases, cytotoxic chemotherapy before death.
Statistical Methods
Comparison of the distribution of clinical factors at random assignment stratified by extent of comorbidity.
Descriptive statistics were used to calculate the distribution of clinical factors for the 206 men in the study cohort stratified by comorbidity subgroup. Across comorbidity subgroups, the continuous covariates of age and PSA DT were compared using a Wilcoxon rank sum test,19 whereas the categorical covariates of comorbidity, risk group, and randomly assigned treatment arm were compared using a Mantel-Haenszel χ2 metric.20 The distributions of the median time to PSA failure and the median follow-up were compared across comorbidity subgroups using a log-rank test.21 PSA DTs in months were calculated on the basis of 85 and 23 men who experienced PSA failure with no or minimal comorbidity and moderate or severe comorbidity, respectively. PSA failure times in years were calculated on the basis of 157 and 49 men with no or minimal comorbidity and moderate or severe comorbidity, respectively, using the Kaplan-Meier method21 and were compared using a log-rank test.21
Risk of ACM.
Cox regression univariable and multivariable analyses22 were performed to evaluate whether the occurrence of PSA failure was associated with an increased risk of ACM among men with no or minimal versus moderate or severe comorbidity when adjusting for age, highest Gleason score, tumor stage, PSA, and randomly assigned treatment arm. Given the known previously reported interaction between treatment with ADT and comorbidity,2 an interaction term between treatment with ADT and comorbidity was included in the model. In addition, to evaluate the effect of the time-dependent covariate of PSA failure on the risk of ACM by comorbidity subgroup, a second interaction term between comorbidity and PSA failure (t) was included in the model. Time zero was defined as the date of random assignment. Unadjusted and adjusted hazard ratios (HRs) with 95% CIs and associated P values were reported for each clinical covariate.
Estimates of ACM stratified by PSA failure status and comorbidity subgroup.
For the purpose of illustration, estimates of ACM after random assignment stratified by men who experienced PSA failure or not were calculated using the extended Kaplan-Meier methodology with time-dependent covariates23 after adjusting for age24 and were displayed for each comorbidity subgroup. These estimates were compared using a Cox time-dependent covariate P value.22 A two-sided P value < .025 was considered statistically significant after adjustment using a Bonferroni correction (n = 2 comorbidity subgroups). Fifteen-year point estimates of ACM with associated 95% CIs were calculated and reported for men with no or minimal and moderate-to-severe comorbidity, stratified by whether PSA failure was or was not observed. SAS (version 9.4 , SAS Institute, Cary, NC) was used for all calculations with the exception of the age-adjusted ACM estimates and 95% CIs with time-dependent covariates, for which R (version 3.2.3, Auckland, New Zealand) was used.
RESULTS
Comparison of the Distribution of Clinical Factors at Random Assignment Stratified by Extent of Comorbidity
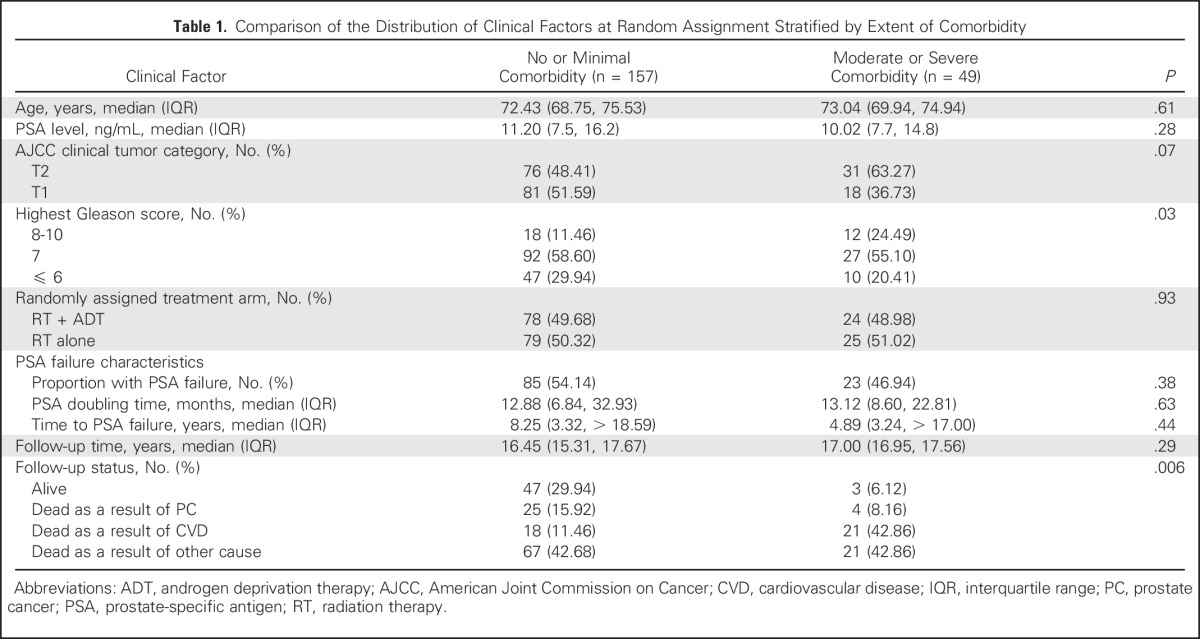
As illustrated in Table 1, there was no significant difference in the distribution of clinical factors at random assignment stratified by comorbidity subgroup for the median age (P = .61), median PSA level (P = .28), American Joint Commission on Cancer tumor (T) stage category (P = .07), randomly assigned treatment arm (P = .93), median follow-up time (P = .29), or the occurrence of (P = .38), time to (P = .44), and DT of (P = .63) PSA failure. There were a greater proportion of men with Gleason 8 to 10 PC among men with moderate-to-severe comorbidity (24.49%) versus men with no-to-minimal comorbidity (11.46%; P = .03). Men with no or minimal, as compared with moderate-to-severe, comorbidity were significantly less likely (P = .006) to have died overall (70.1% v 93.9%) or to have died as a result of cardiovascular disease (11.5% v 42.9%), but were more likely to have died as a result of PC (15.9% v 8.2%).
Table 1.
Comparison of the Distribution of Clinical Factors at Random Assignment Stratified by Extent of Comorbidity
Risk of ACM
After a median follow-up of 16.62 years, 156 men (76%) died, of whom 29 (19%) died as a result of PC. Among men with moderate or severe comorbidity, 46 of 49 (94%) died, compared with 110 of 157 men (70%) with no or minimal comorbidity. Among men with no or minimal comorbidity at random assignment, the risk of ACM was significantly increased in men who were observed to have PSA failure compared with those who were not (HR, 1.59; 95% CI, 1.03 to 2.46; P = .04). However, there was no significant increase in the risk of ACM in men with moderate-to-severe comorbidity irrespective of PSA failure status (HR, 1.75; 95% CI, 0.76 to 3.99; P = .19).
Estimates of ACM Stratified by PSA Failure Status and Comorbidity Subgroup
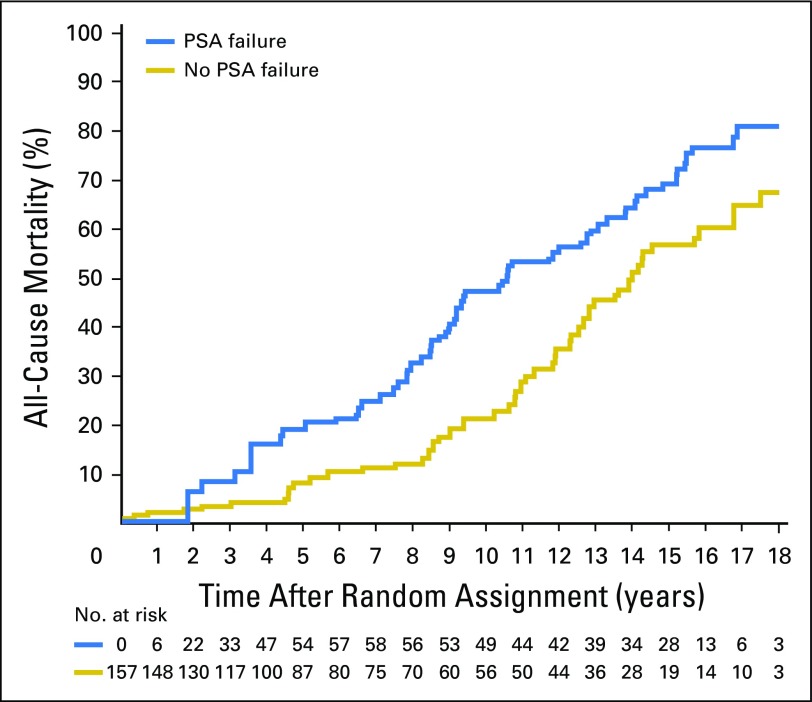
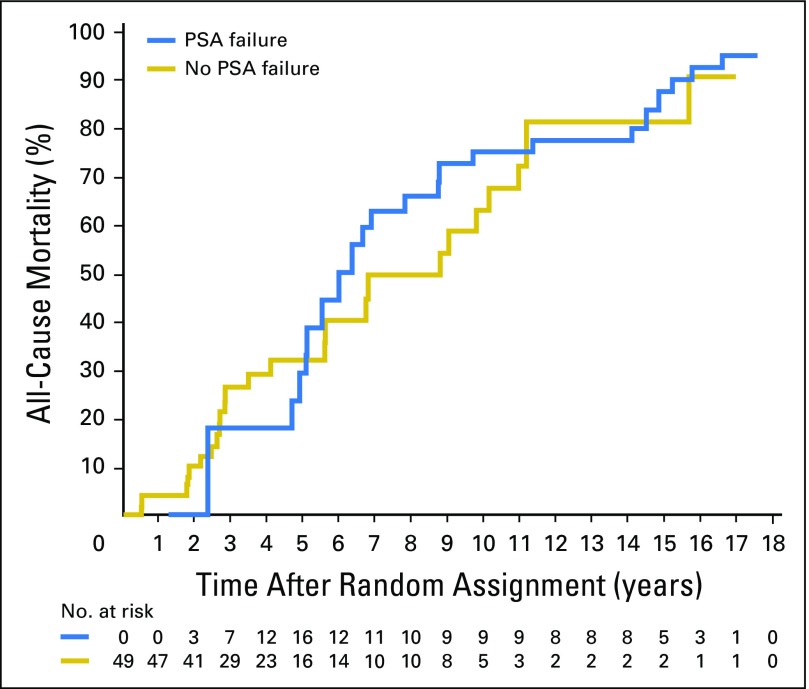
As illustrated in Figure 2, estimates of ACM after adjusting for age were significantly higher (P = .008) among men with no or minimal comorbidity in whom PSA failure was observed, compared with those in whom PAS failure was not observed, whereas no significant difference in ACM estimates after adjusting for age were observed across PSA failure status in men with moderate or severe comorbidity (P = .51; Fig 3). The age-adjusted 15-year estimates of ACM in men with no or minimal comorbidity were 69.1% (95% CI, 59.8 to 78.0) versus 56.6% (95% CI, 45.6 to 68.1) among men in whom PSA failure was observed versus those in whom PSA failure was not observed. These respective estimates were 87.7% (95% CI ,71.0 to 97.1) and 81.4% (95% CI, 61.6 to 94.8) for men with moderate-to-severe comorbidity.
Fig 2.
Estimates of all-cause mortality adjusting for age in the 157 men with no or minimal comorbidity stratified by whether or not PSA failure was observed. Cox P value = .008. PSA, prostate-specific antigen.
Fig 3.
Estimates of all-cause mortality adjusting for age in the 49 men with moderate or severe comorbidity stratified by whether or not PSA failure was observed. Cox P value = .51.
DISCUSSION
We found that although PSA failure was more common in men with no or minimal comorbidity compared with men with moderate-to-severe comorbidity, the occurrence of PSA failure was associated with a significantly increased risk of ACM only in men with no or minimal, but not moderate-to-severe, comorbidity. However, the distributions of the known prognostic factors of time to PSA failure and PSA DT were not significantly different among men with no or minimal versus moderate-to-severe comorbidity, which would lead one to expect that the occurrence of PSA failure in both subgroups should relate similarly to the risk of ACM. The discordance in the association between the occurrence of PSA failure and the subsequent risk of ACM in the adjusted model despite similar prognostic PSA failure profiles provides evidence to support the possibility that competing risks, and not PC death, are the reason for a lack of translation of PSA failure into an increased risk of ACM in men with moderate-to-severe comorbidity.
The clinical significance of these observations is that they provide evidence to support a discussion of treatment approaches that have been shown in the early results of RCTs13-15,25 to provide a PSA failure–free survival benefit in men who have unfavorable-risk PC and no or minimal comorbidity. Conversely, such a discussion may not be appropriate in men with moderate-to-severe comorbidity because of the low likelihood that a PSA failure–free survival benefit will translate into a reduction in the risk of death from ACM in these men, given the extent of their comorbidity and resulting competing risk(s).
Several points require further discussion. First, it is important to note that the evidence provided by the results of this study suggests that PSA failure in men with no or minimal comorbidity is a prognostic factor and not a surrogate for PC death.26 Specifically, having a negative prognostic factor (in this case, PSA failure) does not necessarily dictate that a patient will subsequently die as a result of PC. To validate that PSA failure is a surrogate for PC death in men with no or minimal comorbidity would require a randomized trial in which men are stratified by comorbidity status before random assignment and in whom results documented a PC-specific survival benefit in the experimental arm. In such a trial, Prentice criteria for surrogacy26 could be tested formally. Fortunately, such a trial has been performed, namely, RTOG 0815, in which men were stratified before random assignment using the ACE-27 comorbidity metric (no or minimal v moderate or severe comorbidity as used in this study) and then randomly assigned to receive high-dose RT (79.2 Gy EBRT or 45 Gy EBRT plus a low-dose rate or high-dose rate brachytherapy boost) with or without 6 months of ADT, in an fashion analogous that of to our study. If that study shows both a PC-specific survival and an OS benefit in the subgroup of men with no or minimal comorbidity with the addition of 6 months of ADT to high-dose RT, then formal testing of PSA failure, defined as nadir plus 2 ng/mL as in our study, as a surrogate for both PC-specific mortality and ACM could be performed. Until then, practice-changing measures should only be taken on the basis of OS results, because our study findings raise a hypothesis about the prognostic significance only, and not the surrogacy, of PSA failure for OS in men with various degrees of comorbidity who presented with localized unfavorable-risk PC and were treated with conventional-dose RT and 6 months of ADT.
Nevertheless, it is important to note that the results of our study provide evidence to support the possibility that PSA failure is not a prognostic factor in men with moderate-to-severe comorbidity and as a result cannot be a surrogate for PC death, given that one of the requirements of a surrogate is that the surrogate be a prognostic factor.26 Therefore, applying early results of RCTs that observe a reduction in PSA failure in men with moderate-to-severe comorbidity would be unlikely to translate into a reduction in PC death.
Second, the median age of the patients in our study with no or minimal comorbidity was 72.43 years, and despite this advanced age at random assignment, PSA failure remained prognostic. Therefore, younger men with no or minimal comorbidity who have a longer remaining life expectancy when compared with older men with matched comorbidity profiles27 would be expected to have a higher likelihood that PSA failure would translate into death from PC. Third, our observation of a significant association between PSA failure and ACM in men with no or minimal comorbidity may not be applicable in treatment settings outside the one used in this study, which was of EBRT and 6 months of ADT versus EBRT alone. Therefore, it remains to be studied as to whether the prognostic significance of PSA failure, as observed in our study, is maintained in the postoperative rising PSA setting as well as in the de novo setting, where prescribed treatments are different from those delivered in our study. Finally, the impact of salvage ADT on the risk of ACM in the two comorbidity subgroups was also investigated through the use of an interaction term in our multivariable model between comorbidity and salvage ADT use (Table 2), and this was not found to be significant (adjusted HR, 0.63; 95% CI. 0.26 to 1.53; P = .30). Therefore, it does not seem that systemic therapy after PSA failure affected ACM differently among men with no or minimal versus moderate-to-severe comorbidity. This may be explained by the fact that only 23 men with moderate-to-severe comorbidity developed a PSA failure (compared with 85 men with no or minimal comorbidity), and of these 23 men with moderate-to-severe comorbidity, eight had received ADT as part of their initial treatment (RT + ADT), and of the remaining 15 (who did not receive ADT as part of their initial definitive treatment), only eight received salvage ADT. Thus, if an interaction between ADT and comorbidity was to occur, it already had the opportunity to occur at the time of initial treatment in all but 15 men, only eight of whom received salvage ADT by the date of last follow-up of this analysis (Fig 1).
Table 2.
Unadjusted and Adjusted All-Cause Mortality Hazard Ratios for Each Clinical Factor
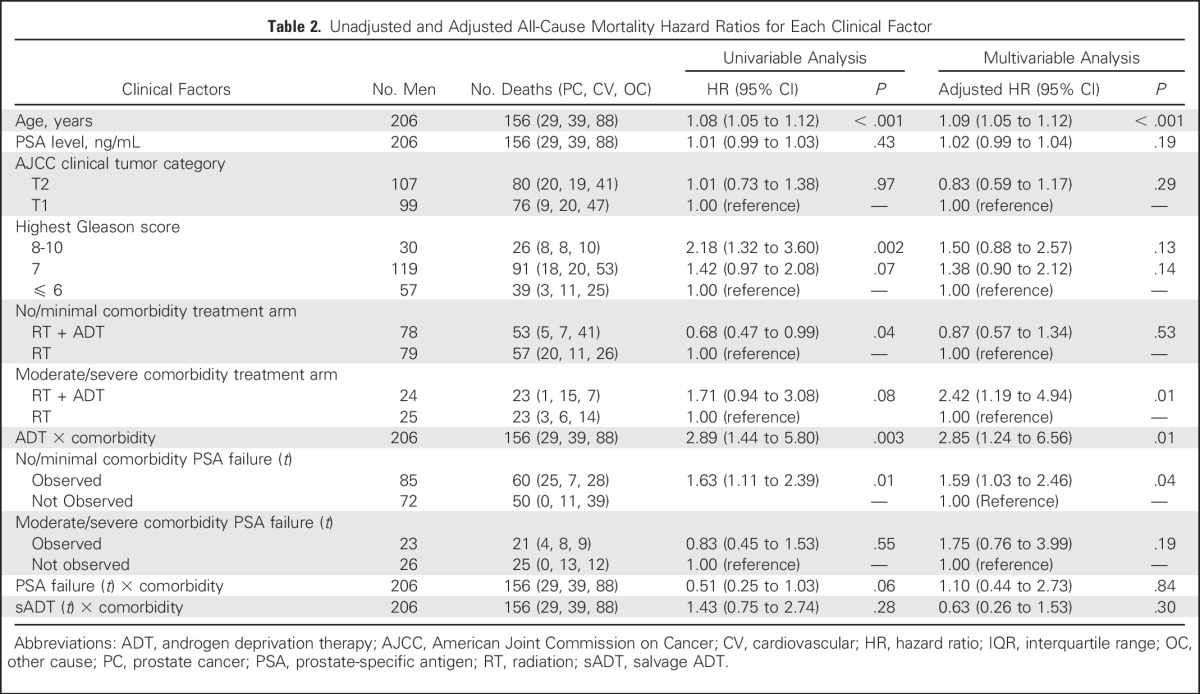
Despite these potential limitations, our data provide evidence to support a significant association between PSA failure and the risk of ACM in men with no or minimal but not moderate-to-severe comorbidity, highlighting the importance of competing risks when discussing early results of RCTs with men diagnosed with unfavorable-risk PC. Therefore, recommending treatment on the basis of the reduced PSA failure observed in early results of RCTs is unlikely to prolong survival in men with moderate-to-severe comorbidity but may prolong survival in men with no or minimal comorbidity, providing evidence to support discussing the early results with these men.
ACKNOWLEDGMENT
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Clinical trial information: NCT00116220.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Anthony V. D'Amico
Administrative support: Anthony V. D'Amico
Collection and assembly of data: Ming-Hui Chen, Andrew Renshaw, Marian Loffredo, Anthony V. D'Amico
Data analysis and interpretation: Nicholas J. Giacalone, Jing Wu, Ming-Hui Chen, Philip W. Kantoff, Anthony V. D'Amico
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prostate-Specific Antigen Failure and Risk of Death Within Comorbidity Subgroups Among Men With Unfavorable-Risk Prostate Cancer Treated in a Randomized Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Nicholas J. Giacalone
No relationship to disclose
Jing Wu
No relationship to disclose
Ming-Hui Chen
No relationship to disclose
Andrew Renshaw
No relationship to disclose
Marian Loffredo
No relationship to disclose
Philip W. Kantoff
Stock or Other Ownership: Bellicum Pharmaceuticals, Blend Therapeutics, Metamark Genetics, Placon
Consulting or Advisory Role: Bavarian Nordic, Janssen Pharmaceuticals, Millennium Pharmaceuticals, MorphoSys, Pfizer, Astellas Pharma, Bellicum Pharmaceuticals, BIND Biosciences, Blend Therapeutics, Endocyte, Metamark Genetics, Medivation, Merck, MTG Therapeutics, OncoCellMDX, Oncogenex, Sotio, Sanofi, Tokai Pharmaceuticals, Bayer AG, Genentech (a member of the Roche Group), Cristal Therapeutics, Ipsen, Omnitura, MorphoSys, GTx
Research Funding: Medivation (Inst), Sanofi (Inst), Oncogenex (Inst), Aragon Pharmaceuticals (Inst), Amgen (Inst), Astellas Pharma (Inst), Bayer AG (Inst), Bavarian Nordic (Inst), Dendreon (Inst), Exelixis (Inst), Janssen Pharmaceuticals (Inst)
Expert Testimony: Sanofi, Janssen Pharmaceuticals
Travel, Accommodations, Expenses: Sanofi, Janssen Pharmaceuticals, BIND Biosciences, Bavarian Nordic, Millennium Pharmaceuticals
Anthony V. D'Amico
No relationship to disclose
REFERENCES
- 1.D’Amico AV, Chen M-H, Renshaw AA, et al. Androgen suppression and radiation vs radiation alone for prostate cancer: A randomized trial. JAMA. 2008;299:289–295. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 2.D’Amico AV, Chen MH, Renshaw A, et al. Long-term follow-up of a randomized trial of radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA. 2015;314:1291–1293. doi: 10.1001/jama.2015.8577. [DOI] [PubMed] [Google Scholar]
- 3.Denham JW, Steigler A, Lamb DS, et al. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol. 2011;12:451–459. doi: 10.1016/S1470-2045(11)70063-8. [DOI] [PubMed] [Google Scholar]
- 4.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107–118. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 5.Pisansky TM, Hunt D, Gomella LG, et al. Duration of androgen suppression before radiotherapy for localized prostate cancer: Radiation therapy oncology group randomized clinical trial 9910. J Clin Oncol. 2015;33:332–339. doi: 10.1200/JCO.2014.58.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 7.Zapatero A, Martin de Vidales C, Arellano R, et al. Updated results of bladder-sparing trimodality approach for invasive bladder cancer. Urol Oncol. 2010;28:368–374. doi: 10.1016/j.urolonc.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 8.Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: A phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 9. Duration of androgen deprivation therapy in high-risk prostate cancer: A randomized trial. J Clin Oncol 31, 2013 (suppl; abstr LBA4510). http://meetinglibrary.asco.org/content/114784-132.
- 10.Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 11.Freedland SJ, Humphreys EB, Mangold LA, et al. Death in patients with recurrent prostate cancer after radical prostatectomy: Prostate-specific antigen doubling time subgroups and their associated contributions to all-cause mortality. J Clin Oncol. 2007;25:1765–1771. doi: 10.1200/JCO.2006.08.0572. [DOI] [PubMed] [Google Scholar]
- 12.Zhou P, Chen M-H, McLeod D, et al. Predictors of prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Clin Oncol. 2005;23:6992–6998. doi: 10.1200/JCO.2005.01.2906. [DOI] [PubMed] [Google Scholar]
- 13. Morris WJ, Tyldesley S, Pai HH, et al: ASCENDE-RT*: A multicenter, randomized trial of dose-escalated external beam radiation therapy (EBRT-B) versus low-dose-rate brachytherapy (LDR-B) for men with unfavorable-risk localized prostate cancer. J Clin Oncol 33, 2015 (suppl 7; abstr 3). http://meetinglibrary.asco.org/content/141698-159.
- 14. Carrie C, Hasbini A, De Laroche G, et al: Interest of short hormonotherapy (HT) associated with radiotherapy (RT) as salvage treatment for biological relapse (BR) after radical prostatectomy (RP): Results of the GETUG-AFU 16 phase III randomized trial– NCT00423475. ASCO Meet Abstr 33:5006, 2015. [Google Scholar]
- 15.Bolla M, Maingon P, Carrie C, et al. Short androgen suppression and radiation dose escalation for intermediate- and high-risk localized prostate cancer: Results of EORTC trial 22991. J Clin Oncol. 2016;34:1748–1756. doi: 10.1200/JCO.2015.64.8055. [DOI] [PubMed] [Google Scholar]
- 16.Beahrs OH, Henson DE, Hutter RVP, et al. American Joint Committee on Cancer Manual for Staging of Cancer Fourth. Philadelphia: J. B. Lippincott Company; 1992. [Google Scholar]
- 17.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 18.Roach M, III, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 19.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 20.Hollander M, Wolfe DA, Chicken E. Nonparametric Statistical Methods. John Wiley & Sons, Hoboken, NJ; 2013. [Google Scholar]
- 21.Agresti A. Categorical Data Analysis. John Wiley & Sons, Hoboken, NJ; 2013. [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 23.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. Springer Science & Business Media; New York: 2003. [Google Scholar]
- 24.Snapinn SM, Jiang Q, Iglewicz B. Illustrating the impact of a time-varying covariate with an extended Kaplan-Meier estimator. Am Stat. 2005;59:301–307. [Google Scholar]
- 25.Cupples LA, Gagnon DR, Ramaswamy R, et al. Age-adjusted survival curves with application in the Framingham Study. Stat Med. 1995;14:1731–1744. doi: 10.1002/sim.4780141603. [DOI] [PubMed] [Google Scholar]
- 26. Michalski JM, Moughan J, Purdy J, et al: A randomized trial of 79.2Gy versus 70.2Gy radiation therapy (RT) for localized prostate cancer. J Clin Oncol 33, 2015 (suppl 7; abstr 4) [Google Scholar]
- 27.Prentice RL. Surrogate endpoints in clinical trials: Definition and operational criteria. Stat Med. 1989;8:431–440. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]