Abstract
Purpose
To determine the effects of carfilzomib, lenalidomide, and dexamethasone (KRd) versus lenalidomide and dexamethasone (Rd) on health-related quality of life (HR-QoL) in the Carfilzomib, Lenalidomide, and Dexamethasone Versus Lenalidomide and Dexamethasone for the Treatment of Patients With Relapsed Multiple Myeloma (ASPIRE) trial.
Methods
Patients with relapsed multiple myeloma were randomly assigned to receive KRd or Rd. The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 and myeloma-specific module were administered at baseline; day 1 of cycles 3, 6, 12, and 18; and after treatment. The Global Health Status/Quality of Life (GHS/QoL) scale and seven subscales (fatigue, nausea and vomiting, pain, physical functioning, role functioning, disease symptoms, and adverse effects of treatment) were compared between groups using a mixed model for repeated measures. The percentages of responders with ≥ 5- or 15-point GHS/QoL improvement at each cycle were compared between groups.
Results
Baseline questionnaire compliance was excellent (94.1% of randomly assigned patients). KRd patients had higher GHS/QoL scores versus Rd patients over 18 treatment cycles (two-sided P < .001). The minimal important difference was met at cycle 12 (5.6 points) and approached at cycle 18 (4.8 points). There was no difference between groups for the other prespecified subscales from ASPIRE. A higher proportion of KRd patients met the GHS/QoL responder definition (≥ 5-point improvement) with statistical differences at cycle 12 (KRd v Rd patients, 25.5% v 17.4%, respectively) and 18 (KRd v Rd patients, 24.2% v 12.9%, respectively).
Conclusion
KRd improves GHS/QoL without negatively affecting patient-reported symptoms when compared with Rd. These data further support the benefit of KRd in patients with relapsed multiple myeloma.
INTRODUCTION
Patients with multiple myeloma (MM) typically report significant impairment in health-related quality of life (HR-QoL), including reduced physical function, fatigue, and pain.1 Improvements in the efficacy of MM treatment, particularly with the approval of newer agents such as thalidomide, lenalidomide, pomalidomide, bortezomib, carfilzomib, ixazomib, panobinostat, daratumumab, and elotuzumab, which each have unique risk-benefit profiles, have made HR-QoL an increasingly important end point in clinical trials and factor in treatment decisions.2
Carfilzomib is an epoxyketone proteasome inhibitor that binds selectively and irreversibly to the constitutive proteasome and immunoproteasome. In the randomized phase III Carfilzomib, Lenalidomide, and Dexamethasone Versus Lenalidomide and Dexamethasone for the Treatment of Patients With Relapsed Multiple Myeloma (ASPIRE) trial (clinical trial information: NCT01080391), patients with relapsed MM treated with carfilzomib, lenalidomide, and dexamethasone (KRd) reported significantly longer progression-free survival (PFS) compared with patients treated with lenalidomide and dexamethasone (Rd; two-sided P < .001; median PFS, 26.3 v 17.6 months, respectively).3 Despite a longer median treatment duration with KRd versus Rd (88.0 v 57.0 weeks, respectively), fewer patients treated with KRd than Rd discontinued treatment as a result of adverse events (15.3% v 17.7%, respectively).3 HR-QoL assessed using the Global Health Status/Quality of Life (GHS/QoL) scale of the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire C30 (QLQ-C30) was a predefined secondary end point in ASPIRE, and improvements were seen with KRd versus Rd over 18 cycles of treatment (two-sided P < .001).3
Here, we present full HR-QoL results from cross-sectional and longitudinal analyses of secondary and exploratory HR-QoL end points. Analyses of patient-reported outcomes (PROs) were prespecified in a separate statistical analysis plan. The primary PRO hypothesis was superiority of KRd over Rd for the GHS/QoL scale (secondary end point). Seven further subscales were prespecified from the QLQ-C30 (fatigue, nausea/vomiting, pain, physical functioning, role functioning) and EORTC myeloma-specific QLQ-MY20 module (disease symptoms, adverse effects of treatment).
METHODS
Study Design
ASPIRE is a prospective, multicenter, open-label, randomized, phase III trial. Full trial details have been published.3
Briefly, patients with relapsed MM (one to three prior regimens) were randomly assigned (1:1) to receive KRd or Rd in 28-day cycles until withdrawal of consent, disease progression, or occurrence of unacceptable toxicity. Random assignment was stratified by β2-microglobulin level (< 2.5 v ≥ 2.5 mg/L), previous bortezomib therapy (no v yes), and previous lenalidomide therapy (no v yes). Carfilzomib (10-minute intravenous infusion) was administered on days 1, 2, 8, 9, 15, and 16 (starting dose, 20 mg/m2 on days 1 and 2 of cycle 1; target dose, 27 mg/m2 thereafter) during cycles 1 through 12 and on days 1, 2, 15, and 16 during cycles 13 through 18. Lenalidomide (25 mg; oral) was administered on days 1 through 21. Dexamethasone (40 mg; oral or intravenous) was administered on days 1, 8, 15, and 22. Patients in both groups received only Rd beyond cycle 18 until disease progression.
Institutional review boards of all participating institutions approved the study protocol. All patients provided written informed consent.
HR-QoL Assessments and End Points
PROs were assessed with two EORTC instruments—the QLQ-C304 and the 20-item MM-specific module QLQ-MY20.5 These instruments are commonly used and are valid and reliable in patients with MM.2,5-12 The instruments were scored using guidelines recommended by the EORTC.13 The QLQ-C30 contains an overall GHS/QoL domain, five functional domains (physical, emotional, cognitive, social, and role functioning), and nine symptom domains (fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties). Each domain is scored from zero to 100; higher scores indicate better QoL, better functioning, or more severe symptoms, respectively. The QLQ-MY20 contains two functional domains (future perspective and body image) and two symptom domains (disease symptoms and adverse effects of treatment); higher scores indicate better functioning and more symptoms, respectively. Patients completed the questionnaires (paper) at baseline (day 1 of cycle 1); on day 1 of cycles 3, 6, 12, and 18; and at the posttreatment visit (30 days after last treatment administration). To focus on the comparison of HR-QoL during the randomly assigned treatments, questionnaires completed at the posttreatment visit were excluded from the main analyses.
Statistical Analyses
Compliance was calculated using the proportion of randomly assigned patients (intent-to-treat population) and the proportion of patients expected to have an assessment (alive and on study treatment). Logistic regression models were used to explore relationships between the probability of missing data and baseline scores (baseline models) and between the probability of missing data and previous scores (previous value models). HR-QoL trajectories grouped by timing of last assessment were plotted by treatment group to assess the relationship of scores to dropout time.
PRO subscales were compared between treatment groups using a restricted maximum likelihood–based mixed model for repeated measures (MMRM) under the assumption of missing at random.14 The model included the fixed, categorical effects of treatment, visit (coded using integers representing cycle number), treatment by visit, and random assignment stratification factors and the fixed, continuous covariates of baseline score and baseline score by visit. The inferential test associated with the secondary efficacy end point of GHS/QoL scale was assessed against an overall one-sided significance level of P = .025 (two-sided significance level of P = .05) under fixed sequence hierarchical testing procedure to adjust for multiplicity.15 Because the result of the interim overall survival analysis did not cross the monitoring boundary, the GHS/QoL end point was not to be tested; thus, reported P values for the GHS/QoL secondary end point are descriptive and unadjusted for multiplicity. A two-sided 5% level was used to compare P values for the exploratory subscales descriptively. Least squares mean differences for each visit are presented with P values. Two sensitivity analyses for GHS/QoL were planned to test the robustness of the primary analysis.14,16 The first model included a simple dropout variable (no v yes) to assess whether treatment effect was consistent with the primary analysis.17 The second pattern-mixture model produced overall averaged estimates across submodels for each missing data pattern. Five missing patterns were defined for this analysis, with patients grouped by the timing of their last assessment (complete case, dropout at cycle 18, dropout at cycle 12, dropout at cycle 6, and dropout at cycle 3).18,19
The minimally important difference (MID) represents the smallest group-level difference in a PRO score considered clinically meaningful.20 A MID of 5 points for between-group differences on the QLQ-C30 GHS/QoL was prespecified.6,21,22 No published MIDs are available for the QLQ-MY20. Therefore, the standard error of measurement (SEM; calculated using the scale reliability, Cronbach’s α) from the baseline questionnaires was used to estimate the MID for the multi-item QLQ-MY20 subscales (ie, disease symptoms, future perspective, and adverse effects of treatment).23
To explore individual patient changes, improvement was defined as a ≥ 5-point change from baseline. A 15-point change was used as a sensitivity analysis to account for a more stringent threshold on the GHS/QoL scale (equivalent to commonly used 10-point change).24 The proportions of patients achieving a response on the GHS/QoL were compared between treatment groups at each time point using the Cochran-Mantel-Haenszel test with modified ridit scores to stratify by random assignment stratification factors. The odds ratio and 95% CI were estimated. Patients with missing data were considered nonresponders, and a sensitivity analysis was conducted excluding missing data. Time to deterioration was analyzed with the same response thresholds, using Cox proportional hazards models to account for the random assignment stratification factors for all prespecified subscales.
Changes from baseline within each treatment group and for patients achieving a partial response (PR) or better were explored post hoc using least squares mean estimates, 95% CIs, and P values from MMRM models. A two-sided 5% significance level was used. Clinical interpretation was based on comparing 95% CIs with the guidelines by Cocks et al25 for interpreting change scores from the EORTC QLQ-C30.
RESULTS
Patient Population
From July 2010 to March 2012, 792 patients were enrolled in North America, Europe, and the Middle East.3 Patients were randomly assigned to KRd (n = 396) or Rd (n = 396). Patient demographics and disease-related characteristics were balanced across treatment groups (Table 1).3 Median patient age was 64 years (range, 31 to 91 years).
Table 1.
Baseline Patient Characteristics and EORTC QLQ-C30 and QLQ-MY20 Scores
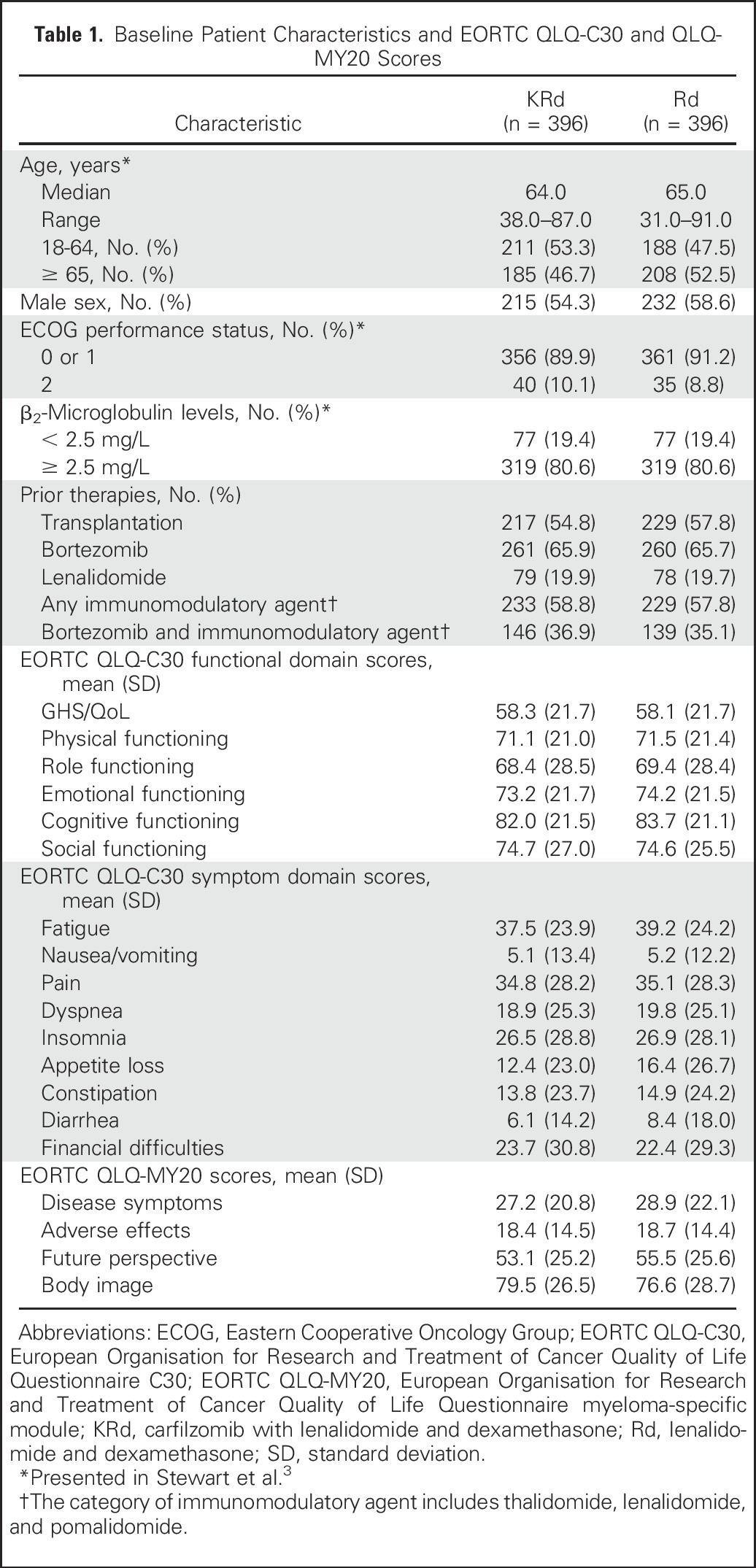
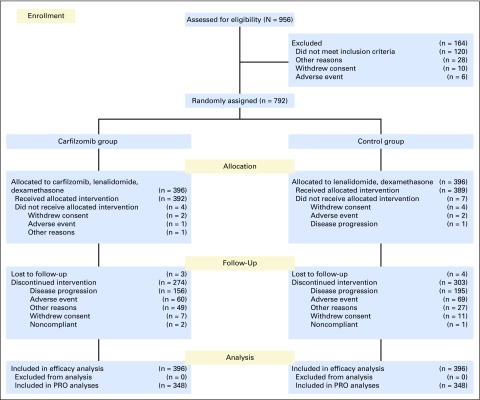
Among the 792 patients in the intent-to-treat population (Fig 1), 713 completed one or more postbaseline PRO assessments and were included in these analyses (KRd, n = 365; Rd, n = 348). Baseline QLQ-C30 and QLQ-MY20 subscale scores were similar between treatment groups (Table 1).
Fig 1.
CONSORT diagram.
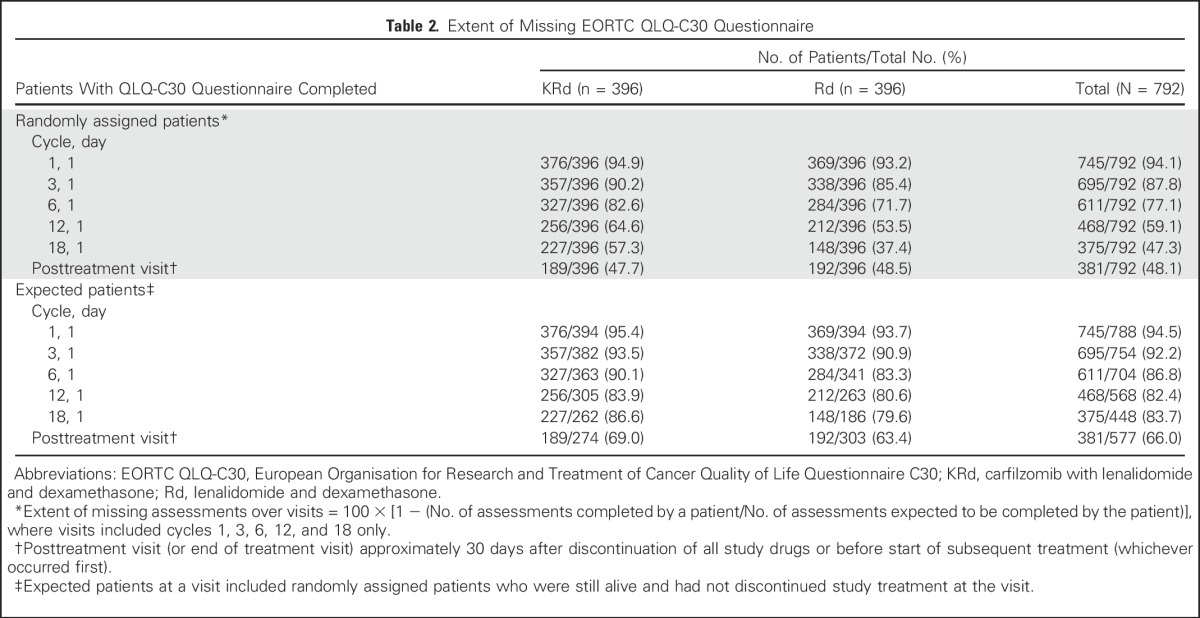
Compliance
Compliance rates were high with 94.1% of all randomly assigned patients completing the QLQ-C30 questionnaire at baseline and with similar compliance across treatment groups (Table 2). Among the randomly assigned study population, more KRd than Rd patients completed the QLQ-C30 questionnaire at each cycle as expected because Rd patients discontinued treatment sooner than KRd patients (median treatment duration, 88.0 weeks for KRd v 57.0 weeks for Rd).3 As a proportion of those expected to have an assessment (alive and on study treatment), compliance was good, with 86.6% and 79.6% of KRd and Rd patients, respectively, returning a questionnaire at cycle 18. Similar compliance rates were observed for the GHS/QoL and other subscales (data not shown).
Table 2.
Extent of Missing EORTC QLQ-C30 Questionnaire
Missing Data
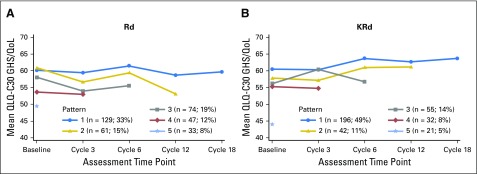
Logistic regression models show that missing data on the GHS/QoL subscale were related to poorer baseline scores and, at later cycles, poorer scores at the previous cycle. Graphs of GHS/QoL over time grouped by the timing of last assessment show slight differences in patterns of dropout across the treatment groups (Appendix Fig A1, online only). Overall, patients with lower GHS/QoL scores at baseline dropped out earlier. These observations suggest the data may not be missing at random, as expected in this setting.
Treatment Group Differences
QLQ-C30 GHS/QoL scores.
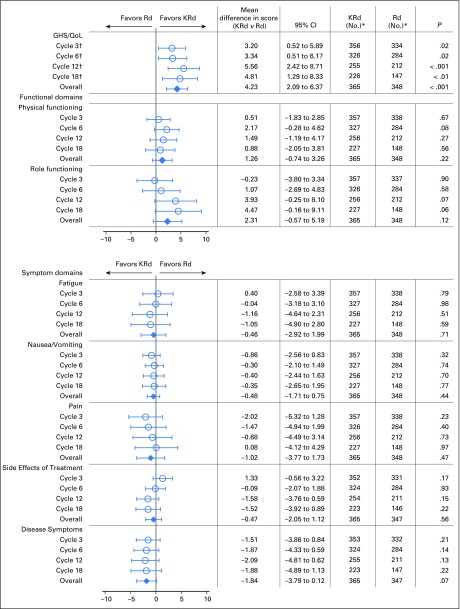
GHS/QoL least squares mean scores and mean treatment differences are shown in Figure 2. As previously reported, using the MMRM model, KRd improved GHS/QoL scores compared with Rd over 18 cycles of treatment (one-sided P < .001; two-sided P < .001).3 The MID was met at cycle 12 (5.6 points) and approached at cycle 18 (4.8 points).3 The overall treatment difference point estimate was calculated as 4.2 (95% CI, 2.1 to 6.4).
Fig 2.
Adjusted least squares mean treatment difference in European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 and myeloma-specific module scores. Scores have been adjusted for baseline score, baseline score by visit interaction, and the random assignment stratification factors (baseline β2-microglobulin levels, prior bortezomib, and prior lenalidomide). For functioning scales, a positive difference is in favor of carfilzomib, lenalidomide, and dexamethasone (KRd). For symptom scales, a negative difference is in favor of KRd. Horizontal bars indicate 95% CIs. (*) Number of patients with data at that time point. (†) Presented in Stewart et al.3 GHS/QoL, Global Health Status/Quality of Life domain; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone.
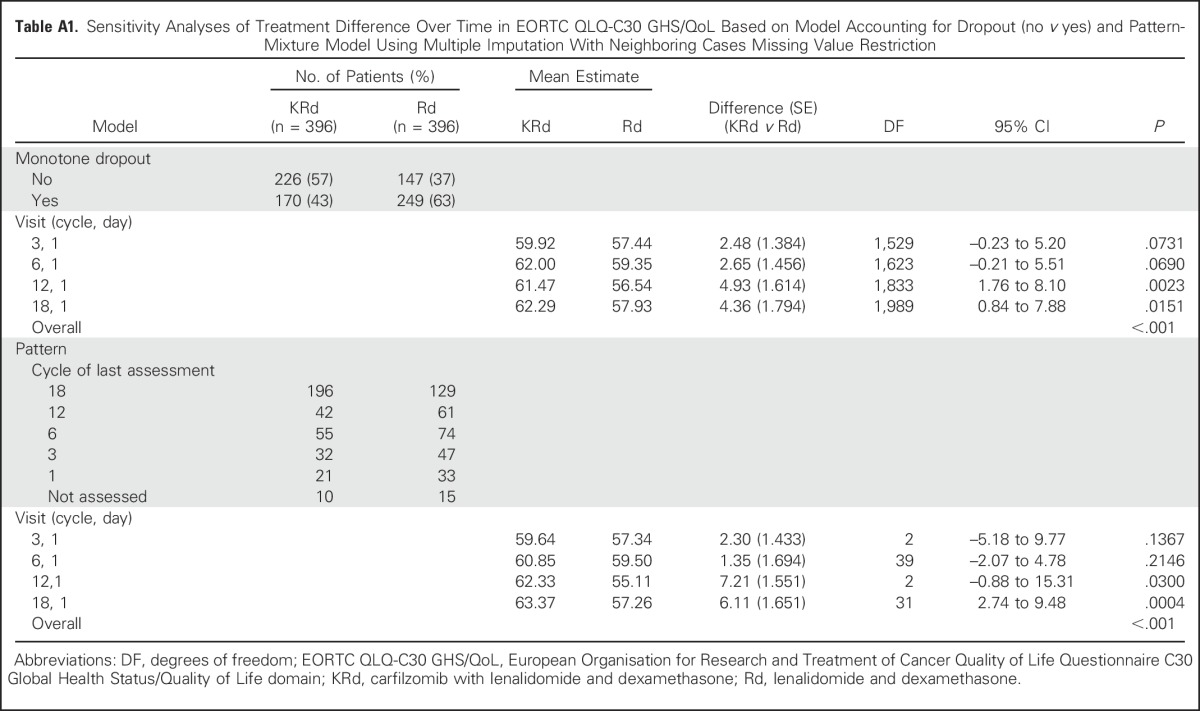
Results from the two sensitivity analyses confirmed findings from the MMRM analysis (Appendix, online only). The model accounting for dropout showed similar results as the primary analysis, in that KRd improved global health status based on the GHS/QoL compared with Rd over 18 cycles of treatment (one-sided P < .001). The pattern-mixture model further supported the primary analysis with an average treatment effect across the missing data patterns having one-sided P < .001. Full results from these analyses are listed in Appendix Table A1 (online only). The results of these sensitivity analyses demonstrate the robustness of the primary analysis result.
A priori subscales of the QLQ-C30 and QLQ-MY20.
Using MMRM models, there was no difference between treatment groups on the other prespecified QLQ-C30 and QLQ-MY20 questionnaire subscales (Fig 2). Point estimates tended to favor KRd but did not reach clinical or statistical significance.
QLQ-MY20 MID.
The SEM was 9 points for disease symptoms, 11 points for future perspectives, and 7 points for adverse effects of treatment. All subscale Cronbach’s α values were greater than .7, indicating good internal consistency of the QLQ-MY20 scales for this study population. These SEMs were similar to those reported in other studies.26
HR-QoL responder analysis.
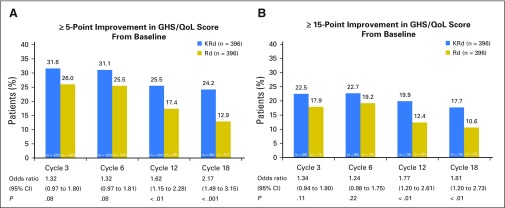
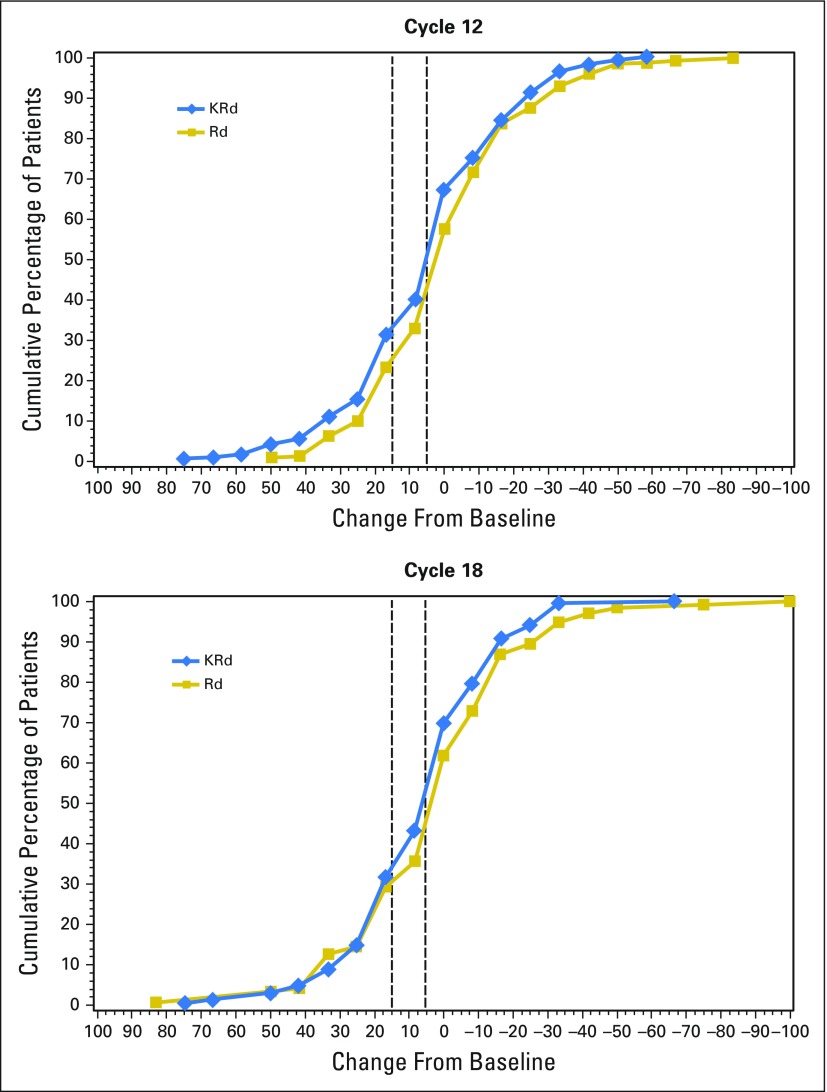
KRd patients had higher response rates than Rd patients in GHS/QoL at all cycles (Fig 3). These differences reached statistical significance at cycles 12 and 18. Findings were not sensitive to choice of benchmark, because similar results were observed using either a ≥ 5- or ≥ 15-point improvement in GHS/QoL score from baseline. Cumulative distribution curves at cycles 12 and 18 (Appendix Fig A2, online only) show the differences are consistent across a range of cutoff points. When excluding patients with missing data, KRd still consistently showed a higher proportion of patients with an improvement compared with Rd. These did not reach statistical significance, but the sensitivity analysis had a smaller sample size.
Fig 3.
Percentage of patient-reported outcome responders for European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (QLQ-C30) Global Health Status/Quality of Life (GHS/QoL) domain at cycles 3, 6, 12, and 18. A responder was defined as having a (A) ≥ 5-point improvement or (B) ≥ 15-point improvement from baseline for QLQ-C30 GHS/QoL. Missing data were assumed to have not reached the required improvement. GHS/QoL, Global Health Status/Quality of Life domain; KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone.
Patients in the KRd group also experienced a longer time to deterioration in GHS/QoL compared with those in the Rd group (hazard ratio from Cox model, 0.80; 95% CI, 0.65 to 0.98; P = .03), with a median time to deterioration (≥ 5-point reduction) of 10.3 v 4.8 months, respectively. A similar hazard ratio (0.79; 95% CI, 0.63 to 0.99; P = .04) was seen for the 15-point threshold (median time to deterioration, 16.6 v 11.9 months for KRd v Rd, respectively). No differences in time to deterioration were observed for six of the prespecified subscales. There was a borderline difference of 1.2 months in favor of the KRd group for physical functioning (P = .05). This was only significant for the larger threshold (10 points).
Longitudinal Changes by Treatment Group for EORTC QLQ-C30 and QLQ-MY20
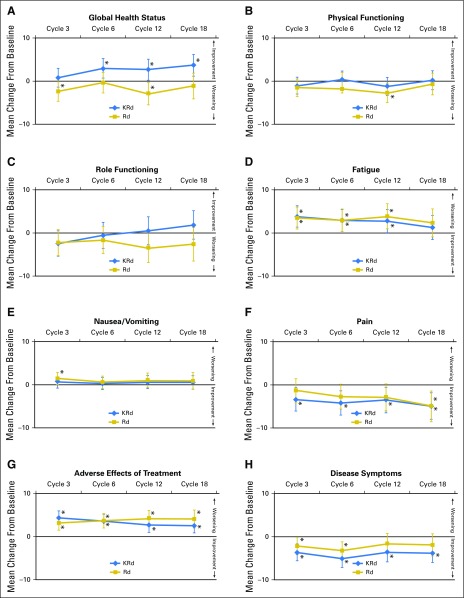
Figure 4 shows the change from baseline for each treatment group. There were some statistically significant changes from baseline within treatment groups, but these did not generally reach clinical significance.
Fig 4.
Adjusted least squares mean change from baseline for the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire C30 and myeloma-specific module scores: (A) Global Health Status, (B) physical functioning, (C) role functioning, (D) fatigue, (E) nausea and vomiting, (F) pain, (G) adverse effects of treatment, and (H) disease symptoms. Scores have been adjusted for baseline score, baseline score by visit interaction, and the random assignment stratification factors (baseline β2-microglobulin levels, prior bortezomib, and prior lenalidomide). Differences between treatments are presented with 95% CIs at each visit. Longitudinal changes within treatment groups and level of significance are also indicated. Vertical bars indicate 95% CIs of the mean. (*) P < .05 change from baseline. KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone.
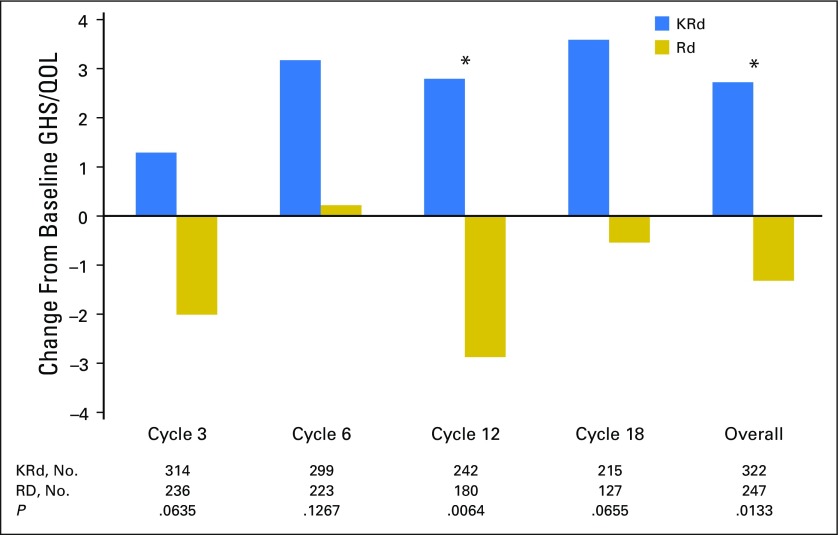
HR-QoL for Patients Achieving a PR or Better
Figure 5 shows the change from baseline GHS/QoL for patients with a PR or better as their best overall response at each cycle and overall. A total of 322 patients treated with KRd and 247 Rd patients treated with Rd achieved a PR or better and had baseline GHS/QoL assessment and at least one postbaseline assessment. The KRd responders consistently showed higher GHS/QoL scores compared with baseline across all cycles. Despite the level of response, changes from baseline in the Rd group indicated little change or declines in GHS/QoL scores. Differences between the groups were statistically significant at cycle 12 and over 18 cycles (overall).
Fig 5.
Change from baseline Global Health Status/Quality of Life (GHS/QoL) domain for patients with a partial response or better. Positive change indicates improved GHS/QoL. (*) P < .05 between-group differences. KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone.
DISCUSSION
Patients with relapsed MM experience the disease as an acute and chronic condition.27-29 Functional impairment from disease- and treatment-related symptoms, combined with the overall burden of a terminal diagnosis, can profoundly affect QoL. Although the goal of new therapies and combinations of therapies is primarily to extend overall survival and PFS, HR-QoL is also of key importance.2,30,31 With longer survival times, patients live longer with what remains an incurable disease. The ASPIRE trial had HR-QoL as a prespecified secondary end point measured by the GHS/QoL subscale, which provides a measure of the overall impact of MM and its treatment from the patient’s perspective.
Between-group improvements in HR-QoL are not routinely observed in oncology clinical trials, particularly when comparing triplet and doublet regimens, as in the ASPIRE trial. However, the QLQ-C30 GHS/QoL subscale scores significantly increased with KRd treatment compared with Rd. Additionally, clinically relevant differences were observed between the KRd and Rd groups. The MID represents the smallest group-level difference in a PRO score considered clinically meaningful. A 5-point MID for between-group differences on the QLQ-C30 GHS/QoL was prespecified. We chose a conservative threshold of 5 points, which is 1 point greater than the actual threshold between trivial (0 to 4 points) and small differences (4 to 10 points).21
Although no statistically significant differences were observed between the KRd and Rd groups for the seven prespecified subscales, these results are important for demonstrating that the addition of carfilzomib to Rd improves GHS/QoL without negatively affecting patient-reported fatigue, nausea and vomiting, disease symptoms, or adverse effects of treatment when compared with Rd. Additionally, although there were no statistically significant differences between patient-reported pain for those treated with KRd or Rd, patients treated with KRd had significant changes from baseline for pain scores at cycles 3, 6, 12, and 18, and patients treated with Rd had significant changes from baseline at cycle 18. These changes may represent trivial to small clinically meaningful improvements in pain scores.25 Importantly, KRd treatment did not result in a decline of physical functioning or role functioning scores, suggesting that combining the intravenous administration of carfilzomib with an oral regimen of Rd did not affect daily living or patients’ ability to perform work. In patients achieving a PR or better, HR-QoL improved from baseline for patients receiving KRd; however, a PR or better in patients receiving Rd did not necessarily translate into an HR-QoL benefit.
Given that more patients experienced progression in the Rd group, the imbalance in compliance rates between the groups is expected. The pattern of dropout varied across treatment groups. Most Rd patients (79%) demonstrated scores similar to or less than baseline scores before dropout, regardless of the cycle in which they dropped out. These results suggest that the condition of the missing Rd patients was deteriorating; therefore, observed GHS/QoL scores may be higher than if all patients were observed through cycle 18. Conversely, in the KRd group, 61% of patients dropped out when their scores were greater than baseline and were on an upward trajectory, suggesting that the observed KRd patients may underestimate GHS/QoL scores. Therefore, the observed differences between groups may have been diluted by missing data and may be conservative regarding the benefit of KRd.
Limitations of the study include the open-label design, because patients were aware of their treatment allocation before completing their baseline assessment. However, both groups had similar baseline completion rates and mean baseline GHS/QoL scores, showing limited evidence of bias. Another limitation was that there was differential attrition across groups. However, this does not necessarily mean that results will be biased.32 Careful consideration of the nature of missing data and the analysis strategy sought to minimize this risk. Congruence of the primary and sensitivity analyses of the GHS/QoL scores suggests that the improvement in the KRd group versus the Rd group is a robust finding, and analyses of the missing data patterns also suggest that the observed differences may be conservative.
In conclusion, this is the first report of PRO data in a population with relapsed MM treated with KRd. The results demonstrated that KRd is superior to Rd on the QLQ-C30 GHS/QoL scale, whereas exploratory analyses showed that the addition of carfilzomib to Rd resulted in no evidence of a negative impact on key subscales of HR-QoL related to disease symptoms and adverse effects of treatment. The aims of MM treatment are to control disease, prolong survival, and maximize patient well-being. Moreover, if survival is extended, it is equally important that efficacy gains are not at the cost of impaired QoL, particularly with the use of triplet combination therapies. Results from the ASPIRE study confirm that the clinical benefits of the KRd triplet regimen, compared with the Rd doublet regimen, are associated with significant improvements in GHS/QoL, and there was no evidence of a detrimental impact from the triplet regimen on other aspects of HR-QoL.
ACKNOWLEDGMENT
Editorial assistance was provided by Cheryl Chun, CMPP, and Meredith Kalish, CMPP at BlueMomentum, an Ashfield business. Programming support was provided by IDDI.
Appendix
Two sensitivity analyses were performed to account for possible nonignorable missing data.14,16 In the first analysis, a simple covariate representing dropout (no v yes) was added to the mixed model. Patients who miss all scheduled assessments after the last nonmissing assessment were considered as having dropped out, regardless of reasons.17 The overall treatment effect and P value were re-estimated while accounting for this dropout variable and are provided in Appendix Table A1.
In the second analysis, a pattern-mixture model was performed. The following five missing patterns were defined for this analysis using the timing of the last assessment: patients with no missing assessments; patients who drop out at cycle 18; patients who drop out at cycle 12; patients who drop out at cycle 6; and patients who drop out at cycle 3.18,19 The pattern-mixture model initially estimates treatment effect within each pattern. This allows the baseline value and rate of change of the scores to vary for each group of patients with the same pattern. The overall treatment effect is then obtained by averaging across the patterns, weighted by the number of patients with each pattern. The overall model results are listed in Table A1. Within patterns, it is necessary to impute data; there are various methods available to do so. For this model, we used the neighboring case missing values restriction. This means data from patients in the neighboring pattern were used to impute means for missing data.
Fig A1.
Mean European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (QLQ-C30) Global Health Status/Quality of Life (GHS/QoL) domain scores over time, stratified by the time of last assessment. Pattern 1 shows mean scores at each visit for patients with a GHS/QoL score at all visits to cycle 18. Pattern 2 shows mean scores for patients with GHS/QoL scores up to cycle 12. Pattern 3 shows mean scores for patients with GHS/QoL scores up to cycle 6. Pattern 4 shows mean scores for patients with GHS/QoL scores up to cycle 3. Pattern 5 shows mean scores for patients with baseline GHS/QoL scores only. Graphs do not contain intermittent missing patterns. Percentages are based on the total number of patients in the carfilzomib, lenalidomide, and dexamethasone (KRd; n = 396) and lenalidomide and dexamethasone (Rd; n = 396) treatment groups.
Fig A2.
Cumulative distribution of change from baseline in European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 Global Health Status/Quality of Life domain scores. Positive change indicates improved health-related quality of life. KRd, carfilzomib, lenalidomide, and dexamethasone; Rd, lenalidomide and dexamethasone.
Table A1.
Sensitivity Analyses of Treatment Difference Over Time in EORTC QLQ-C30 GHS/QoL Based on Model Accounting for Dropout (no v yes) and Pattern-Mixture Model Using Multiple Imputation With Neighboring Cases Missing Value Restriction
Footnotes
Listen to the podcast by Dr Landgren at www.jco.org/podcasts
Supported by Onyx Pharmaceuticals, an Amgen subsidiary.
Results from this study have been previously presented in part in Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med 372:142-152, 2015.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01080391.
AUTHOR CONTRIBUTIONS
Conception and design: A. Keith Stewart, Meletios A. Dimopoulos, David S. Siegel, Kim Cocks, Naseem Zojwalla, Antonio Palumbo
Provision of study materials or patients: Ruben Niesvizky, Jesus F. San-Miguel, Heinz Ludwig
Collection and assembly of data: Meletios A. Dimopoulos, Ivan Špička, Albert Oriol, Roman Hájek, Laura Rosiñol, David S. Siegel, Ruben Niesvizky, Andrzej J. Jakubowiak, Naseem Zojwalla, Margaret Tonda
Data analysis and interpretation: A. Keith Stewart, Meletios A. Dimopoulos, Tamás Masszi, Albert Oriol, David S. Siegel, Ruben Niesvizky, Andrzej J. Jakubowiak, Jesus F. San-Miguel, Heinz Ludwig, Jacqui Buchanan, Kim Cocks, Xinqun Yang, Biao Xing, Naseem Zojwalla, Margaret Tonda, Philippe Moreau, Antonio Palumbo
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Health-Related Quality-of-Life Results From the Open-Label, Randomized, Phase III ASPIRE Trial Evaluating Carfilzomib, Lenalidomide, and Dexamethasone Versus Lenalidomide and Dexamethasone in Patients With Relapsed Multiple Myeloma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
A. Keith Stewart
Consulting or Advisory Role: Celgene, Novartis, Bristol-Myers Squibb, Amgen, Janssen, Takeda
Meletios A. Dimopoulos
Honoraria: Amgen, Novartis, Celgene, Takeda, Genesis Pharmaceuticals, Janssen-Cilag, Bristol-Myers Squibb
Consulting or Advisory Role: Amgen, Janssen-Cilag, Takeda
Research Funding: Janssen-Cilag (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Janssen-Ortho, Genesis Pharmaceuticals
Tamás Masszi
No relationship to disclose
Ivan Špička
Honoraria: Celgene, Janssen-Cilag, Bristol-Myers Squibb, Amgen
Consulting or Advisory Role: Celgene, Janssen-Cilag, Amgen, Bristol-Myers Squibb, Millennium
Speakers' Bureau: Celgene, Janssen-Cilag, Amgen
Research Funding: Celgene
Albert Oriol
Consulting or Advisory Role: Celgene, Janssen
Speakers' Bureau: Celgene, Janssen
Roman Hájek
Honoraria: Celgene, Janssen, Amgen, Takeda, Bristol-Myers Squibb
Consulting or Advisory Role: Amgen, Takeda
Research Funding: Amgen (Inst), Celgene (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Amgen, Celgene, Takeda, Janssen-Cilag, Bristol-Myers Squibb
Laura Rosiñol
Honoraria: Celgene, Janssen
David S. Siegel
Honoraria: Celgene, Amgen, Millennium
Speakers' Bureau: Celgene, Amgen, Millennium
Ruben Niesvizky
Consulting or Advisory Role: Celgene, Takeda, Amgen, Bristol-Myers Squibb, Janssen Oncology
Speakers' Bureau: Celgene, Amgen, Takeda, Bristol-Myers Squibb, Janssen Oncology
Research Funding: Celgene, Amgen, Takeda, Bristol-Myers Squibb, Janssen Oncology
Travel, Accommodations, Expenses: Celgene, Amgen, Takeda
Andrzej J. Jakubowiak
Honoraria: Amgen, Celgene
Consulting or Advisory Role: Amgen, Celgene
Research Funding: Amgen (Inst), Celgene (Inst)
Jesus F. San-Miguel
Consulting or Advisory Role: Millennium, Celgene, Novartis, Onyx Pharmaceuticals, Janssen, Bristol-Myers Squibb, Merck Sharp & Dohme, Amgen
Heinz Ludwig
Speakers' Bureau: Celgene, Takeda, Bristol-Myers Squibb, Amgen, Janssen-Cilag
Jacqui Buchanan
Employment: Amgen, Silk Road Medical (I)
Stock or Other Ownership: Amgen, Silk Road Medical (I)
Kim Cocks
Employment: Adelphi Values
Consulting or Advisory Role: Onyx Pharmaceuticals, Creo Medical, Endomag, Diasolve, SmartMatrix, Amgen, Celgene, Bristol-Myers Squibb
Xinqun Yang
Employment: Onyx Pharmaceuticals, BioMarin Pharmaceutical
Stock or Other Ownership: Amgen, BioMarin Pharmaceutical
Biao Xing
Employment: Amgen
Stock or Other Ownership: Amgen
Naseem Zojwalla
Employment: Onyx Pharmaceuticals, Peloton Therapeutics
Leadership: Peloton Therapeutics
Stock or Other Ownership: Peloton Therapeutics, Onyx Pharmaceuticals
Travel, Accommodations, Expenses: Onyx Pharmaceuticals, Peloton Therapeutics
Margaret Tonda
Employment: Amgen
Stock or Other Ownership: Amgen
Philippe Moreau
Consulting or Advisory Role: Celgene, Takeda, Janssen, Novartis, Amgen
Antonio Palumbo
Honoraria: Amgen, Novartis, Bristol-Myers Squibb, Genmab, Celgene, Janssen-Cilag, Millennium, Onyx Pharmaceuticals, Sanofi
Consulting or Advisory Role: Amgen, Bristol-Myers Squibb, Genmab, Celgene, Janssen-Cilag, Millennium, Onyx Pharmaceuticals
REFERENCES
- 1.Gulbrandsen N, Hjermstad MJ, Wisløff F. Interpretation of quality of life scores in multiple myeloma by comparison with a reference population and assessment of the clinical importance of score differences. Eur J Haematol. 2004;72:172–180. doi: 10.1046/j.0902-4441.2003.00195.x. [DOI] [PubMed] [Google Scholar]
- 2.Sonneveld P, Verelst SG, Lewis P, et al. Review of health-related quality of life data in multiple myeloma patients treated with novel agents. Leukemia. 2013;27:1959–1969. doi: 10.1038/leu.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–152. doi: 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- 4.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 5.Cocks K, Cohen D, Wisløff F, et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-MY20) in assessing the quality of life of patients with multiple myeloma. Eur J Cancer. 2007;43:1670–1678. doi: 10.1016/j.ejca.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Kvam AK, Fayers PM, Wisloff F. Responsiveness and minimal important score differences in quality-of-life questionnaires: A comparison of the EORTC QLQ-C30 cancer-specific questionnaire to the generic utility questionnaires EQ-5D and 15D in patients with multiple myeloma. Eur J Haematol. 2011;87:330–337. doi: 10.1111/j.1600-0609.2011.01665.x. [DOI] [PubMed] [Google Scholar]
- 7.Stead ML, Brown JM, Velikova G, et al. Development of an EORTC questionnaire module to be used in health-related quality-of-life assessment for patients with multiple myeloma. Br J Haematol. 1999;104:605–611. doi: 10.1046/j.1365-2141.1999.01206.x. [DOI] [PubMed] [Google Scholar]
- 8.Wisløff F, Eika S, Hippe E, et al. Measurement of health-related quality of life in multiple myeloma. Br J Haematol. 1996;92:604–613. doi: 10.1046/j.1365-2141.1996.352889.x. [DOI] [PubMed] [Google Scholar]
- 9. Leleu X, Petrucci MT, Welslau M, et al: Psychometric performance of the EORTC Quality-of-Life Core questionnaire (QLQ-C30) and QLQ-Multiple Myeloma (QLQ-MY20) in relapsed/refractory multiple myeloma (RRMM). Blood 122, 2013 (abstr 1721)
- 10.Gulbrandsen N, Wisløff F, Brinch L, et al. Health-related quality of life in multiple myeloma patients receiving high-dose chemotherapy with autologous blood stem-cell support. Med Oncol. 2001;18:65–77. doi: 10.1385/MO:18:1:65. [DOI] [PubMed] [Google Scholar]
- 11.Uyl-de Groot CA, Buijt I, Gloudemans IJ, et al. Health related quality of life in patients with multiple myeloma undergoing a double transplantation. Eur J Haematol. 2005;74:136–143. doi: 10.1111/j.1600-0609.2004.00346.x. [DOI] [PubMed] [Google Scholar]
- 12.Wisløff F, Hjorth M, Nordic Myeloma Study Group Health-related quality of life assessed before and during chemotherapy predicts for survival in multiple myeloma. Br J Haematol. 1997;97:29–37. doi: 10.1046/j.1365-2141.1997.222667.x. [DOI] [PubMed] [Google Scholar]
- 13.Fayers PM, Aaronson NK, Bjordal K, et al. The EORTC QLQ-C30 Scoring Manual. (ed 3). Brussels, Belgium: European Organisation for Research and Treatment of Cancer; 2001. [Google Scholar]
- 14.Mallinckrod CH, Lane PW, Schnell D, et al. Recommendations for the primary analysis of continuous endpoints in longitudinal clinical trials. Drug Inf J. 2008;42:303–319. [Google Scholar]
- 15. Dmitrienko A, Tamhane AC, Bretz F (eds): Multiple Testing Problems in Pharmaceutical Statistics. Boca Raton, FL, CRC Press, 2009. [Google Scholar]
- 16.Bell ML, Fairclough DL. Practical and statistical issues in missing data for longitudinal patient-reported outcomes. Stat Methods Med Res. 2014;23:440–459. doi: 10.1177/0962280213476378. [DOI] [PubMed] [Google Scholar]
- 17.Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol Methods. 1997;2:64–78. [Google Scholar]
- 18.Fairclough DL. Design and Analysis of Quality of Life Studies in Clinical Trials. Boca Raton, FL: Chapman & Hall/CRC; 2002. [Google Scholar]
- 19.Thijs H, Molenberghs G, Michiels B, et al. Strategies to fit pattern-mixture models. Biostatistics. 2002;3:245–265. doi: 10.1093/biostatistics/3.2.245. [DOI] [PubMed] [Google Scholar]
- 20.Jaeschke R, Singer J, Guyatt GH. Measurement of health status: Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 21.Cocks K, King MT, Velikova G, et al. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol. 2011;29:89–96. doi: 10.1200/JCO.2010.28.0107. [DOI] [PubMed] [Google Scholar]
- 22.Delforge M, Dhawan R, Robinson D, Jr, et al. Health-related quality of life in elderly, newly diagnosed multiple myeloma patients treated with VMP vs. MP: Results from the VISTA trial. Eur J Haematol. 2012;89:16–27. doi: 10.1111/j.1600-0609.2012.01788.x. [DOI] [PubMed] [Google Scholar]
- 23.Nunnally J, Bernstein I. Psychometric Theory. (ed 3). New York, NY: McGraw-Hill; 1994. [Google Scholar]
- 24. Cocks K, Buchanan J: Defining responders on the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (30-item core module) (QLQ-C30) subscales. Presented at 22nd Annual Conference for the International Society for Quality of Life Research, Vancouver, British Columbia, Canada, October 21-24, 2015.
- 25.Cocks K, King MT, Velikova G, et al. Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur J Cancer. 2012;48:1713–1721. doi: 10.1016/j.ejca.2012.02.059. [DOI] [PubMed] [Google Scholar]
- 26.Dimopoulos MA, Delforge M, Hájek R, et al. Lenalidomide, melphalan, and prednisone, followed by lenalidomide maintenance, improves health-related quality of life in newly diagnosed multiple myeloma patients aged 65 years or older: Results of a randomized phase III trial. Haematologica. 2013;98:784–788. doi: 10.3324/haematol.2012.074534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephens M, McKenzie H, Jordens CF. The work of living with a rare cancer: Multiple myeloma. J Adv Nurs. 2014;70:2800–2809. doi: 10.1111/jan.12430. [DOI] [PubMed] [Google Scholar]
- 28.Potrata B, Cavet J, Blair S, et al. Understanding distress and distressing experiences in patients living with multiple myeloma: An exploratory study. Psychooncology. 2011;20:127–134. doi: 10.1002/pon.1715. [DOI] [PubMed] [Google Scholar]
- 29.Molassiotis A, Wilson B, Blair S, et al. Living with multiple myeloma: Experiences of patients and their informal caregivers. Support Care Cancer. 2011;19:101–111. doi: 10.1007/s00520-009-0793-1. [DOI] [PubMed] [Google Scholar]
- 30.Kvam AK, Waage A. Health-related quality of life in patients with multiple myeloma: Does it matter? Haematologica. 2015;100:704–705. doi: 10.3324/haematol.2015.127860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gadó K, Domján G: Quality of life issues of patients with multiple myeloma. http://www.intechopen.com/books/multiple-myeloma-a-quick-reflection-on-the-fast-progress/quality-of-life-issues-of-patients-with-multiple-myeloma.
- 32.Bell ML, Kenward MG, Fairclough DL, et al. Differential dropout and bias in randomised controlled trials: When it matters and when it may not. BMJ. 2013;346:e8668. doi: 10.1136/bmj.e8668. [DOI] [PMC free article] [PubMed] [Google Scholar]