ABSTRACT
Apoptosis regulation by Bcl-2 proteins is pivotal for mammalians, not only because it is key for development but also because aberrant apoptosis is prerequisite to severe diseases, like cancer. Recently, we quantified interactions within the Bcl-2 protein network in solution and membranes, and addressed membrane recruitment, preference of interaction partners and the consequences for Bax activation and inhibition.
KEYWORDS: Bcl-2 proteins, mitochondrial apoptosis
The Bcl-2 protein family regulates mitochondrial outer membrane permeabilization (MOMP) and cell death. Several family members have become targets for anti-cancer drug development as well as in cancer diagnostics. However, in order to predict and manipulate cancer cell death by apoptosis, a deep and detailed understanding of the underlying mechanisms is necessary, including the structure, function and interactions between the different Bcl-2 family members. We addressed these questions in previous studies, with our most recent study addressing the integration of interaction network in the cytosol and membrane compartment, which provides a new integrated model how the Bcl-2 family regulates apoptosis.1
About twenty Bcl-2 proteins are known and interact with each other to form a complex network. Contacts are made in two very different environments: the hydrophilic 3D cytosol and the hydrophobic 2D outer mitochondrial membrane. We focused on three representative Bcl-2 proteins cBid (Caspase 8 cleaved Bid), Bax and Bcl-xL that are all able to adopt soluble or membrane-embedded conformations.2,3 One would also need to consider the protein concentrations in the human body to integrate the network, but existing data suggest a large variation between different cell types and individual humans.4
For proper understanding of MOMP regulation and execution, the transformation from soluble, inactive Bax (or its homolog Bak) to the oligomeric, membrane-embedded form inducing MOMP is crucial and needs to be addressed beyond all others. All other players in the network activate, accelerate or inhibit this conformational changes. Bax conformational changes involve at least five steps: 1) Soluble Bax binds loosely to the membrane2 and afterwards 2) it inserts deeply into the membrane.5,6 3) Then homo-dimers are formed6–8 that 4) assemble into higher order oligomers,6,7 which 5) provokes changes in the lipid bilayer that enable stable, toroidal pores9,10 (Figure 1A). Based on these five transitions, six conformational forms of Bax exist (States a-f see Figure 1A).
Figure 1.
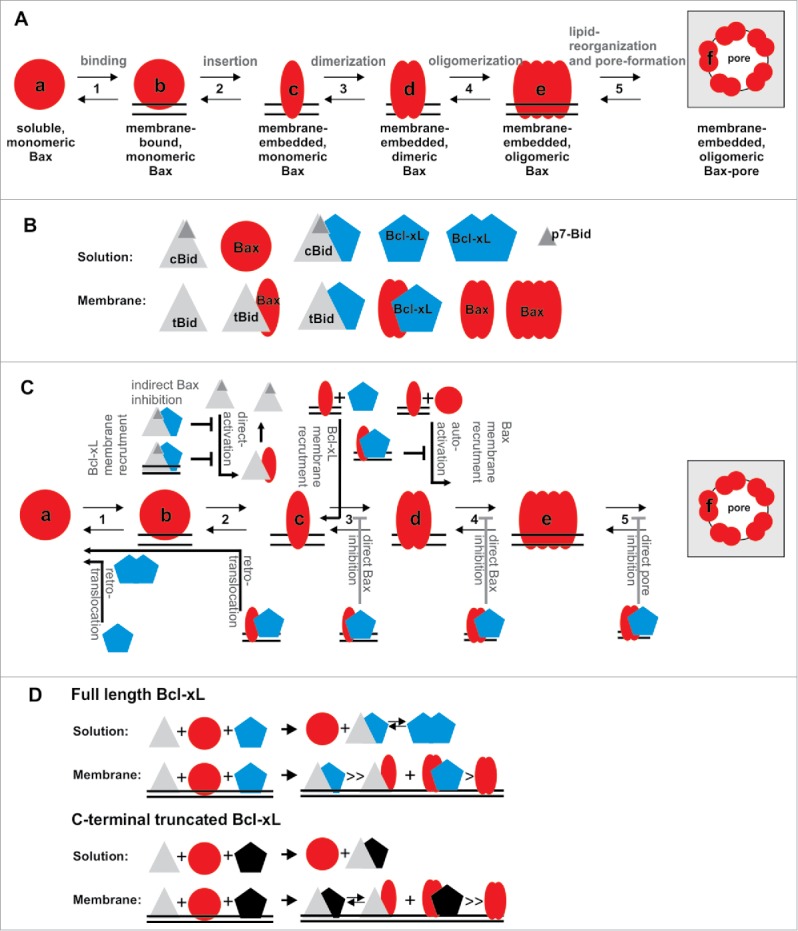
Regulation and mutual interaction of Bcl-2 family proteins. A) Conformational changes during Bax activation. To transform from the soluble to the pore forming conformation, Bax needs to undergo at least five transitions (transition 1 to 5), so that at least six conformational states (a-f) exist. B) Schematic drawing of the twelve spatially-resolved protein/protein complexes identified upon co-incubation of cBid, Bax and Bcl-xL1. C) Diagram on how cBid and Bcl-xL, as well as Bax itself, regulate the five conformational transitions of Bax introduced in A). D) Schematic drawing of preferential complexes formed by full length and C-terminus-truncated Bcl-xL.
Our recent work addresses the interactions between Bax, it best-studied activator cBid and the apoptosis inhibitor Bcl-xL. We investigated them in their soluble and membrane-embedded states using a minimalistic model membrane setup,1 which allowed us a tight control over each factor (e.g. protein concentration, lipid composition, pH and salt concentration), so that the impact of disturbances or variations can be directly followed. In cells this is impossible, due to the complexity of parameters and the existence of unknown effects.
Upon co-incubation, cBid, Bax and Bcl-xL form a network of at least twelve components/complexes with six of them detected in solution and the other six in the membrane (Figure 1B). In solution, Bcl-xL assembles into very stable homodimers that are in equilibrium with cBid/Bcl-xL heterodimers. In contrast, Bax is monomeric in solution.1 This soluble state “a” is in equilibrium with a loosely membrane-attached state “b” of Bax, which is the prerequisite for the transition into the membrane-embedded states c-f. One mechanism how Bcl-xL inhibits Bax is by translocating it back from the membrane into solution. Two mechanisms have been suggested for this retro-translocation (Figure 1C): Schellenberg et al. suggest that Bcl-xL shifts the equilibrium between Bax state a and b towards a, so that the transition into state c-f is hindered2 while Edlich et al. implies that Bcl-xL is able retro-translocate membrane inserted Bax.3 For the first time, we detected retro-translocation in a reconstituted model-membrane system1, which demonstrated that Bcl-xL alone is sufficient, but we could not clarify the mechanism. However, the formation of very stable Bcl-xL dimers in solution could be the driving force for separating Bcl-xL/Bax complexes once they leave the membrane.
Additionally, we examined how the transition from states b to c takes place (Fig. 1C). Since more than decade it is heavily discussed whether Bax needs a direct activator or is constitutively activated in absence of inhibitors. In the meantime we learned that both mechanisms take place: Bax activation is strongly enhanced by direct activators like cBid,8 but in their absence Bax can also auto-activate itself.6,11 State c is likely transient and could only observed using biochemical tricks.5,6 We hypothesize that the transient, membrane-bound cBid/Bax complex detected is the most relevant stage between Bax membrane insertion and homo-dimerization1 (Fig. 1C). Membrane-embedded Bax can then recruit additional soluble or membrane-bound Bax molecules into the membrane-embedded states. We call this process recruitment, as this mechanism is also observed for other Bcl-2 proteins: cBid recruits Bax and Bcl-xL to insert into the membrane;8 membrane-embedded Bax recruits soluble Bax1 and our new data show that membrane-embedded Bax additionally recruits Bcl-xL1 (Fig. 1C).
Bcl-xL inhibition of Bax takes place by least are two more mechanisms additionally to the retro-translocation: indirectly by sequestering cBid and directly by interaction with membrane-embedded Bax.1 However, which transitions in the process of Bax activation are concretely inhibited by the Bax/Bcl-xL complex formation remain unknown (Fig. 1C).
For a detailed understanding of the network the preference of interaction partners needs to be addressed and here we made a surprising observation. In solution full length Bcl-xL formed homo- and heterodimers with cBid with similar affinities. However, when the C-terminal transmembrane helix of Bcl-xL was removed homodimer formation was not detectable1 (Fig. 1D). Moreover, the C-terminal truncation affects the preference of interaction partners in membrane-embedded Bcl-xL. While the full length protein preferentially forms cBid/Bcl-xL over Bax/Bcl-xL complexes,1 the truncated form loses this preference1 (Fig. 1D). This supports the role of the C-terminal tails in complex formation and brings a note of caution that truncated protein variants should be used with care.
Next steps on the road towards manipulation of cells with aberrant apoptosis should be a detailed analysis of the structures of homo-and hetero-complexes in their transition to the membrane-embedded conformations.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosure.
Acknowledgments
AJGS is supported by University of Tübingen, the European Research Council (ERC-2012-StG 309966) as well as the Forschergruppe 2036. SB is supported by the Cluster of Excellence RESOLV (EXC 1069) funded by the Deutsche Forschungsgemeinschaft.
References
- 1.Bleicken S, Hantusch A, Das KK, Frickey T, Garcia-Saez AJ. Quantitative interactome of a membrane Bcl-2 network identifies a hierarchy of complexes for apoptosis regulation. Nat Commun. 2017;8:73. doi: 10.1038/s41467-017-00086-6. PMID:28706229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schellenberg B, Wang P, Keeble JA, Rodriguez-Enriquez R, Walker S, Owens TW, Foster F, Tanianis-Hughes J, Brennan K, Streuli CH, et al.. Bax Exists in a Dynamic Equilibrium between the Cytosol and Mitochondria to Control Apoptotic Priming. Mol Cell. 2013;49:959–971. doi:https://doi.org/ 10.1016/j.molcel.2012.12.022. PMID:23375500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edlich F, Banerjee S, Suzuki M, Cleland MM, Arnoult D, Wang C, Neutzner A, Tjandra N, Youle RJ. Bcl-x(L) Retrotranslocates Bax from the Mitochondria into the Cytosol. Cell. 2011;145:104–116. doi: 10.1016/j.cell.2011.02.034. PMID:21458670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindner AU, Concannon CG, Boukes GJ, Cannon MD, Llambi F, Ryan D, Boland K, Kehoe J, McNamara DA, Murray F, et al.. Systems Analysis of BCL2 Protein Family Interactions Establishes a Model to Predict Responses to Chemotherapy. Cancer Res. 2013;73:519–528. doi: 10.1158/0008-5472.CAN-12-2269. PMID:23329644 [DOI] [PubMed] [Google Scholar]
- 5.Niu X, Brahmbhatt H, Mergenthaler P, Zhang Z, Sang J, Daude M, Ehlert FGR, Diederich WE, Wong E, Zhu W, Pogmore J, et al.. A Small-Molecule Inhibitor of Bax and Bak Oligomerization Prevents Genotoxic Cell Death and Promotes Neuroprotection. Cell chem Biol. 2017;24:493−506.e495. doi: 10.1016/j.chembiol.2017.03.011. PMID:28392146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subburaj Y, Cosentino K, Axmann M, Pedrueza-Villalmanzo E, Hermann E, Bleicken S, Spatz J, García-Sáez AJ, et al.. Bax monomers form dimer units in the membrane that further self-assemble into multiple oligomeric species. Nat Commun. 2015;6:.doi: 10.1038/ncomms9042. PMID:26271728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bleicken S, Jeschke G, Stegmueller C, Salvador-Gallego R, García-Sáez AJ, Bordignon E. Structural Model of Active Bax at the Membrane. Mol Cell. 2014;56:496–505. doi: 10.1016/j.molcel.2014.09.022. PMID:25458844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, Andrews DW. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. [pii] 10.1016/j.cell.2008.11.010. doi: 10.1016/j.cell.2008.11.010. PMID:19062087 [DOI] [PubMed] [Google Scholar]
- 9.Bleicken S, Landeta O, Landajuela A, Basanez G, Garcia-Saez AJ. Proapoptotic Bax and Bak form stable protein-permeable pores of tunable size. J Biol Chem. 2013;288:33241–33252. doi: 10.1074/jbc.M113.512087. PMID:24100034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleicken S, Hofhaus G, Ugarte-Uribe B, Schroder R, Garcia-Saez AJ. cBid, Bax and Bcl-xL exhibit opposite membrane remodeling activities. Cell Death Dis. 2016;7:e2121. doi: 10.1038/cddis.2016.34. PMID:26913610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Neill KL, Huang K, Zhang J, Chen Y, Luo X. Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane. Genes Dev. 2016;30:973–988. doi: 10.1101/gad.276725.115. PMID:27056669 [DOI] [PMC free article] [PubMed] [Google Scholar]


