ABSTRACT
Mortality in ovarian cancer is predominantly due to acquired chemoresistance and tumor recurrence. UBIQUITIN CONJUGATING ENZYME E2 or RAD6 expression increases in cell lines and patient tumors in response to platinum-based chemotherapy and promotes both activation of DNA damage response pathways and expression of stemness genes and a stem cell-like phenotype driving ovarian cancer chemoresistance.
KEYWORDS: RAD6, ovarian cancer, DNA repair, cancer stem cells, chemoresistance
Chemoresistance in cancer can be attributed to several factors including enhanced DNA repair or damage tolerance (DDT) activities and/or development of a stem cell-like phenotype – which can include quiescence to evade impact of chemotherapeutic drugs, increased efflux pumps or aldehyde dehydrogenase (ALDH) activity to remove or inactivate the drug, or impaired apoptosis to survive the cytotoxic effects of chemotherapy. Acquired chemoresistance is a major factor contributing to poor survival rates in ovarian cancer patients. We recently identified UBIQUITIN CONJUGATING ENZYME E2 (UBE2), commonly known as RAD6, as a key regulator of acquired chemoresistance in ovarian cancer.1 RAD6 is an ubiquitin-conjugating enzyme that, through interaction with different ubiquitin ligase partners, acts to regulate multiple DNA repair and damage tolerance pathways. Furthermore, RAD6 is capable of regulating gene expression through pro-transcription histone modifications.2 Humans have two RAD6 proteins (RAD6A & B or UBE2A & B) and both are frequently overexpressed in various tumor types, and overexpression of RAD6 promotes tumorigenesis, proliferation, invasion and chemoresistance in normal, immortalized cells.3
Our previous work has shown a connection between RAD6 expression and chemoresistance and acquisition of a stem cell-like phenotype in ovarian cancer cell lines.4 In our manuscript we followed up on these findings and showed that RAD6 levels are high in ovarian cancer and increase in response to treatment with chemotherapeutic agents in ovarian cancer cell lines as well as patient tumors.1 Mortality in ovarian cancer patients is typically attributed to development of a recurrent, chemoresistant tumor following chemotherapy and RAD6 correlated with progression free survival (PFS) in ovarian cancer patients. These findings suggest RAD6 could be an important factor for resistance to treatment and poor PFS. Therefore, to investigate the role of RAD6 in acquired chemoresistance in ovarian cancer, we knocked-down or inhibited both RAD6A and RAD6B in multiple ovarian cancer cell lines. These cells showed marked decreases in expression of cancer stem cell (CSC) markers and activation of DNA damage response (DDR) proteins and concomitant sensitivity to carboplatin treatment. These findings strongly suggest that RAD6 expression is stimulated in response to chemotherapy and in turn promotes chemoresistance in cancer cells through at least two mechanisms; stimulating expression of CSC genes and enhancing DNA repair activity in response to chemotherapeutic agents (Fig. 1). While the mechanisms of RAD6-mediated activation of DNA repair are well-known, RAD6 regulation of CSC gene expression has not been explored. RAD6 promotes stabilization and nuclear localization of CTNNB1, typically called β-catenin5 and functions in conjunction with ring finger protein 20/40 (RNF20/40) to monoubiquitinate histone H2B at lysine 120 which leads to further histone modification resulting in a localized open conformation that allows increased gene transcription.2 Neither of these mechanisms has been shown to promote CSC gene expression in cancer cells; therefore, we examined the levels monoubiquitinated H2B within the promoters of two CSC genes that we determined to be transcriptionally regulated by RAD6 and that also correlated with chemoresistance and poor PFS in ovarian cancer patients [Aldehyde Dehydrogenase 1 Family Member A1 (ALDH1A1) and SRY-box 2 (SOX2)]. We found that the levels of monoubiquitinated H2B decreased when RAD6 was depleted or inhibited, while control regions in promoters of genes not regulated by RAD6 were unchanged. The promoters of both ALDH1A1 and SOX2 contain putative β-catenin binding sites, and while β-catenin was not found to be associated with these sites in untreated cells, it did bind the ALDH1A1 promoter in response to carboplatin treatment and this interaction was dependent upon the presence of RAD6. This establishes for the first time direct RAD6-mediated regulation of CSC gene expression in response to chemotherapeutic agents.
Figure 1.
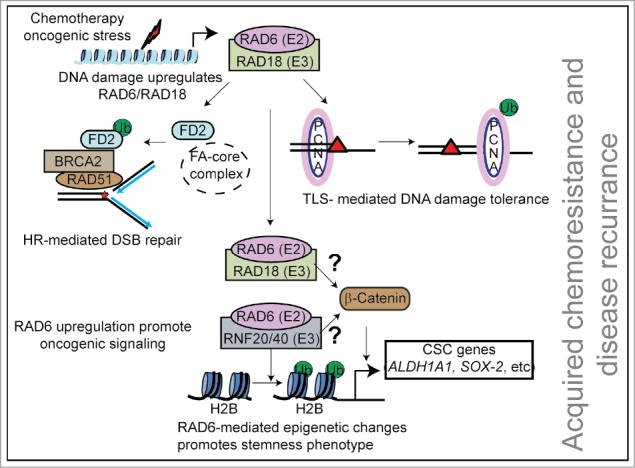
Main RAD6 functions in cancer cells. RAD6 is an E2 ubiquitin-conjugating enzyme that partners with multiple ubiquitin ligases to regulate DNA repair and gene transcription. RAD6 is overexpressed in many cancers and is further induced by chemotherapy-induced DNA damage. Increased RAD6 promotes cancer cell survival by working with RAD18 to activate DNA repair by the Fanconi anemia and homologous recombination pathways and DNA damage tolerance by translesion synthesis. Furthermore, RAD6 in conjunction with RNF20/40 promote transcription of stemness factors, such as SOX2 and ALDH1A1, by monoubiquitination of histone H2B in the vicinity of these genes. Histone H2B monoubiquitination leads to further epigenetic modifications and open chromatin structures enhancing gene transcription. RAD6 also stabilizes and promotes nuclear localization of the pro-stemness transcription factor β-catenin, by an unknown mechanism. Increased expression of stemness factors assists survival of the cancer cell in response to treatment with chemotherapeutic agents.
We demonstrated that the RAD6-specific small molecule inhibitor TZ9 [4-Amino-6-(phenylamino)-[1,3,5]triazin-2-yl)methyl-4-nitrobenzoate] strongly sensitized multiple ovarian cancer cell lines to carboplatin, a drug commonly utilized for treating ovarian cancer by blocking activation of DNA repair and DDT as well as preventing expression of CSC proteins in response to carboplatin treatment. Because RAD6 is at the center of multiple pathways that contribute to acquired chemoresistance in ovarian cancer (and potentially many other neoplasms) it is an attractive option as a future therapeutic target. Several RAD6 inhibitors have been tested in recent years; however, they have not been very effective in humans (including TZ9) and new, more efficient compounds need to be developed. Toward this goal we have begun developing multiple RAD6 inhibitors based upon TZ9 as well as other unrelated compounds.6
RAD6 partners with RAD18 to activate either faithful DNA repair, such as through homologous recombination,7 or DDT like translesion synthesis,8 which are suspected of contributing to the mutagenesis associated with development and/or propagation and metastasis of cancer. RAD6/RAD18 activity could be the key determinant of which pathway is chosen and this could have a profound impact on cancer-related mutagenesis. Furthermore, RAD18 has recently been demonstrated to have vastly different actions in normal and cancer cells;9 therefore, it is imperative that the roles of RAD6 be thoroughly explored in normal versus cancer cells. These differences could be exploited for developing new chemotherapeutic agents targeting RAD6/RAD18-dependent ubiquitin signaling pathways that specifically target cancer cells.
Acknowledgments
This work was supported by Abraham Mitchell Cancer Research Scholar Endowment grant, and by National Institutes of Health grant [R01GM098956] to K.P.
References
- 1.Somasagara RR, Spencer SM, Tripathi K, Clark DW, Mani C, Madeira da Silva L, Scalici J, Kothayer H, Westwell AD, Rocconi RP, et al.. RAD6 promotes DNA repair and stem cell signaling in ovarian cancer and is a promising therapeutic target to prevent and treat acquired chemoresistance. Oncogene. 2017; [Epub ahead of print]. doi: 10.1038/onc.2017.279. PMID:28806395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kao C-F, Hillyer C, Tsukuda T, Henry K, Berger S, Osley MA. Rad6 plays a role in transcriptional activation through ubiquitylation of histone H2B. Genes Dev. 2004;18:184‐95. doi: 10.1101/gad.1149604. PMID:14752010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyakhovich A, Shekhar MPV. RAD6B overexpression confers chemoresistance: RAD6 expression during cell cycle and its redistribution to chromatin during DNA damage-induced response. Oncogene. 2004;23:3097‐106. doi: 10.1038/sj.onc.1207449. PMID:14981545. [DOI] [PubMed] [Google Scholar]
- 4.Somasagara RR, Tripathi K, Spencer SM, Clark DW, Barnett R, Bachaboina L, Scalici J, Rocconi RP, Piazza GA, Palle K. Rad6 upregulation promotes stem cell-like characteristics and platinum resistance in ovarian cancer. Biochem Biophys Res Commun. 2016;469:449‐55. doi: 10.1016/j.bbrc.2015.11.134. PMID:26679603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shekhar MPV, Gerard B, Pauley RJ, Williams BO, Tait L. Rad6B is a positive regulator of beta-catenin stabilization. Cancer Res. 2008;68:1741‐50. doi: 10.1158/0008-5472.CAN-07-2111. PMID:18339854. [DOI] [PubMed] [Google Scholar]
- 6.Kothayer H, Spencer SM, Tripathi K, Westwell AD, Palle K. Synthesis and in vitro anticancer evaluation of some 4,6-diamino-1,3,5-triazine-2-carbohydrazides as Rad6 ubiquitin conjugating enzyme inhibitors. Bioorg Med Chem Lett. 2016;26:2030‐4. doi: 10.1016/j.bmcl.2016.02.085. PMID:26965855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Game JC, Chernikova SB. The role of RAD6 in recombinational repair, checkpoints and meiosis via histone modification. DNA Repair. 2009;8:470‐82. doi: 10.1016/j.dnarep.2009.01.007. PMID:19230796. [DOI] [PubMed] [Google Scholar]
- 8.Hoege C, Pfander B, Moldovan G-L, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135‐41. doi: 10.1038/nature00991. PMID:12226657. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Poe JC, Yang L, Fedoriw A, Desai S, Magnuson T, Li Z, Fedoriw Y, Araki K, Gao Y, et al.. Rad18 confers hematopoietic progenitor cell DNA damage tolerance independently of the Fanconi Anemia pathway in vivo. Nucleic Acids Res. 2016;44:4174‐88. doi: 10.1093/nar/gkw072. PMID:26883629. [DOI] [PMC free article] [PubMed] [Google Scholar]


