Abstract
This review highlights the recent advances in our understanding of adipocyte contributions to carcinogenesis or cancer disease progression for cancers in the bone.
Purpose
In this review, we aim to describe bone marrow adipose tissue and discuss the soluble adipocyte-derived cytokines (adipokines) or endocrine factors, adipocyte-derived lipids, and the actual or putative juxtacrine signaling between bone marrow adipocytes and tumor cells in the bone marrow. This relationship likely affects tumor cell initiation, proliferation, metastasis, and/or drug resistance.
Recent Findings
Bone marrow adipose may affect tumor proliferation, drug resistance, or cancer-induced bone disease and hence may be a new target in the fight against cancer.
Summary
Overall, evidence is mixed regarding the role of bone marrow adipose and adipocytes in cancer progression, and more research in this arena is necessary to determine how these bone marrow microenvironmental cells contribute to malignancies in the marrow to identify novel, potentially targetable pathways.
Keywords: Cancer, Bone Marrow Adipocytes, Adipokine, Bone Marrow Adipose Tissue
1. Obesity, Adipose Tissue and Cancer
Many researchers are exploring the systemic links between obesity and cancer. Ties with systemic metabolic disorders, such as diabetes and pre-diabetes, make disentangling effects of obesity and systemic inflammation on cancer occurrence and progression a daunting task. It is becoming clear, however, that obesity and diabetes can accelerate cancer progression, tumor growth or drug resistance in the marrow. Specifically for multiple myeloma (MM), although individual studies have disagreed [1], the most recent pooled analysis of 20 prospective studies found clear associations between increased risk of death from MM in patients with a higher body mass index (BMI) during early adulthood, as well as higher BMI and higher waist circumferences at study entry [2]. In addition, Bredella et al. found that patients who were recently diagnosed with MM had higher abdominal fat cross-sectional areas and higher fat metabolic activity compared to patients with Monoclonal Gammopathy of Undetermined Significance (MGUS) [3]. Obesity in children is also associated with a 50% increased recurrence of acute lymphoblastic leukemia (ALL) in high-risk patients, and similarly, obesity in mouse ALL models increased the rate of relapse after monotherapy with vincristine [4]. Cell culture studies demonstrated that adipocyte-ALL co-cultures were able to model this response, demonstrating that the adipocyte itself, rather than systemic endocrine alterations exclusively, may contribute to tumor growth or drug resistance [4].
Obesity may also contribute to other bone cancers. Prostate cancer cells injected intratibially in mice fed a high fat diet (HFD) grew faster than the same cells injected into the tibia of mice on a low fat diet [5]. The authors confirmed that the HFD mice had profoundly increased marrow adipose tissue (MAT), and went on to demonstrate that the marrow adipocyte itself was a major contributor to the increased tumor growth in the mice, as further detailed below [5]. They also found that obesity led to increased growth of the cells when implanted subcutaneously, but observed that the genes upregulated in the tumor cells in intratibial injection model were different from those upregulated in the subcutaneous model, suggesting that effects of increased MAT caused unique, specific changes in tumor cells (e.g., increases in interleukin -1β (IL-1β), heme oxygenase 1 (HMOX-1), and the fatty acid chaperone fatty acid binding protein4 (FABP4) expression) [5]. Others have since confirmed both clinically and in mice that obesity also correlates with increased MAT [6], and hence researchers are now exploring if in obesity, the corresponding increased MAT may contribute to acceleration of malignancies in the bone marrow (BM) through localized actions within the microenvironment.
2. Bone Marrow Adipocytes are Distinct from White Adipocytes
To understand the diverse effects of bone marrow adipocytes (BMAs) on tumor progression, it is essential to examine the differences between BMAs and white adipocytes. White adipose tissue (WAT) is derived from Pref-1 positive mesenchymal progenitor cells, which are chameleon cells able to undergo cell division, apoptosis, or differentiation down an array of pathways [7]. Pref-1 appears to turn on even before peroxisome proliferator-activated receptor-γ (PPARγ) or zinc finger protein 423 (ZFP423) in adipogenic differentiation [7]. Like white adipocytes, BMAs also appear to derive from Pref-1 positive mesenchymal progenitor cells, but WAT and MAT are quite different. Although both produce adipokines, their secreted protein profiles and stress responses are unique. For example, while WAT shrinks during starvation, MAT expands in number and size of BMAs, perhaps due to evolutionary adaptations to starvation [8]. While in certain cases (e.g., obesity and ovariectomy) WAT and BMAT correlate [9], and both can be reduced with exercise [10], no relationship between WAT and MAT was found in type 2 diabetic patients [11]. MAT also increases with age [12,13] and mesenchymal stem cells (MSCs) from older individuals (rodent and human) are more prone to adipogenic differentiation rather than osteogenic or chondrogenic differentiation [14,15]. Therefore, as myeloma also correlates with aging, it is of great interest to determine if this relationship is correlative or also has a causative nature. Thus, due to the differences between WAT and MAT signaling and phenotype, and correlations between MAT and myeloma, we set out to describe what is known specifically about the effects of MAT on myeloma disease progression.
3. MAT Adipokines, Lipids, and Cell-Cell Contact Supports Cancers in the Bone Marrow
There are many ways in which adipokines from local bone marrow could potentially stimulate cancer progression and the development of drug resistant clones. A handful of studies in myeloma have found that MAT produces factors that support myeloma disease progression, although certain adipokines, such as adiponectin [16], have been found to inhibit myeloma, confounding the roles of MAT in myeloma [17,18]. Cell-derived factors (molecules, lipids, small metabolites, or microRNAs) may be free or embedded in exosomes or other microvesicles and transferred between BMAs and myeloma cells as one method of communication [19]. BMA factors that have been shown to support myeloma recruitment to the BM include monocyte chemotactic protein-1 (MCP-1) and stromal cell-derived factor-1a (SDF1α) [20]. BMA-derived leptin and adipsin have been shown to support chemotherapy survival through inducing autophagy and reducing apoptosis in myeloma cells [21].
Leptin appears to be an adipocyte-derived driver of MM proliferation. Caers et al. observed leptin-receptor expression on MM cells and they found that leptin increased myeloma cell proliferation [22]. They also analyzed biopsies of 50 newly-diagnosed MM patients using leptin receptor (LepR) immunohistochemistryand found strong immunoactivity in half of the biopsies. Although LepR expression did not correlate with prognostic measure or staging, progression-free survival after first therapy was longer, and drug response was better, in patients with weaker LepR expression [22]. Other researchers later confirmed that leptin induces myeloma proliferation. For example, in one study researchers analyzed levels of adipokines including leptin and adiponectin in 28 MM patients and identified significantly higher leptin compared with controls [23]. Leptin levels were positively correlated with clinical stage, bone marrow plasma cells percentage, and levels of immunoglobulin G (IgG), beta-2 microglobulin (β2MG), and erythrocyte sedimentation rate [23]. In MM cell and adipocyte co-culture, increased growth of myeloma cells and reduced toxicity of bortezomib were observed, along with increased expression of cyclinD1, B-cell lymphoma 2 (Bcl-2) and decreased caspase-3 expression. Phosphorylated protein kinase B (AKT) and Signal transducer and activator of transcription 3 (STAT3) were stimulated by leptin treatment and leptin signaling via these pathways induced myeloma cell proliferation [23]. Overall, it appears that leptin might be a potential therapeutic target for treating myeloma.
FABP4 also holds great promise as a signaling molecule between tumor and BM adipocytes. Upregulation of FABP4 has been linked to activation of the β-oxidation pathway in ovarian cancer, which has already been identified as a predominant source of energy for prostate cancer cells at the primary site and thus suggests that FABP4 is a driver of increased β-oxidation in the BM as well [24]. Interestingly, co-culture of ovarian cancer cells and white adipocytes leads to increased migration, homing, and invasion (stimulated in part by adipocyte-derived IL-8), and upregulation of FABP4 and the β-oxidation pathway in ovarian cells, with corresponding lipolysis in adipocytes [24]. Surprisingly, although FABP4 is a PPARγ target gene whose expression in adipocytes typically correlates with PPAR activation, PC3 cells treated with Adipo conditioned media in fact displayed a downregulation of PPARγ signaling, with significant decreases in mRNA and DNA binding activity of PPARγ. However, as the authors explain, FABP4 can also reside upstream of PPAR signaling and regulate the transcriptional activity of PPARγ by delivering nuclear ligands, so it is possible that the observed PPARγ downregulation is due to overstimulation of the receptor from excessive FABP4-transported ligands [5]. This would explain why treatment with the PPARγ antagonist GW9662 or an FABP4 inhibitor restored PPARγ signaling in these cells [5]. It is also possible that FABP4-mediated activation of c-Jun N-terminal kinase/mitogen activated protein kinase (JNK/MAPK) pathways or MAP kinase/mitogen-activated protein kinase (MEK/ERK)-mediated PPAR phosphorylation (established mechanisms of PPARy inactivation) were at play [5]. Therapeutically targeting metabolic pathways, PPAR signaling, or FABP4 itself in tumor cells are interesting areas of future research.
To understand the potential roles of MAT in MM, it is helpful to review what has been observed in other cancers (see Table 1 for an overview of effects of MAT in different cancer types). Herroon et. al demonstrated that conditioned media from murine BMAs (Adipo-CM) increased cell proliferation of certain prostate (C42B and PC3) and bone-metastatic breast cancer (MDA-231 BO) cell lines when grown in collagen I gels. Invasion was also increased in prostate and breast cancer cells exposed to Adipo-CM [5]. Interestingly, the group observed that certain breast and prostate cancer cells demonstrated Adipo-CM-derived lipid uptake using BIODIPY staining of lipid droplets [5] and increased expression of lipid droplet-associated genes (CD36, the lipid droplet marker Perilipin 2, and FABP4) when exposed to MAT-derived factors [5]. This expression of adipogenic genes in tumor cells, or “adipomimicy”, may have important ramifications for tumor cells. In vitro, Adipo-CM was also found to induce gene expression related to inflammation and oxidative stress (e.g., (Interleukin 1 Receptor Accessory Protein (IL1RAP), Chemokine (C-C motif) ligand 20 (CCL20), IL-1α, Hypoxia inducible factor-1α (HIF-1α), and Vascular endothelial growth factor (VEGF)) in PC3 tumor cells and some of these were also found to be upregulated in tumor cells in a high BMA microenvironment in vivo (HMOX-1, IL-1β, and FABP4) at both protein and gene levels [5]. Co-culture of PC3 cells with Adipo-CM and the FABP4 or IL-1β inhibitors demonstrated that these are the major factors in Adipo-CM driving increased invasion of tumor cells. In bone metastatic breast cancer cells (MDA-231BO), PPARy was induced via exposure to adipocyte CM and also led to upregulation of FABP4 and expression of (IL-1β and HMOX-1) that supported tumor cell growth and invasion [5]. Similarly, others have shown that BM-adipocytes support PC3 invasion and migration, and find that BMAs act as a source of the poly-unsaturated fatty acid (PUFA) arachidonic acid (AA), a potent stimulator of prostate cancer invasion [25].
Table 1.
The Roles of Adipocytes in an Array of Cancer Types
| Type of Cancer Cell | Adipocyte Roles | References |
|---|---|---|
| Multiple Myeloma | Adiponectin has an inhibitory effect. MAT appears to support myeloma proliferation and recruitment via MCP-1 and SDF1α. Leptin and Adipsin support chemoresistance & MM proliferation. |
16, 17, 28 18,20,21,22 |
| Breast Cancer | BMAs support proliferation, invasion, adipomimicry. | 5 |
| Prostate Cancer | BMAs support proliferation and invasion, adipomimicry. | 5 |
| Ovarian Cancer (Note: this is not BMA specific). | Adipocytes support migration, homing, and invasion in part via IL-8, FABP4, beta-oxidation. | 24 |
| Leukemia (ALL and AML) | Adipocytes support drug resistance, survival, proliferation. | 4,32 |
Abbreviations: Multiple Myeloma (MM) Marrow Adipose Tissue (MAT); Bone Marrow Adipocyte (BMA); monocyte chemotactic protein-1 (MCP-1) and stromal cell-derived factor-1a (SDF1α); acute lymphoblastic leukemia (ALL); acute monocytic leukemia (AML).
The role of BMAT in myeloma cell homing to the bone marrow is also greatly understudied. The adipokines leptin and IL-1β have been reported as significant chemoattractants for MDA-MB-231 breast cancer cells, but the role for IL-1β in myeloma-BMAT homing is yet unknown [26]. Caers et. al [22] have demonstrated the preference for myeloma cells (5T33MM) to migrate to BMA 14F1.1 and 3T3L1 adipocytes. They also found that many soluble factors are secreted by the murine 14F1.1 BM cell line using multi-analyte profile testing (interleukins: (IL-1 β, IL-6, IL- 10, IL-12p70, IL-18), oncostatin M, Vascular cell adhesion protein 1 (VCAM-1), Tumor necrosis factorα (TNFα), basic fibroblast growth factor (bFGF), Leukemia inhibitory factor (LIF), MCP-1, MCP-3, Macrophage Inflammatory Protein (MIP-1α, and MIP-1β)). Interestingly, some of these factors were much more highly expressed by 14F1.1 BMAs than MSCs, including leptin, stem cell factor, VEGF, fibroblast growth factor 9 (FGF-9), and MIP-1γ. However, the secretome from 14F1.1 may not accurately recapitulate the secretome from mouse or human MAT suggesting that future characterization of the secretome from MAT should be pursued. Caers et. al [22] also found that cell-cell contact between 14F1.1 adipocytes and myeloma cells resulted in increased proliferation of 5T2MM cells, and protection from apoptosis, whereas soluble factors had no effect, indicating that cell-cell contact-dependent mechanisms were required to induce survival in MM cells [22].
Diet-induced obesity has also been found to contribute to myeloma progression, potentially due to WAT and/or BMAT influences. Utilizing C57BL/6 mice, these scientists found that diet-induced obesity created a permissive environment for myeloma cell engraftment and growth, which was not present in chow-fed mice [27]. The team found that IL-6 was not altered in diet-induced obesity or ob/ob obese mice, but did find that obesity increased levels of insulin growth factor-1 (IGF-1) in diet-induced obesity mice [27]. Interestingly, no IGF-1 was detected in ob/ob mice, which suggested that IGF-1, a potent myeloma growth factor, was specific to diet-induced obesity. Edwards et al. also found that adiponectin can inhibit myeloma growth and associated osteolytic bone disease in vivo [16]. Building on this work, others have found that adiponectin-induced myeloma inhibition may be via Protein kinase A/AMP-activated protein kinase (PKA/AMPK) signaling [28]. Adiponectin is a circulating insulin-sensitizing hormone derived from both WAT and BMAT, and is increased in BMAT during caloric restriction, but unlike most adipokines, adiponectin is decreased in obese individuals. [29]. Thus adiponectin may hold potential for a treatment for obesity-associated myeloma.
There is high potential for MAT to induce drug resistance in myeloma cells, much like has been observed in ALL cells in response to adipocytes [4], via induction of two prosurvival signals: Bcl-2 and Proviral Integrations of Moloney virus 2 (Pim-2). Although the existence and mechanism of drug resistance induced in myeloma cells by MAT is currently unknown, it is possible that this is driven by chromatin modulation rather than by induced mutations. For example, MAT may induce epigenetic regulation of tumor cells, such as histone demethylation driven by the histone demethylate KDM5A/RBP2/Jarid1A, which has been shown to be important in drug tolerance in a few cell types, but which hasn’t been studied with MAT [30]. Indeed, bivalency and other chromosome methylation states have proven important for drug resistance in other cells and may be important in myeloma as well [31]. Our understanding of drug resistance and drug-resistant “persister cells” is truly a new field, and we are only now starting to understand the fluid nature of drug resistance, and the dependence on the microenvironment for this cell property [32]. More research into how MAT may induce MM cells to transition into a transient, pre-resistant state and how adding a drug may initiate a cellular reprogramming cascade to “burn-in” a stable, resistant phenotype is needed. Importantly, transient and genetic causes of resistance are not mutually exclusive and these resistance mechanisms may synergize. Further elucidating the plasticity of drug resistance and reprogramming capacity of tumor cells via their microenvironment will likely open new avenues for therapeutic targeting in myeloma and other cancers. It is important to consider that MAT would likely also be affected when pursuing epigenetic targets of tumor cells in the bone marrow, because epigenetic regulation also has a larget influence on adipocyte differentiation from bone marrow mesenchymal stem cells and on changes in mature adipocytes [33–39].
Drug resistance can also be imparted via alterations in cellular metabolism. Tabe et al. [40] found that BMAs support acute monocytic leukemia (AML) cells by regulating their metabolic energy balance. The scientists observed that MAT decreased AML cell spontaneous apoptosis by increasing their fatty acid β-oxidation (FAO), which reduced reactive oxygen species production. When FAO was blocked using Etomoxir in AML cells in co-culture with BMAs, the antiapoptotic effects of BMAs on AML cells was lost, indicating that the protection was due to increased FAO. BMA-induced FAO in AML also decreased integrated stress response mediator ATF4 and reactive oxygen species (ROS) production. Interestingly, they observed that FAO driven by BMA-derived fatty acids induced AML cells to exhibit adipomimicry by expressing adipocytic transcription factors PPARγ and FABP4.
Similarly, Shafat et al. found that leukemic cells induced BMAs to generate a protumor environment to support their survival and proliferation. [41] They also found that AML cells were able to induce FABP4 expression and phosphorylation of hormone-sensitive lipase, and hence lipolysis, in adipocytes. This led to the transfer of fatty acids from adipocytes to myeloma cells. They identified FABP4 expression as increased both in AML and BMA cells, and found that knocking this down in AML cells prevented their proliferation on BMAs in direct co-culture, but also increased survival, suggesting that dormancy or quiescence could be activated by BM adipocytes [41]. Similarly, 3T3-L1 adipocyte co-culture with mouse leukemic cells imparted resistance to the antileukemia drug vincristine, as well as three other chemotherapies. Interestingly, this protection was independent of cell-cell contact, and it extended to human leukemia cell lines as well. Adipocytes protected against chemotherapy-induced apoptosis and increased expression of the two prosurvival signals Bcl-2 and Pim-2 [4]. These data underscore the role of the adipocyte in fostering leukemic chemotherapy resistance; how similar the interactions between adipocytes and leukemic cells are to other cancer cells and their interactions with BMAs, remains to be determined. Clinically, the roles of bone marrow adipose in drug resistance are unknown and should be investigated.
4. Adipocyte and Adipokine Effects on Other Cells in the Tumor Niche
To analyze the effects of BMAs on myeloma progression, it is crucial to recognize that these cells also interact with other cells in the BM, which may cause indirect effects on myeloma or myeloma-induced bone disease. We have recently shown that the BM extracellular matrix (ECM) and MSC compartments [42] are significantly different between MM patients’ and healthy donors’ BM [42,43]. These alterations in the host microenvironment ultimately support tumor growth, bone disease, and drug resistance (for example, through induction of MUC1 as recently demonstrated by Bar-Natan et al. [44]), and it is very likely that MAT effects MSCs and the BM ECM. Furthermore, MAT has been found to accelerate osteoclastogenesis through secretion of Receptor activator of nuclear factor kappa-B ligand (RANKL) [45–47] as well as chemokine ligand 1 and 2 (CXCL1 and CXCL2) [48], which suggests that MAT may contribute to cancer-induced osteolysis via activation of osteoclast bone resorption. Other researchers have shown that osteoclasts play a key role in determining if a myeloma cell will remain dormant or proliferate [49]. Osteocytes also dictate myeloma cell fate and bone remodeling [50–52], and we have shown that targeting osteocyte-derived sclerostin is an effective way to combat myeloma-induced bone disease in mouse models as well as decrease MAT [53,54].
The effect of BMAs on bone and osteoblasts is an emerging research field. Although researchers have observed that osteoporosis, aging and anorexia, all conditions of bone loss, often correlate with increased MAT, the causative relationships here are still a relatively new realm of research [13]. Both clinically and in mice, it has been shown that MAT correlates with reduced bone strength and mass [55,56]. Many researchers have speculated that MAT impedes bone development due to direct effects on mature osteoblasts or MSCs, but direct evidence is lacking due to the challenges of specifically modulating MAT. Interestingly, many of the signaling pathways in MSCs that induce osteogenic differentiation inhibit adipogenic differentiation, such as Wnt [54,57] and parathyroid hormone (PTH) signaling [47,58]. Thus, increased adipogenesis may decrease the pool of progenitor cells able to undergo osteogenesis, which would decrease the number of osteoblasts in the niche and likely decrease bone volume fractions in cortical and trabecular bone regions. Local BMAs are currently believed to decrease bone mineral density and volume by inhibiting osteoblast function, which, if true, would further compound bone loss in osteolytic cancers. If MAT is inhibitory towards bone integrity and function, it is possible that decreased lipid content of MAT could be positive for bone health.
On the other hand, evidence also suggests that adipokines, such as leptin, can increase osteoblast differentiation [59,60]. Moreover, in certain diseases with increased MAT and bone disease, decreasing MAT using a PPARγ antagonist was not able to prevent bone loss [61]. Thus it remains unclear if MAT itself is detrimental to bones. Interestingly, the connection between MAT and bone strength may be reliant upon a third cellular compartment: the hematopoetic compartment. Naveiras et al. observed that fatless mice [62] (A-ZIP/F1 mice that had a dominant-negative form of CCAAT-enhancer-binding protein (C/EBP) under the FABP4 promotor) display increased trabecular bone in femurs compared to wild-type controls, after irradiation. The group demonstrated that MAT had a direct inhibitory effect on hematopoietic cells through the release of diffusible inhibitors of hematopoiesis in vitro [62]. They concluded, by comparing different regions of the spine in mice, that MAT-rich marrow is associated with lower absolute and relative numbers of hematopoietic progenitors. Also, MAT appeared to contain less hematopoetic cells in the replicating phases of the cell cycle (S/G2/M). Importantly, they found that preventing the formation of BMAs alone did not lead to osteogenesis, rather, osteogenesis was stimulated only upon ablation of both BM adipocytes and hematopoietic compartments [61,62]. Thus, there appeared to be a three-way co-regulation of hematopoiesis, osteogenesis and adipogenesis within the BM compartment, and this likely also governs the relationship between myeloma cells and other BM cells. However, later studies by the Morrison group found evidence that MAT may be the first line of support for the recovery of hematopoiesis after injury such as irradiation, via the secretion of molecules such as stem cell factor (SCF) to regrow blood vessels into the niche [48]. They conclude that BMAs are a niche component that promotes hematopoietic regeneration, but consequences for bone were not examined [48]. Thus, the role of BMAT is debatable even in non-cancerous BM niches. Overall, there is still controversy in the field as to the true effect of MAT on bone, because, despite the correlations between diseases with poor bone health and elevated MAT, MAT may in fact have the potential to act as a supportive tissue for failing bones and disrupted hematopoiesis [48].
The multitude of cell-cell relationships in the marrow confounds our understanding of the roles of these cells and requires even more detailed, controlled studies and experiments to unravel the full mystery of the cells in the BM. Importantly, changes in bone cells, vasculature, osteoclasts, and MSCs will alter immune cell populations and function, which will unavoidably have significant consequences on bone cells as well as myeloma cells, as reviewed elsewhere [63]. The effects of MAT on vasculogenesis may have large implications for MM, as we and others have shown the importance of the vessel content, endothelial cell structure, and adhesion ligands for myeloma extravasation and growth in the marrow [64–66]. There may also be a link between MAT and vasculogenesis [48], and tumor cells exposed to bone marrow adipocyte conditioned media overexpress VEGF [5]; these combined results suggest that MAT may contribute to tumor angiogenesis and thus progression in the bone. In sum, BMAT induces changes in cell composition of the marrow, which would thus indirectly affect a tumor cell’s ability to home to, colonize, and survive in the marrow.
5. Reciprocal Interactions: Tumor Cell Effects on BMAT
MAT-myeloma signaling is not a one-way street; it is essential to understand the influences of myeloma cells on MAT to completely understand their signaling relationship. Outside of the marrow, peritumoral adipocytes, sometimes known as cancer-associated adipocytes (CAAs) are evidenced to support tumor cells more than healthy adipocytes, so it is probable that tumor cells alter and hijack their neighboring adipocytes to transform them into more supportive stromal cells in the BM as well. This field is still developing, especially regarding BM-CAAs. Little is known about how BM-CAAs differ from healthy BMAs, in their own phenotype or in their support of tumor cells in the BM. However, there are data showing that CAAs contain less lipid content and/or undergo a de-differentiation process that significantly changes their phenotype, at least in co-culture with breast cancer cells [67] and prostate cancer cells [5,25]. In breast cancer, CAAs have been shown to have an “activated” phenotype, with higher expression of inflammatory cytokines such as IL-6 and IL-1β and proteases (matrix metalloproteinase-11 (MMP-11)) [67]. These CAAs also displayed decreased adipogenic marker expression and delipidation, perhaps due to lipid utilization or release [67]. Adipogenic markers such as FABP4, Resistin and Adiponectin were shown to be decreased in mouse MAT cultured in transwells with prostate cancer cells [5]. It is unclear how tumor cells affect differentiation of adipocytes, but transwell experiments suggest that a prostate-cancer cell derived soluble mediator may be responsible for the delipidation of CAAs [5]. Replacement of peritoneal adipose by tumor cells in ovarian cancer metastasis models has also been observed and is suggested to lead to a release of lipids to fuel tumor cell proliferation [68]. In sum, there appears to be bi-directional interaction between tumor cells and BMAs that suggests that by utilizing BMA-derived lipids, tumor cells may alter their own metabolic function, and that of fat cells in the bone marrow. Uncovering the specific mechanisms involved in this bi-directional interaction will be crucial in understanding more fully how tumor cells hijack their local BM niche and use it to fuel their proliferation, evolution, and survival.
6. Conclusions and Future Directions
Herein we focused on the effects of local BMAs on the progression of cancer in the marrow (Figure 1). Overall, there are a multitude of ways in which MAT and BMAs may contribute to tumor growth, and clinically targeting MAT is an interesting and innovative concept. It is possible that therapies that build bone may also inhibit MAT, as we have recently demonstrated with anti-sclerostin antibodies [54,57], and as others have found with PTH [47] and leptin [69]. The FGF family members may also hold promise for targeting to modulate not only bone, but also MAT. Exercise is capable of modulating MAT in obese mice [70] and mice treated with a PPARγ agonists, so it is possible that using exercise as a therapy in myeloma could inhibit MAT and increase bone strength [71]. As opposed to osteoporosis, targeting MAT in cancers of the BM may prove much more challenging as scientists and clinicians must be certain that their therapies do not inadvertently enhance tumor growth. Still, with more research in this area, better therapies to target MAT, or bone and MAT together, will likely emerge that do not accelerate tumor growth, as McDonald and our lab have recently demonstrated with anti-sclerostin antibodies pre-clinically [53]. Overall, it appears that adipocyte-cancer cell cross-talk has metabolic implications for both cellular compartments resulting in increased mitochondrial metabolism in tumor cells and enhanced lipolysis/delipidation in cancer-associated adipocytes that provides net pro-tumorigenic effects in most cases described. Currently, more research is needed specifically in determining the roles of BMAs in cancer to reveal unique therapeutic targets and treatment options for these typically incurable cancers.
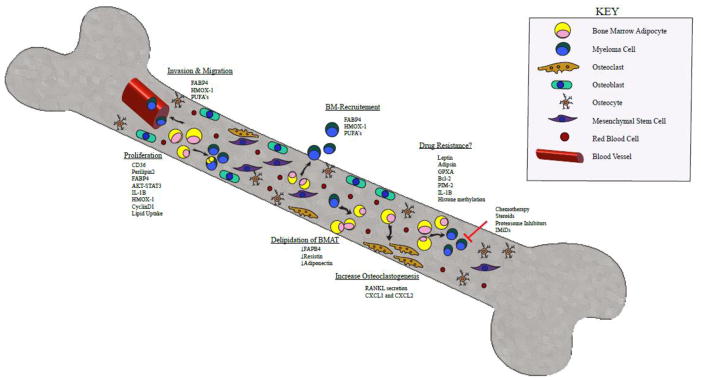
Figure 1. Multistep Signaling between Bone Marrow Adipocytes and Multiple Myeloma.
A schematic overview of the signals sent and received between BMAs and myeloma cells within the bone marrow microenvironment. MAT may promote MM progression, and MM cells may lead to MAT destruction. Signaling from MAT through FABP4, HMOX-1, and PUFAs promotes MM invasion and migration through blood vessel walls into the bone marrow. In addition, the same signals aid in MM recruitment to the bone marrow. MAT can also induce MM cell proliferation through multiple mediators such as CD36, perilipin2, FABP4, AKT-STAT3, IL-1β, HMOX-1, CyclinD1 and direct uptake of lipids. We hypothesize that MAT may play a role in MM drug resistance through mechanisms involving leptin, adipisin, GPXA, Bcl-2, PIM-2, IL-1β and modification of histone methylation. Not only can MAT influence MM cells, it has been shown to increase osteoclastogenesis through secretion of RANKL and CXCL1/CXCL2. Lastly, MM cells can induce MAT delipidation potentially by downregulating FABP4, adiponectin and resistin.
Abbreviations: BMA: Bone Marrow Adipocyte. FABP4: fatty acid binding protein 4; HMOX-1: Heme Oxygenase 1; PUFAs: polyunsaturated fatty acids; CD36: platelet glycoprotein 4; IL-1B: Interleukin 1 Beta; GPXA: glutathione peroxidase related gene; Bcl-2: B-cell lymphoma 2; PIM-2: Proviral Integrations of Moloney virus 2; RANKL: Receptor activator of nuclear factor kappa-B ligand; CXCL1/CXCL2: chemokine ligand 1 and 2.
Acknowledgments
The authors’ work is supported by MMCRI Start-up funds, a pilot project grant from NIH/NIGMS (P30GM106391), the NIH/NIDDK (R24DK092759-01), and the COBRE grant from the NIH/NIGMS (P20GM121301). This work was also funded in part by pilots from NIGMS/NIH P30 GM106391 and the American Cancer Society (Research Grant #IRG-16-191-33). This review was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number P30AR066261. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
Carolyne Falank, Heather Fairfield, and Michaela R. Reagan each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article contains no studies with human or animal subjects performed by any of the authors.
References
- 1.Beason TS, Chang S-H, Sanfilippo KM, Luo S, Colditz GA, Vij R, et al. Influence of Body Mass Index on Survival in Veterans With Multiple Myeloma. Oncologist. 2013;18:1074–9. doi: 10.1634/theoncologist.2013-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teras LR, Kitahara CM, Birmann BM, Hartge PA, Wang SS, Robien K, et al. Body size and multiple myeloma mortality: a pooled analysis of 20 prospective studies. Br J Haematol. 2014;166:667–76. doi: 10.1111/bjh.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *3.Veld J, O’Donnell EK, Reagan MR, Yee AJ, Torriani M, Rosen CJ, et al. Abdominal adipose tissue in MGUS and multiple myeloma. Skeletal Radiol. 2016;45:1277–83. doi: 10.1007/s00256-016-2425-4. This important research was the first to demonstrate a connection between abdominal adiposity and progression from MGUS to MM. [DOI] [PubMed] [Google Scholar]
- 4.Behan JW, Yun JP, Proektor MP, Ehsanipour EA, Arutyunyan A, Moses AS, et al. Adipocytes impair leukemia treatment in mice. Cancer Res. 2009;69:7867–74. doi: 10.1158/0008-5472.CAN-09-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herroon MK, Rajagurubandara E, Hardaway AL, Powell K, Turchick A, Feldmann D, et al. Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget. 2013;4:2108–23. doi: 10.18632/oncotarget.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devlin MJ, Rosen CJ. The bone-fat interface: basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol. 2015;3:141–7. doi: 10.1016/S2213-8587(14)70007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudak CS, Gulyaeva O, Wang Y, Park S-M, Lee L, Kang C, et al. Pref-1 marks very early mesenchymal precursors required for adipose tissue development and expansion. Cell Rep. 2014;8:678–87. doi: 10.1016/j.celrep.2014.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devlin MJ. Why does starvation make bones fat? Am J Hum Biol. 2011;23:577–85. doi: 10.1002/ajhb.21202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnamoorthy D, Frechette DM, Adler BJ, Green DE, Chan ME, Rubin CT. Marrow adipogenesis and bone loss that parallels estrogen deficiency is slowed by low-intensity mechanical signals. Osteoporos Int. 2015 doi: 10.1007/s00198-015-3289-5. [DOI] [PubMed] [Google Scholar]
- 10.Styner M, Thompson WR, Galior K, Uzer G, Wu X, Kadari S, et al. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone. 2014;64:39–46. doi: 10.1016/j.bone.2014.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Araújo IM, Salmon CEG, Nahas AK, Nogueira-Barbosa MH, Elias J, de Paula FJA. Marrow adipose tissue spectrum in obesity and type 2 diabetes mellitus. Eur J Endocrinol. 2017;176:21–30. doi: 10.1530/EJE-16-0448. [DOI] [PubMed] [Google Scholar]
- 12.Singh L, Brennan TA, Russell E, Kim J-H, Chen Q, Brad Johnson F, et al. Aging alters bone-fat reciprocity by shifting in vivo mesenchymal precursor cell fate towards an adipogenic lineage. Bone. 2016;85:29–36. doi: 10.1016/j.bone.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–71. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 14.Kretlow JD, Jin Y-Q, Liu W, Zhang WJ, Hong T-H, Zhou G, et al. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lecka-Czernik B, Rosen CJ, Kawai M. Skeletal aging and the adipocyte program: New insights from an “old” molecule. Cell Cycle. 2010;9:3648–54. doi: 10.4161/cc.9.18.13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **16.Fowler JA, Lwin ST, Drake MT, Edwards JR, Kyle RA, Mundy GR, et al. Host-derived adiponectin is tumor-suppressive and a novel therapeutic target for multiple myeloma and the associated bone disease. Blood. 2011;118:5872–82. doi: 10.1182/blood-2011-01-330407. This groundbreaking finding was the first to show that adiponectin has anti-myeloma properties. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falank C, Fairfield H, Reagan MR. Signaling Interplay between Bone Marrow Adipose Tissue and Multiple Myeloma cells. Front Endocrinol (Lausanne) 2016;7:67. doi: 10.3389/fendo.2016.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald MM, Fairfield H, Falank C, Reagan MR. Adipose, Bone, and Myeloma: Contributions from the Microenvironment. Calcif Tissue Int. 2016 doi: 10.1007/s00223-016-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soley L, Falank C, Reagan MR. MicroRNA Transfer Between Bone Marrow Adipose and Multiple Myeloma Cells. Curr Osteoporos Rep. 2017;15:162–70. doi: 10.1007/s11914-017-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trotter TN, Gibson JT, Sherpa TL, Gowda PS, Peker D, Yang Y. Adipocyte-Lineage Cells Support Growth and Dissemination of Multiple Myeloma in Bone. Am J Pathol. 2016;186:3054–63. doi: 10.1016/j.ajpath.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z, Xu J, He J, Liu H, Lin P, Wan X, et al. Mature adipocytes in bone marrow protect myeloma cells against chemotherapy through autophagy activation. Oncotarget. 2015;6:34329–41. doi: 10.18632/oncotarget.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *22.Caers J, Deleu S, Belaid Z, De Raeve H, Van Valckenborgh E, De Bruyne E, et al. Neighboring adipocytes participate in the bone marrow microenvironment of multiple myeloma cells. Leukemia. 2007;21:1580–4. doi: 10.1038/sj.leu.2404658. This seminal piece of research first drew attention to the potential for bone marrow adipose tissue to contribute to myeloma. [DOI] [PubMed] [Google Scholar]
- *23.Yu W, Cao D-D, Li Q, Mei H, Hu Y, Guo T. Adipocytes secreted leptin is a pro-tumor factor for survival of multiple myeloma under chemotherapy. Oncotarget. 2016;7:86075–86. doi: 10.18632/oncotarget.13342. This important manuscript demonstrates that leptin, derived from adipocytes, has tumor survival effects on myeloma cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown MD, Hart C, Gazi E, Gardner P, Lockyer N, Clarke N. Influence of omega-6 PUFA arachidonic acid and bone marrow adipocytes on metastatic spread from prostate cancer. Br J Cancer. 2010;102:403–13. doi: 10.1038/sj.bjc.6605481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Templeton ZS, Lie W-R, Wang W, Rosenberg-Hasson Y, Alluri RV, Tamaresis JS, et al. Breast Cancer Cell Colonization of the Human Bone Marrow Adipose Tissue Niche. Neoplasia. 2015;17:849–61. doi: 10.1016/j.neo.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **27.Lwin ST, Olechnowicz SWZ, Fowler JA, Edwards CM. Diet-induced obesity promotes a myeloma-like condition in vivo. Leukemia. 2015;29:507–10. doi: 10.1038/leu.2014.295. This important manuscript demonstrates that obesity can induce greater myeloma survival and proliferation in the bone marrow and that this may be via increased IGF-1. [DOI] [PubMed] [Google Scholar]
- 28.Medina EA, Oberheu K, Polusani SR, Ortega V, Velagaleti GVN, Oyajobi BO. PKA/AMPK signaling in relation to adiponectin’s antiproliferative effect on multiple myeloma cells. Leukemia. 2014;28:2080–9. doi: 10.1038/leu.2014.112. [DOI] [PubMed] [Google Scholar]
- 29.Sulston RJ, Learman BS, Zhang B, Scheller EL, Parlee SD, Simon BR, et al. Increased Circulating Adiponectin in Response to Thiazolidinediones: Investigating the Role of Bone Marrow Adipose Tissue. Front Endocrinol (Lausanne) 2016;7:128. doi: 10.3389/fendo.2016.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, et al. A Chromatin-Mediated Reversible Drug-Tolerant State in Cancer Cell Subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messier TL, Boyd JR, Gordon JAR, Stein JL, Lian JB, Stein GS. Oncofetal Epigenetic Bivalency in Breast Cancer Cells: H3K4 and H3K27 Tri-Methylation as a Biomarker for Phenotypic Plasticity. J Cell Physiol. 2016;231:2474–81. doi: 10.1002/jcp.25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaffer SM, Dunagin MC, Torborg SR, Torre EA, Emert B, Krepler C, et al. Rare cell variability and drug-induced reprogramming as a mode of cancer drug resistance. Nature. 2017;546:431–5. doi: 10.1038/nature22794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zych J, Stimamiglio MA, Senegaglia AC, Brofman PRS, Dallagiovanna B, Goldenberg S, et al. The epigenetic modifiers 5-aza-2′-deoxycytidine and trichostatin A influence adipocyte differentiation in human mesenchymal stem cells. Brazilian J Med Biol Res. 2013;46:405–16. doi: 10.1590/1414-431X20132893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer MB, Benkusky NA, Sen B, Rubin J, Pike JW. Epigenetic Plasticity Drives Adipogenic and Osteogenic Differentiation of Marrow-Derived Mesenchymal Stem Cells. n.d doi: 10.1074/jbc.M116.736538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fani N, Ziadlou R, Shahhoseini M, Baghaban Eslaminejad M. Comparative epigenetic influence of autologous versus fetal bovine serum on mesenchymal stem cells through in vitro osteogenic and adipogenic differentiation. Exp Cell Res. 2016;344:176–82. doi: 10.1016/j.yexcr.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Li G, Yao W, Jiang H. Short-Chain Fatty Acids Enhance Adipocyte Differentiation in the Stromal Vascular Fraction of Porcine Adipose Tissue. J Nutr. 2014;144:1887–95. doi: 10.3945/jn.114.198531. [DOI] [PubMed] [Google Scholar]
- 37.Rumberger JM, Arch JRS, Green A. Butyrate and other short-chain fatty acids increase the rate of lipolysis in 3T3-L1 adipocytes. PeerJ. 2014;2:e611. doi: 10.7717/peerj.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bricambert J, Favre D, Brajkovic S, Bonnefond A, Boutry R, Salvi R, et al. Impaired histone deacetylases 5 and 6 expression mimics the effects of obesity and hypoxia on adipocyte function. Mol Metab. 2016;5:1200–7. doi: 10.1016/j.molmet.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ali D, Alshammari H, Vishnubalaji R, Chalisserry EP, Hamam R, Alfayez M, et al. CUDC-907 Promotes Bone Marrow Adipocytic Differentiation Through Inhibition of Histone Deacetylase and Regulation of Cell Cycle. Stem Cells Dev. 2017;26:353–62. doi: 10.1089/scd.2016.0183. [DOI] [PubMed] [Google Scholar]
- 40.Tabe Y, Yamamoto S, Saitoh K, Sekihara K, Monma N, Ikeo K, et al. Bone Marrow Adipocytes Facilitate Fatty Acid Oxidation Activating AMPK and a Transcriptional Network Supporting Survival of Acute Monocytic Leukemia Cells. Cancer Res. 2017;77:1453–64. doi: 10.1158/0008-5472.CAN-16-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shafat MS, Oellerich T, Mohr S, Robinson SD, Edwards DR, Marlein CR, et al. Leukemic blasts program bone marrow adipocytes to generate a protumoral microenvironment. Blood. 2017;129:1320–32. doi: 10.1182/blood-2016-08-734798. [DOI] [PubMed] [Google Scholar]
- 42.Reagan MR, Mishima Y, Glavey SV, Zhang YY, Manier S, Lu ZN, et al. Investigating osteogenic differentiation in multiple myeloma using a novel 3D bone marrow niche model. Blood. 2014;124:3250–9. doi: 10.1182/blood-2014-02-558007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glavey SV, Naba A, Manier S, Clauser K, Tahri S, Park J, et al. Proteomic characterization of human multiple myeloma bone marrow extracellular matrix. Leukemia. 2017 doi: 10.1038/leu.2017.102. [DOI] [PubMed] [Google Scholar]
- 44.Bar-Natan M, Stroopinsky D, Luptakova K, Coll MD, Apel A, Rajabi H, et al. Bone marrow stroma protects myeloma cells from cytotoxic damage via induction of the oncoprotein MUC1. Br J Haematol. 2017;176:929–38. doi: 10.1111/bjh.14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan Y, Bi R, Densmore MJ, Sato T, Kobayashi T, Yuan Q, et al. Parathyroid hormone 1 receptor is essential to induce FGF23 production and maintain systemic mineral ion homeostasis. FASEB J. 2015 doi: 10.1096/fj.15-278184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takeshita S, Fumoto T, Naoe Y, Ikeda K. Age-related marrow adipogenesis is linked to increased expression of RANKL. J Biol Chem. 2014;289:16699–710. doi: 10.1074/jbc.M114.547919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan Y, Hanai J, Le PT, Bi R, Maridas D, DeMambro V, et al. Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate. Cell Metab. 2017;25:661–72. doi: 10.1016/j.cmet.2017.01.001. doi: http://dx.doi.org/10.1016/j.cmet.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou BO, Yu H, Yue R, Zhao Z, Rios JJ, Naveiras O, et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol. 2017;19:891–903. doi: 10.1038/ncb3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lawson MA, McDonald MM, Kovacic NN, Khoo WH, Terry RTL, Down J, et al. Osteoclasts Control Re-activation of Dormant Myeloma Cells by Remodeling the Endosteal Niche. Nat Commun. 2015;6:8983. doi: 10.1038/ncomms9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delgado-Calle J, Anderson J, Cregor MD, Hiasa M, Chirgwin JM, Carlesso N, et al. Bidirectional Notch signaling and osteocyte-derived factors in the bone marrow microenvironment promote tumor cell proliferation and bone destruction in multiple myeloma. Cancer Res. 2016;76:1089–100. doi: 10.1158/0008-5472.CAN-15-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giuliani N, Ferretti M, Bolzoni M, Storti P, Lazzaretti M, Dalla Palma B, et al. Increased osteocyte death in multiple myeloma patients: role in myeloma-induced osteoclast formation. Leukemia. 2012;26:1391–401. doi: 10.1038/leu.2011.381. [DOI] [PubMed] [Google Scholar]
- 52.Trotter TN, Fok M, Gibson JT, Peker D, Javed A, Yang Y. ASH Annu Meet Abstr. San Diego, CA: ASH Oral Presentation; 2016. Osteocyte Apoptosis Attracts Myeloma Cells to Bone and Supports Progression through Regulation of the Bone Marrow Microenvironment. #484. Session: 651. Myeloma: Biology and Pathophysiology. [Google Scholar]
- 53.McDonald MM, Reagan MR, Youlten SE, Mohanty ST, Seckinger A, Terry RL, et al. Inhibiting the osteocyte specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma. Blood. 2017 doi: 10.1182/blood-2017-03-773341. blood-2017-03-773341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fairfield H, Falank C, Harris E, Demambro V, McDonald M, Pettitt JA, et al. The skeletal cell-derived molecule sclerostin drives bone marrow adipogenesis. J Cell Physiol. 2017 doi: 10.1002/jcp.25976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheller EL, Rosen What’s the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci. 2014;1311:14–30. doi: 10.1111/nyas.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suchacki KJ, Cawthorn WP, Rosen CJ. Bone marrow adipose tissue: Formation, function and regulation. Curr Opin Pharmacol. 2016;28:50–6. doi: 10.1016/j.coph.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fairfield H, Rosen CJ, Reagan MR. Connecting Bone and Fat: the Potential Role for Sclerostin. Curr Mol Biol Reports. 2017;3:114–21. doi: 10.1007/s40610-017-0057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balani DH, Ono N, Kronenberg HM. Parathyroid hormone regulates fates of murine osteoblast precursors in vivo. J Clin Invest. 2017 doi: 10.1172/JCI91699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Philbrick KA, Wong CP, Branscum AJ, Turner RT, Iwaniec UT. Leptin stimulates bone formation in ob/ob mice at doses having minimal impact on energy metabolism. J Endocrinol. 2017;232:461–74. doi: 10.1530/JOE-16-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu J-C, Wu G-H, Zhou L-L, Yang X-J, Liu J-T. Leptin improves osteoblast differentiation of human bone marrow stroma stem cells. Eur Rev Med Pharmacol Sci. 2016;20:3507–13. [PubMed] [Google Scholar]
- 61.Botolin S, McCabe LR. Inhibition of PPARgamma prevents type I diabetic bone marrow adiposity but not bone loss. J Cell Physiol. 2006;209:967–76. doi: 10.1002/jcp.20804. [DOI] [PubMed] [Google Scholar]
- 62.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–63. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawano Y, Roccaro A, Azzi J, Ghobrial I. Multiple Myeloma and the immune microenvironment. Curr Cancer Drug Targets. 2017;17:1–1. doi: 10.2174/1568009617666170214102301. [DOI] [PubMed] [Google Scholar]
- 64.Moschetta M, Mishima Y, Sahin I, Manier S, Glavey S, Vacca A, et al. Role of endothelial progenitor cells in cancer progression. Biochim Biophys Acta. 2014;1846:26–39. doi: 10.1016/j.bbcan.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 65.Glavey SV, Manier S, Natoni A, Sacco A, Moschetta M, Reagan MR, et al. The sialyltransferase ST3GAL6 influences homing and survival in multiple myeloma. Blood. 2014;124:1765–76. doi: 10.1182/blood-2014-03-560862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moschetta M, Mishima Y, Kawano Y, Manier S, Paiva B, Palomera L, et al. Targeting vasculogenesis to prevent progression in multiple myeloma. Leukemia. 2016;30:1103–15. doi: 10.1038/leu.2016.3. [DOI] [PubMed] [Google Scholar]
- 67.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–65. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 68.Clark R, Krishnan V, Schoof M, Rodriguez I, Theriault B, Chekmareva M, et al. Milky spots promote ovarian cancer metastatic colonization of peritoneal adipose in experimental models. Am J Pathol. 2013;183:576–91. doi: 10.1016/j.ajpath.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yue R, Zhou BO, Shimada IS, Zhao Z, Morrison SJ. Leptin Receptor Promotes Adipogenesis and Reduces Osteogenesis by Regulating Mesenchymal Stromal Cells in Adult Bone Marrow. Cell Stem Cell. 2016;18:782–96. doi: 10.1016/j.stem.2016.02.015. [DOI] [PubMed] [Google Scholar]
- *70.Styner M, Pagnotti GM, McGrath C, Wu X, Sen B, Uzer G, et al. Exercise decreases Marrow Adipose Tissue though β-oxidation in Obese Running Mice. J Bone Miner Res. 2017 doi: 10.1002/jbmr.3159. This excellent work demonstrates that bone marrow adipose can be targeted with exercise, suggesting non-pharmacological ways to modulate bone marrow adipose tissue and providing new persepctives of how and why exercise may be so beneficial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Styner M, Pagnotti GM, Galior K, Wu X, Thompson WR, Uzer G, et al. Exercise Regulation of Marrow Fat in the Setting of PPARγ Agonist Treatment in Female C57BL/6 Mice. Endocrinology. 2015;156:2753–61. doi: 10.1210/en.2015-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]