Abstract
Neural stem cells (NSCs) are a valuable cell source for tissue engineering, regenerative medicine, disease modeling, and drug screening applications. Analogous to other stem cells, NSCs are tightly regulated by their microenvironmental niche, and prior work utilizing NSCs as a model system with engineered biomaterials has offered valuable insights into how biophysical inputs can regulate stem cell proliferation, differentiation, and maturation. In this review, we highlight recent exciting studies with innovative material platforms that enable narrow stiffness gradients, mechanical stretching, temporal stiffness switching, and three-dimensional culture to study NSCs. These studies have significantly advanced our knowledge of how stem cells respond to an array of different biophysical inputs and the underlying mechanosensitive mechanisms. In addition, we discuss efforts to utilize engineered material scaffolds to improve NSC-based translational efforts and the importance of mechanobiology in tissue engineering applications.
Graphical abstract
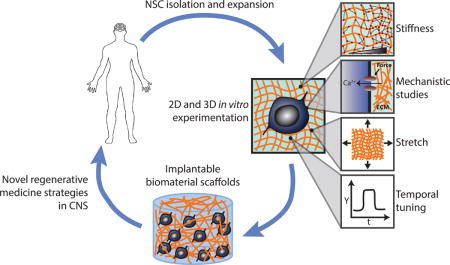
Introduction
Neural stem cells (NSCs) have been established as important mediators and effectors of plasticity, learning, and memory in the adult nervous system [1] and are envisioned as a potential source for transplantation in neurodegenerative diseases [2,3,4]. NSCs reside in two specific regions of the adult mammalian brain, the subventricular zone (SVZ) of the lateral ventricles and subgranular zone (SGZ) within the dentate gyrus of the hippocampus [1]. Hippocampal neural stem cells in particular have been indicated to play critical roles in learning, memory formation, behavioral regulation, and disease pathology, important processes that motivate a deeper basic understanding of NSC behavior [5]. Since the landmark discovery of these cells in mammals [6], the development of long-term NSC culture methods have enabled basic investigations of their behavioral regulation as well as exploration of their therapeutic potential to treat neurodegenerative disease, traumatic brain injury (TBI), spinal cord injury (SCI), and stroke [7].
NSCs, like other stem cells, are tightly regulated by the extracellular microenvironment within their resident tissues, collectively termed the stem cell niche [8]. Within these tissues, NSCs reside in proximity to more mature neural progenitor cell progeny, and it is possible to isolate and culture these populations. Throughout this review we will refer to cultured cells as “NSCs”, though a recognized caveat is that cultures may contain mixed populations. Previous research has revealed that biochemical cues present in the niche can strikingly direct NSC behavior in vitro and in vivo [9, 10]. However, biophysical and specifically mechanical cues have been more recently implicated as a potentially important but relatively poorly understood signal input for NSCs [11]. Early in vitro work showed that softer 2D substrates that more closely emulate brain tissue stiffness (< 1 kPa) promote neuronal differentiation of NSCs, whereas stiffer substrates (> 1 kPa) suppress neurogenesis [12,13], of strong potential interest given the presence of tissue stiffness gradients within the hippocampus [14]. However, the mechanisms that govern this behavior are progressively being elucidated, and further studies are needed to confirm whether NSCs are similarly mechanosensitive in 3D, in vivo contexts. Furthermore, NSCs encounter and are regulated by many other types of mechanical input besides stiffness during development, injury, or disease. Therefore, it is necessary to investigate impacts of these inputs is needed to assemble a more complete understanding of how biophysical cues regulate NSCs.
Within this field, biomaterials have not only played a role in enabling in vitro investigation of mechanobiology, but have also been harnessed as scaffolds to address common challenges in stem cell regenerative medicine such as inefficient expansion and differentiation, widespread death of transplanted cells, and limited homing to or retention in the desired site [15]. Scaffolds have a distinct advantage over the injection of dissociated “bolus” cell suspensions since it is possible to engineer “synthetic microenvironments” that support NSC survival and differentiation upon transplantation. Although scaffolds have shown promise in improving the engraftment of NSCs into the central nervous system (CNS), increased understanding of the mechanical effects of these scaffolds is again needed to enable precise tuning of NSC behavior [16].
In this review, we highlight recent studies in which innovative biomaterial systems have been engineered and exploited to further illuminate how NSCs respond to and process mechanical inputs. In addition, several relevant signaling mechanisms that respond to these material systems will be discussed, though a more thorough overview of NSC mechanotransductive pathways may be found elsewhere [17]. We begin by covering recent work describing novel effects of substrate stiffness and the impact of stretch stimuli on NSC fate commitment, neuronal maturation, and other cellular behaviors. We then discuss insights gleaned from a new generation of culture platforms that enable reversible tuning of substrate stiffness or incorporation of 3D architecture. Finally, we will discuss how engineered material systems are being used to improve translational strategies for neurodegenerative disease and neurological injury. While there have been important advancements in scaffold-based treatments in the spinal cord [18], this review will focus on recent efforts for application in the brain.
Biophysical regulation of NSCs
We and others have previously reported that substrate stiffness can specifically direct NSC fate commitment – soft substrates promote neurogenic differentiation of NSCs, while stiffer substrates suppress neurogenesis and increase astrocytic differentiation [13,19]. Moreover, we have identified Rho GTPase-mediated cytoskeletal dynamics and the transcriptional co-activator Yes-Associated Protein (YAP) as key players in stiffness-instructed NSC differentiation [20], and other work in the field has demonstrated the importance of focal adhesion proteins such as vinculin [21,22]. Adding to those findings, novel approaches have further explored the extent of NSC sensitivity to various biophysical inputs and the mechanisms that actuate mechanosensitive NSC behavior.
Substrate stiffness regulates NSCs through various intracellular signals
Although it is clear that substrate stiffness can strongly influence NSCs, many initial studies including some of our own [12] have primarily examined discrete stiffnesses that span orders of magnitude (e.g. 0.1 to 10 kPa). While such studies have clearly established that NSC proliferation and differentiation are mechanosensitive, the underlying experimental systems do not precisely elucidate the continuous, dynamic impact of stiffness on NSC behavior. Mosley et al addressed this shortcoming by using a hydrogel that was engineered to have a continuous stiffness gradient [23]. Excising small circular portions down the length of this initial material generated gels with much finer relative differences in stiffness than those reliably achieved by conventional methods. Using these gels, they found that neurites from human induced pluripotent stem cell-derived NSCs cultured in neural differentiation media for 14 days were significantly longer on 0.9 kPa gels than on 1.44 kPa gels and that the expression of neuronal markers Tuj1 and MAP2 were affected by small differences in stiffness that could be missed in typical studies. Differences in neurite extension suggest differences in underlying cytoskeletal organization, which is a key mechanosensitive response in stem cells. Although many studies have identified broad regimes of stiffness that promote certain NSC behavior (e.g. stiffness < 1 kPa as measured by shear rheology or AFM promotes neurogenesis [12,19,24]), this study strikingly implies that NSCs are much more finely tuned to stiffness cues than previously appreciated. An important general caveat with these studies is that various methods such as shear rheology or AFM are used to measure the elastic moduli of culture substrates, which does limit the extent to which measurements can be quantitatively compared between reports.
Previous studies have identified specific mechanosensitive molecules such as integrin and CD44 cell surface receptors that translate stiffness cues into intracellular signaling cascades in both stem and non-stem cells [25,26]. Continuing work has progressively revealed important new pathways that can direct stem cell fate commitment in response to stiffness input. For example, a growing body of evidence has indicated that intracellular calcium can regulate stem cell behavior [27,28] via mechanisms that are only starting to be investigated. In a recent study [29], Pathak and colleagues reported that Piezo1 (stretch-activated cationic channel) was expressed in human fetal-derived cortical NSCs and could induce spontaneous calcium influxes in a stiffness-dependent manner such that stiffer silicone elastomeric substrates (3.7 and 750 kPa) elicited greater Ca2+ activity compared to softer substrates (0.4 and 0.7 kPa). Notably, and contrary to earlier studies, the authors reported increased neuronal differentiation on stiffer substrates. They attributed this unexpected finding to the difference in species, tissue, and maturation state of the cells used, and supported this interpretation by successfully replicating findings from previous reports. In addition, they found that RNAi-mediated knockdown of Piezo1 resulted in inhibition of YAP, which is normally active on stiffer substrates, and that knockdown or chemical inhibition of Piezo1 increased astrocytic differentiation and decreased neurogenesis in hNSCs. Therefore, Piezo1 and other mechanically-gated ionic channels may be critical regulators of stiffness-mediated NSC differentiation that impinge on known and yet undiscovered mechanosensitive pathways.
Mechanical stretch and NSCs
In addition to substrate elastic modulus and stiffness, stem cells encounter many other mechanical inputs that can affect their behavior [30,31,32]. In MSCs, stretch stimuli influence gene expression and fate commitment to promote osteogenic and myogenic differentiation [33,34,35,36]. Further insight has revealed that stretch-induced responses can be age-dependent [37], induce differentiation in the absence of growth factors [38], and be orchestrated by the TGF-β signaling pathway [39]. Mechanical stretching is also an important regulatory cue for NSCs, as they encounter a plethora of active forces during tissue morphogenesis and due to cyclic deformation of vasculature by blood flow. Arulmoli et al recently found that a 10% static equibiaxial strain significantly reduced oligodendrocyte fate commitment (Figure 1A, B), whereas neurogenesis and astrogenesis were unaffected [40], representing the first report that static stretch can impact NSC behavior in vitro. Importantly, the authors included a “prestretched” condition in which cells were seeded after substrate stretching to distinguish between the effects of the stretch stimulus and the ~100-fold increase in stiffness induced by the stretch (1.6 MPa vs 10 kPa). Interestingly, they found that stiffer (i.e. pre-stretched) substrates significantly increased oligodendrocytic differentiation, but a single stretch stimulus applied during the early onset of differentiation reversed this effect, even though the ultimate stiffness was the same in both cases. This approach elegantly illustrates that different mechanical stimuli can direct NSC differentiation in varied ways, potentially via different signaling cascades. This report also further supports other studies suggesting that the timing of mechanical stimuli is significantly important in stem cell fate commitment [41,20].
Figure 1.
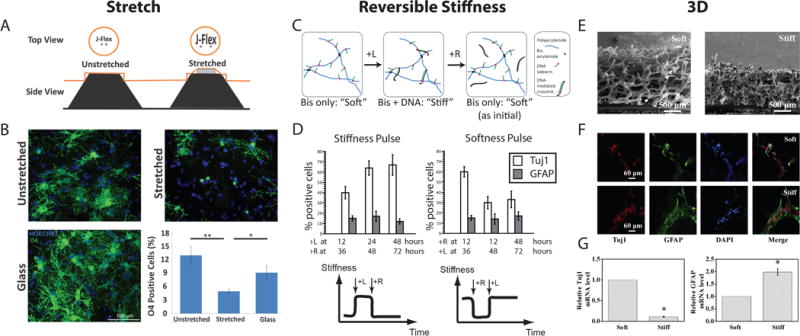
Novel material platforms have been used to investigate NSC mechanosensitive differentiation. The “J-Flex”, a tension-based stretch culture system (A), shows that a stretch cue decreases oligodendrocytic differentiation (O4 marker staining) and is independent from stiffness inputs (B). A DNA-crosslinked polyacrylamide gel system enables reversible stiffness tuning (C), which reveals a 12–36 hour time window for mechanosensitive fate commitment in NSCs (D). Stiffer graphene-based 3D materials (E) decrease neurogenesis (Tuj1) and increase astrocytic differentiation (GFAP) as measured by staining (F) and qPCR (G). Reproduced with permission from [33], [16], and [57].
Importantly, stretch is a relevant mechanical stimulus for modeling traumatic brain injury (TBI) in vitro. In fact, stretch inputs are among the most widely used in vitro models for the initial mechanical injury in TBI [42], and simple stretch injury culture systems have already offered insights into TBI disease pathology and drug screening [43,44,45]. Victims of TBI unfortunately often suffer from cognitive, behavioral, and memory problems, functions that are at least partially modulated by hippocampal NSCs [46]. Therefore, in combination with other platforms that modulate other mechanical inputs relevant to TBI, such as shear stress, a stretch-induced in vitro model of TBI may represent a valuable additional system to study NSC mechanobiology in a pathological context.
Recent advanced materials to probe and control NSC mechanotransduction
In vitro platforms to study stem cell mechanobiology have largely used two-dimensional, irreversibly crosslinked polymeric substrates such as collagen, hyaluronic acid (HA), polyacrylamide, and polydimethylsiloxane (PDMS). While these studies have been extremely useful for studying NSC mechanotransduction, recent developments in materials design – particularly ones enabling dynamic stiffness tuning and three-dimensional culture – are expanding our experimental capabilities and understanding of how NSCs are controlled by their physical environments.
Temporally tunable mechanics
During development, the dynamic activity of precise intracellular pathways in response to extracellular cues is crucial for proper morphogenesis [47]. In addition, cell migration can expose stem cells and their progeny to a range of mechanical environments. Importantly, the timing of mechanical cues and cellular responses has been shown to strongly influence MSC mechanosensitive behavior using hydrogels that can either be irreversibly softened or stiffened after initial cell seeding [48,49]. These materials have yielded important insights, such as temporal “points of no return” beyond which differentiation is no longer mechanosensitive. That said, the development of materials capable of reversible stiffening or softening could open access to an entirely new set of questions, such as the importance of the onset timing and duration of exposure to a given stiffness in guiding fate determination. To address this need, our group adopted a DNA-crosslinking approach [50] to develop a hydrogel that can be reversibly softened and re-stiffened, or vice versa (Figure 1C), over an order of magnitude spanning a relevant stiffness range for NSCs (0.3 – 3 kPa) [20]. Using this system, we identified a “mechanosensitive time window” of 12–36 hours after initiation of hippocampal NSC differentiation, such that stiffness cues only within this time window instructed neuro/astrocytic fate commitment, analyzed after 6 days of differentiation (Figure 1D). Furthermore, we found that YAP activity mediated fate commitment within this early time window, though via a mechanism distinct from its reported role in mechanosensitive MSC differentiation. Specifically, YAP inhibited β-catenin – a neurogenic transcription factor and Wnt signaling pathway effector – rather than functioning via canonical YAP/TEAD-mediated gene regulation in the nucleus as suggested for MSCs. These findings indicate that the temporal dynamics of mechanotransductive signaling, both extracellularly and intracellularly, are critical to understanding how stem cells respond to their physical microenvironment. Temporally dynamic materials exemplified by this study and others [51,52,53] are powerful tools that can enable innovative investigations into stem cell mechanobiology.
Three dimensional materials
Although 2D hydrogels have immense value in the study of cellular behavior, they lack the 3D architectures of endogenous tissue niches, which are expected to strongly influence cell behavior [54]. To address this issue in other systems, a variety of natural and synthetic 3D culture systems have been developed [55,56,57,58,59]. 3D hydrogels have also been recently used to study biophysical regulation of encapsulated NSCs. In the first such study [60], we previously found that encapsulation in softer alginate gels (183 Pa) promoted NSC proliferation and neurogenesis compared to all other stiffnesses tested (1, 1.7, 19.7 kPa). These results were supported in a later study [61] in which others reported increased expression of neuronal marker β-III tubulin on 1.5 kPa vs. 7.2 kPa 3D HA hydrogels. Notably, both studies cultured NSCs in the absence of any differentiation-inducing factors, demonstrating that mechanical cues can potently direct NSC differentiation in 3D.
A recently introduced three-dimensional graphene foam (“3D-GF”) could also support NSC culture, proliferation, and differentiation into neurons and astrocytes (Figure 1E, F). In addition, as a carbon nanomaterial, 3D-GFs potentially allow precise incorporation and actuation of topographical and electrical cues. Indeed, 3D-GFs were shown to increase NSC differentiation compared to 2D graphene films and act as an electrically conductive scaffold [62]. In a follow-up study [63], stiffer 3D-GFs (64 kPa) promoted cell adhesion and proliferation compared to softer 3D-GFs (30 kPa), as measured by qPCR and western blotting (WB) of functional markers (e.g. vinculin and integrin for adhesion, Ki67 for proliferation). Strikingly, NSCs displayed similar stiffness-sensitive differentiation as previously reported in 2D studies: stiff substrates suppressed mRNA expression of neuronal marker Tuj1 by 10-fold while promoting a 2-fold increase in astrocytic marker GFAP expression (Figure 1G). This finding is consistent with a mechanism in which the pores of the 3D-GFs present quasi-2D culture surfaces. Although 3D materials can be challenging to integrate with conventional assays due to the decreased accessibility of the cells (e.g. lysate collection for western blotting or RNA isolation for qPCR), these systems offer novel insights into how the biophysical microenvironment affects NSC behavior in microenvironments that capture defining features of the in vivo niche.
Engineered materials for improved neural tissue engineering
Biomaterial scaffolds currently represent one of the most promising approaches to successfully implant NSCs and neurons in the CNS. In an exciting recent study, iPSC-derived neurons implanted within hippocampal slices exhibited enhanced neurite outgrowth and electrical excitability when delivered in microscale fibrous 3D scaffolds [64]. When transplanted into mice, scaffold-supported cells also survived more frequently than transplanted dissociated cells (5.74% vs. 0.15%). Crucially, post-synaptic density protein 95 (PSD95) was detected adjacent to transplanted cells expressing pre-synaptic protein synaptophysin, indicating integration of the transplant with the host neural network. Although increased physical protection and the presence of an adherent substrate are likely at least partially responsible for the improved performance of scaffold-supported cellular transplants in this study, its findings indicate that maintaining the proper biomechanical context may be an important factor for successful cell- based therapy in the CNS. Indeed, other studies have demonstrated that the stiffness of 3D scaffolds can influence stem cell behavior [65, 66].
Successfully delivering NSCs within a physical scaffold is not trivial. The ideal substrate should accurately mimic the biochemical and mechanical properties of endogenous tissue, support NSC survival, promote appropriate differentiation and neurite outgrowth, and ultimately guide functional integration [67]. Significant cell death or damage due to shear forces during injection is a significant difficulty for scaffold-based tissue engineering strategies in the CNS. To tackle these challenges, researchers have developed injectable hydrogels that are biocompatible, can be readily co-delivered with cell populations, and gel in situ [68,69,70]. While such injectable gels have some disadvantages compared to other scaffold strategies, including the inability to precisely define nano- and micro-topography, they do have specific advantages such as robust protection against shear forces during injection and the ability to co-deliver the gel precursor and cells in a minimally invasive fashion. Recent studies have gone beyond feasibility testing of injectable gels with NSCs and towards optimization, demonstrating the exciting potential of this approach. For example, Farrell et al [71] explored a variety of 3D gel formulations composed of natural ECM components that were physically crosslinked as a means to drive increased NSC survival and function with a more natural microenvironment. By comparing various gel compositions that included type I collagen, HA, chondroitin sulfate proteoglycan, and laminin, they found that pure 1% w/v HA gels promoted neuronal differentiation and neurite outgrowth to a greater extent than other gel compositions, and that these effects could be moderated by the cell surface HA receptor CD44 and β1 integrin. In another recent study [72], Wei et al delivered NSCs with self-healing polysaccharide-based hydrogels, which involved injecting cell-loaded gel fragments that subsequently coalesced within the injection site [73,74]. Their engineered material robustly supported NSC survival and proliferation while eliciting increased neurogenesis compared to 2D culture controls. However, cells that stained positive for Tuj1 in the self-healing gels did not extend neurites like those on 2D substrates, potentially highlighting the importance of tuning mechanical cues to promote not only optimal differentiation but also functional morphology.
We have recently reported that a rationally-designed 3D HA scaffold could support the maturation and implantation of fragile midbrain dopaminergic (mDA) neurons differentiated from hESCs [75]. The HA scaffold offered distinct advantages over culturing cells in 2D or injecting a dissociated cell suspension, including brain-mimetic mechanical tuning (350 Pa), conjugation of chemical factors that promote cellular adhesion and neurite extension (RGD and heparin), and physical protection during injection. In general, NSC mechanobiology in such translatable contexts has not yet been exhaustively studied, but these recent findings demonstrate exciting potential for materials-based approaches to replacing lost neural populations and aiding in functional recovery. Importantly, these studies reported the stiffness of these implantable scaffolds to be within the neurogenic range as determined by earlier studies (0.1 – 1 kPa), indicating that basic research into NSC mechanobiology can directly inform the effective design of materials for translational tissue engineering applications.
Conclusions and future outlook
In the 25 years since the discovery that NSCs mediate the continuous integration of new neurons into the adult brain, there have been significant advances in our understanding of how extracellular cues from the niche control NSC behavior in vivo and in vitro. Originating in embryonic development and persisting though adult neurogenesis, NSCs are regulated by a complex repertoire of soluble and physical cues from the microenvironment. While previous and ongoing studies have investigated the effect of biochemical cues, recent discoveries of the significance of mechanical input are further enriching our understanding of stem cell regulation. A major next step will be to understand how these two classes of inputs are integrated to direct NSC behavior under physiological conditions, as well as how they can go awry in disease or injury. Encouragingly, there have already been studies that have reported the synergistic effect of tuned matrix stiffness and biochemical stimulation in MSCs [76, 77]. However, current culture platforms preclude high-throughput study of the additional parameter space. Towards addressing this challenge, various groups including ours [78] are beginning to develop next-generation materials to enable precise spatial patterning of multiple mechanical and biochemical parameters, such as stiffness and ECM ligand density [79]. Mechanistically, these various extracellular signals are ultimately transduced by biochemical signaling cascades to control cell behavior, but the complexity of these pathways render them challenging to dissect with conventional candidate-based genetic tools. In addition to candidate approaches, genome-wide screening including powerful approaches based on CRISPR/Cas9 [80,81] may enable further advances. Coupled with powerful tools such as next generation sequencing, such technologies may aid in the identification of novel genes that are crucial for mechanosensitive NSC behavior, guiding future advancements in tissue engineering and regenerative medicine applications in the CNS.
Highlights.
Neural stem cells (NSCs) are a promising resource for tissue engineering and regenerative medicine
Various mechanical cues can affect NSC differentiation, proliferation, and maturation
Biomaterial advancements are driving new discoveries in NSC mechanotransduction
Understanding biophysical regulation of NSCs is crucial for designing novel cell replacement strategies
Acknowledgments
The authors gratefully acknowledge support from the NIH (5R01NS074831 to D.V.S. and S.K; R21EB025017 and R21EB016359 to S.K) and CIRM (RT3-07800 to D.V.S. and S.K., and DISC2-08982 to D.V.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lledo P-M, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. [Internet] Nat Rev Neurosci. 2006;7:179–93. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 2.Gage FH, Temple S. Neural stem cells: Generating and regenerating the brain. Neuron. 2013;80:588–601. doi: 10.1016/j.neuron.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 3.Kim SU, Lee HJ, Kim YB. Neural stem cell-based treatment for neurodegenerative diseases. Neuropathology. 2013;33:491–504. doi: 10.1111/neup.12020. [DOI] [PubMed] [Google Scholar]
- 4.Bonnamain V, Neveu I, Naveilhan P. Neural stem/progenitor cells as a promising candidate for regenerative therapy of the central nervous system [Internet] Front Cell Neurosci. 2012;6:17. doi: 10.3389/fncel.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rolando C, Taylor V. Neural Stem Cell of the Hippocampus. Development, Physiology Regulation, and Dysfunction in Disease. Curr Top Dev Biol. 2014;107:183–206. doi: 10.1016/B978-0-12-416022-4.00007-X. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. [Internet] Science (80−) 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 7.Martino G, Pluchino S. The therapeutic potential of neural stem cells. [Internet] Nat Rev Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 8.Keung AJ, Kumar S, Schaffer DV. Presentation Counts: Microenvironmental Regulation of Stem Cells by Biophysical and Material Cues [Internet] Annu Rev Cell Dev Biol. 2010;26:533–556. doi: 10.1146/annurev-cellbio-100109-104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams BP, Park JK, Alberta JA, Muhlebach SG, Hwang GY, Roberts TM, Stiles CD. A PDGF-Regulated Immediate Early Gene Response Initiates Neuronal Differentiation in Ventricular Zone Progenitor Cells. Neuron. 1997;18:553–562. doi: 10.1016/s0896-6273(00)80297-4. [DOI] [PubMed] [Google Scholar]
- 10.Lie D-C, Colamarino SA, Song H-J, Désiré L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 11.Reekmans K, Praet J, Daans J, Reumers V, Pauwels P, van der Linden A, Berneman ZN, Ponsaerts P. Current Challenges for the Advancement of Neural Stem Cell Biology and Transplantation Research. Stem Cell Rev Reports. 2012;8:262–278. doi: 10.1007/s12015-011-9266-2. [DOI] [PubMed] [Google Scholar]
- 12.Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. Substrate Modulus Directs Neural Stem Cell Behavior [Internet] Biophys J. 2008;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leipzig ND, Shoichet MS. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials. 2009;30:6867–6878. doi: 10.1016/j.biomaterials.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Luque T, Kang MS, Schaffer DV, Kumar S. Microelastic mapping of the rat dentate gyrus. R Soc open Sci. 2016;3:150702. doi: 10.1098/rsos.150702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi C, Yan X, Huang C, Melerzanov A, Du Y. Biomaterials as carrier, barrier and reactor for cell-based regenerative medicine. Protein Cell. 2015;6:638–653. doi: 10.1007/s13238-015-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skop NB, Calderon F, Cho CH, Gandhi CD, Levison SW. Improvements in biomaterial matrices for neural precursor cell transplantation. [Internet] Mol Cell Ther. 2014;2:19. doi: 10.1186/2052-8426-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stukel JM, Willits RK. Mechanotransduction of Neural Cells Through Cell-Substrate Interactions [Internet] Tissue Eng Part B Rev. 2016;22:173–182. doi: 10.1089/ten.teb.2015.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsintou M, Dalamagkas K, Seifalian AM. Advances in regenerative therapies for spinal cord injury: A biomaterials approach. Neural Regen Res. 2015;10:726–742. doi: 10.4103/1673-5374.156966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keung AJ, De Juan-Pardo EM, Schaffer DV, Kumar S. Rho GTPases mediate the mechanosensitive lineage commitment of neural stem cells. Stem Cells. 2011;29:1886–1897. doi: 10.1002/stem.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **20.Rammensee S, Kang MS, Georgiou K, Kumar S, Schaffer DV. Dynamics of Mechanosensitive Neural Stem Cell Differentiation [Internet] Stem Cells. 2016 doi: 10.1002/stem.2489. This study developed an improved DNA-crosslinked polyacrylamide hydrogel culture platform that enables reversible stiffness-switching. With this system, a 12–36 hour time window was discovered in which NSCs were most sensitive to stiffness inputs following induction of differentiation. During this time window, stiff matrices increase levels of YAP, which inhibits neurogenesis by binding and antagonizing β-catenin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holle AW, Tang X, Vijayraghavan D, Vincent LG, Fuhrmann A, Choi YS, Del Alamo JC, Engler ÁJ. In situ mechanotransduction via vinculin regulates stem cell differentiation. Stem Cells. 2013;31:2467–2477. doi: 10.1002/stem.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gautrot JE, Malmström J, Sundh M, Margadant C, Sonnenberg A, Sutherland DS. The nanoscale geometrical maturation of focal adhesions controls stem cell differentiation and mechanotransduction. Nano Lett. 2014;14:3945–3952. doi: 10.1021/nl501248y. [DOI] [PubMed] [Google Scholar]
- *23.Mosley MC, Lim HJ, Chen J, Yang Y-H, Li S, Liu Y, Smith Callahan LA. Neurite extension and neuronal differentiation of human induced pluripotent stem cell derived neural stem cells on polyethylene glycol hydrogels containing a continuous Young’s Modulus gradient [Internet] J Biomed Mater Res Part A. 2017;105:824–833. doi: 10.1002/jbm.a.35955. This report describes a unique approach to fabricate hydrogels with very fine differences in stiffness to precisely interrogate NSC mechanotransduction. The authors demonstrate that the extent of neurogenesis and neurite growth were sensitive to these small differences, implicating that NSCs are very finely tuned to stiffness input. [DOI] [PubMed] [Google Scholar]
- 24.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 25.Lv H, Li L, Sun M, Zhang Y, Chen L, Rong Y, Li Y. Mechanism of regulation of stem cell differentiation by matrix stiffness. [Internet] Stem Cell Res Ther. 2015;6:103. doi: 10.1186/s13287-015-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim Y, Kumar S. CD44-mediated adhesion to hyaluronic acid contributes to mechanosensing and invasive motility. [Internet] Mol Cancer Res. 2014;12:1416–29. doi: 10.1158/1541-7786.MCR-13-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buonanno A, Fields RD. Gene regulation by patterned electrical activity during neural and skeletal muscle development. Curr Opin Neurobiol. 1999;9:110–120. doi: 10.1016/s0959-4388(99)80014-2. [DOI] [PubMed] [Google Scholar]
- 28.Kawano S, Shoji S, Ichinose S, Yamagata K, Tagami M, Hiraoka M. Characterization of Ca2+ signaling pathways in human mesenchymal stem cells. Cell Calcium. 2002;32:165–174. doi: 10.1016/s0143416002001240. [DOI] [PubMed] [Google Scholar]
- 29.Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Le DTT, Bernardis E, Flanagan LA, Tombola F. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells [Internet] Proc Natl Acad Sci. 2014;111:16148–16153. doi: 10.1073/pnas.1409802111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conway A. Biophysical regulation of stem cell behavior within the niche [Internet] Stem Cell Res Ther. 2012;3:50. doi: 10.1186/scrt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yim EKF, Sheetz MP. Force-dependent cell signaling in stem cell differentiation. Stem Cell Res Ther. 2012;3:41. doi: 10.1186/scrt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Angelo F, Tiribuzi R, Armentano I, Kenny JM, Martino S, Orlacchio A. Mechanotransduction: Tuning Stem Cells Fate [Internet] J Funct Biomater. 2011;2:67–87. doi: 10.3390/jfb2020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurpinski K, Chu J, Hashi C, Li S. Anisotropic mechanosensing by mesenchymal stem cells [Internet] Proc Natl Acad Sci. 2006;103:16095–16100. doi: 10.1073/pnas.0604182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurpinski K, Chu J, Wang D, Li S. Proteomic Profiling of Mesenchymal Stem Cell Responses to Mechanical Strain and TGF-beta1 [Internet] Cell Mol Bioeng. 2009;2:606–614. doi: 10.1007/s12195-009-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim IS, Song YM, Hwang SJ. Osteogenic responses of human mesenchymal stromal cells to static stretch. [Internet] J Dent Res. 2010;89:1129–34. doi: 10.1177/0022034510375283. [DOI] [PubMed] [Google Scholar]
- 36.Ghazanfari S, Tafazzoli-Shadpour M, Shokrgozar MA. Effects of cyclic stretch on proliferation of mesenchymal stem cells and their differentiation to smooth muscle cells [Internet] Biochem Biophys Res Commun. 2009;388:601–605. doi: 10.1016/j.bbrc.2009.08.072. [DOI] [PubMed] [Google Scholar]
- 37.Tan J, Xu X, Tong Z, Lin J, Yu Q, Lin Y, Kuang W. Decreased osteogenesis of adult mesenchymal stem cells by reactive oxygen species under cyclic stretch: a possible mechanism of age related osteoporosis. [Internet] Bone Res. 2015;3:15003. doi: 10.1038/boneres.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothdiener M, Hegemann M, Uynuk-Ool T, Walters B, Papugy P, Nguyen P, Claus V, Seeger T, Stoeckle U, Boehme KA, et al. Stretching human mesenchymal stromal cells on stiffness-customized collagen type I generates a smooth muscle marker profile without growth factor addition [Internet] Sci Rep. 2016;6:35840. doi: 10.1038/srep35840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li R, Liang L, Dou Y, Huang Z, Mo H, Wang Y, Yu B. Mechanical stretch inhibits mesenchymal stem cell adipogenic differentiation through TGFβ1/Smad2 signaling. J Biomech. 2015;48:3665–3671. doi: 10.1016/j.jbiomech.2015.08.013. [DOI] [PubMed] [Google Scholar]
- **40.Arulmoli J, Pathak MM, McDonnell LP, Nourse JL, Tombola F, Earthman JC, Flanagan LA. Static stretch affects neural stem cell differentiation in an extracellular matrix-dependent manner [Internet] Sci Rep. 2015;5:8499. doi: 10.1038/srep08499. The authors report that a 10% static stretch during NSC culture can strikingly influence oligodendrocytic differentiation. This stretch effect stretch-specific and triggered by laminin but not fibronectin. This study demonstrates that NSCs are sensitive to dynamic mechanical cues that may be triggered by specific adhesion receptors, which in turn may be valuable for understanding and controlling NSC differentiation in culture and in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. [Internet] Nat Mater. 2014 doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morrison B, Elkin BS, Dollé J-P, Yarmush ML. In Vitro Models of Traumatic Brain Injury [Internet] Annu Rev Biomed Eng. 2011;13:91–126. doi: 10.1146/annurev-bioeng-071910-124706. [DOI] [PubMed] [Google Scholar]
- 43.Ellis EF, McKinney JS, Willoughby KA, Liang S, Povlishock JT. A new model for rapid stretch-induced injury of cells in culture: characterization of the model using astrocytes. [Internet] J Neurotrauma. 1995;12:325–39. doi: 10.1089/neu.1995.12.325. [DOI] [PubMed] [Google Scholar]
- 44.Tweedie D, Rachmany L, Rubovitch V, Li Y, Holloway HW, Lehrmann E, Zhang Y, Becker KG, Perez E, Hoffer BJ, et al. Blast traumatic brain injury-induced cognitive deficits are attenuated by preinjury or postinjury treatment with the glucagon-like peptide-1 receptor agonist, exendin-4. Alzheimer’s Dement. 2016;12:34–48. doi: 10.1016/j.jalz.2015.07.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hatic H, Kane MJ, Saykally JN, Citron BA. Modulation of transcription factor Nrf2 in an in vitro model of traumatic brain injury. J Neurotrauma. 2012;29:1188–1196. doi: 10.1089/neu.2011.1806. [DOI] [PubMed] [Google Scholar]
- 46.Girgis F, Pace J, Sweet J, Miller JP. Hippocampal Neurophysiologic Changes after Mild Traumatic Brain Injury and Potential Neuromodulation Treatment Approaches [Internet] Front Syst Neurosci. 2016;10 doi: 10.3389/fnsys.2016.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perrimon N, Pitsouli C, Shilo B-Z. Signaling mechanisms controlling cell fate and embryonic patterning. [Internet] Cold Spring Harb Perspect Biol. 2012;4:a005975. doi: 10.1101/cshperspect.a005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guvendiren M, Burdick JA. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics [Internet] Nat Commun. 2012;3:792. doi: 10.1038/ncomms1792. [DOI] [PubMed] [Google Scholar]
- 49.Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, Chen CS. Mechanical regulation of cell function with geometrically modulated elastomeric substrates [Internet] Nat Methods. 2010;7:733–736. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin DC, Yurke B, Langrana NA. Mechanical properties of a reversible, DNA-crosslinked polyacrylamide hydrogel. J Biomech Eng. 2004;126:104–110. doi: 10.1115/1.1645529. [DOI] [PubMed] [Google Scholar]
- 51.Stowers RS, Allen SC, Suggs LJ. Dynamic phototuning of 3D hydrogel stiffness [Internet] Proc Natl Acad Sci. 2015;112:1953–1958. doi: 10.1073/pnas.1421897112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shih H, Lin C-C. Tuning stiffness of cell-laden hydrogel via host-guest interactions [Internet] J Mater Chem B. 2016;4:4969–4974. doi: 10.1039/c6tb00890a. [DOI] [PubMed] [Google Scholar]
- 53.Kamimura M, Sugawara M, Yamamoto S, Yamaguchi K, Nakanishi J. Dynamic control of cell adhesion on a stiffness-tunable substrate for analyzing the mechanobiology of collective cell migration [Internet] Biomater Sci. 2016;4:933–937. doi: 10.1039/c6bm00100a. [DOI] [PubMed] [Google Scholar]
- 54.Harunaga JS, Yamada KM. Cell-matrix adhesions in 3D. Matrix Biol. 2011;30:363–368. doi: 10.1016/j.matbio.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim J, Sachdev P, Sidhu K. Alginate microcapsule as a 3D platform for the efficient differentiation of human embryonic stem cells to dopamine neurons. Stem Cell Res. 2013;11:978–989. doi: 10.1016/j.scr.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Chatterjee K, Lin-Gibson S, Wallace WE, Parekh SH, Lee YJ, Cicerone MT, Young MF, Simon CG. The effect of 3D hydrogel scaffold modulus on osteoblast differentiation and mineralization revealed by combinatorial screening. Biomaterials. 2010;31:5051–5062. doi: 10.1016/j.biomaterials.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song W, Lu Y-C, Frankel AS, An D, Schwartz RE, Ma M. Engraftment of human induced pluripotent stem cell-derived hepatocytes in immunocompetent mice via 3D co-aggregation and encapsulation [Internet] Sci Rep. 2015;5:16884. doi: 10.1038/srep16884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hassan W, Dong Y, Wang W. Encapsulation and 3D culture of human adipose-derived stem cells in an in-situ crosslinked hybrid hydrogel composed of PEG-based hyperbranched copolymer and hyaluronic acid [Internet] Stem Cell Res Ther. 2013;4:32. doi: 10.1186/scrt182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao S, Xu Z, Wang H, Reese BE, Gushchina LV, Jiang M, Agarwal P, Xu J, Zhang M, Shen R, et al. Bioengineering of injectable encapsulated aggregates of pluripotent stem cells for therapy of myocardial infarction [Internet] Nat Commun. 2016;7:13306. doi: 10.1038/ncomms13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Banerjee A, Arha M, Choudhary S, Ashton RS, Bhatia SR, Schaffer DV, Kane RS. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials. 2009;30:4695–4699. doi: 10.1016/j.biomaterials.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seidlits SK, Khaing ZZ, Petersen RR, Nickels JD, Vanscoy JE, Shear JB, Schmidt CE. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials. 2010;31:3930–3940. doi: 10.1016/j.biomaterials.2010.01.125. [DOI] [PubMed] [Google Scholar]
- 62.Li N, Zhang Q, Gao S, Song Q, Huang R, Wang L, Liu L, Dai J, Tang M, Cheng G. Threedimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells [Internet] Sci Rep. 2013;3:1604. doi: 10.1038/srep01604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **63.Ma Q, Yang L, Jiang Z, Song Q, Xiao M, Zhang D, Ma X, Wen T, Cheng G. ThreeDimensional Stiff Graphene Scaffold on Neural Stem Cells Behavior. ACS Appl Mater Interfaces. 2016;8:34227–34233. doi: 10.1021/acsami.6b12305. This report further characterizes a previously reported novel graphene-based scaffold for NSC culture. In particular, the authors utilized an extruded Ni foam template before chemical vapor deposition of graphene to create 2X stiffer 3D scaffolds, which promoted NSC proliferation and astrocytic differentiation compared to softer scaffolds. This study describes a novel porous 3D material platform that could enable future attempts to tune NSC responses to programmable mechanical and electrical cues from their microenvironment. [DOI] [PubMed] [Google Scholar]
- *64.Carlson AL, Bennett NK, Francis NL, Halikere A, Clarke S, Moore JC, Hart RP, Paradiso K, Wernig M, Kohn J, et al. Generation and transplantation of reprogrammed human neurons in the brain using 3D microtopographic scaffolds [Internet] Nat Commun. 2016;7:10862. doi: 10.1038/ncomms10862. In this study, the authors directly reprogrammed neurons from iPSCs and tested the impact of utilizing a 3D scaffold for transplantation in vivo. They found that the scaffolds significantly improved cellular function and post-engraftment survival compared to the injection of cells alone. In addition, scaffold-delivered neurons displayed evidence of successful integration with the host neural circuitry. These studies demonstrate that biomaterial-supported delivery of cells has the potential to significantly improve regenerative medicine therapies and that further study of material-cell interactions is crucially important. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen G, Dong C, Yang L, Lv Y. 3D Scaffolds with Different Stiffness but the Same Microstructure for Bone Tissue Engineering. ACS Appl Mater Interfaces. 2015;7:15790–15802. doi: 10.1021/acsami.5b02662. [DOI] [PubMed] [Google Scholar]
- 66.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. [Internet] Nat Mater. 2010;9:518–26. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Einstein O, Ben-Hur T. The changing face of neural stem cell therapy in neurologic diseases. Arch Neurol. 2008;65:452–456. doi: 10.1001/archneur.65.4.452. [DOI] [PubMed] [Google Scholar]
- 68.Balakrishnan B, Jayakrishnan A. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials. 2005;26:3941–3951. doi: 10.1016/j.biomaterials.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 69.Cheng TY, Chen MH, Chang WH, Huang MY, Wang TW. Neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering. Biomaterials. 2013;34:2005–2016. doi: 10.1016/j.biomaterials.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 70.Marquardt LM, Heilshorn SC. Design of Injectable Materials to Improve Stem Cell Transplantation [Internet] Curr Stem Cell Reports. 2016 doi: 10.1007/s40778-016-0058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **71.Farrell K, Joshi J, Kothapalli CR. Injectable uncrosslinked biomimetic hydrogels as candidate scaffolds for neural stem cell delivery. J Biomed Mater Res - Part A. 2017;105:790–805. doi: 10.1002/jbm.a.35956. This study explored the use of natural polymeric macromolecules that physically crosslink in situ to deliver NSCs as an injectable solution. This approach addresses drawbacks of traditional scaffolds such as biological incompatibilities of chemical crosslinkers while having the advantages of easier delivery, lower invasiveness, and the ability to conform to the anatomy of the desired site. They characterized various gel compositions and discovered that certain formulations could promote cell survival, neurogenesis, and neurite outgrowth via integrin and CD44 receptor interactions with the materials. Importantly, the gels maintained their integrity after injection and matched the stiffness of endogenous tissue. [DOI] [PubMed] [Google Scholar]
- **72.Wei Z, Zhao J, Chen YM, Zhang P, Zhang Q. Self-healing polysaccharide-based hydrogels as injectable carriers for neural stem cells [Internet] Sci Rep. 2016;6:37841. doi: 10.1038/srep37841. In this study, the authors developed a self-healing injectable hydrogel system that could facilitate the delivery of NSCs. This material builds on the advantages of injectable hydrogels while also allowing greater control of the cells before injection and active maintenance of gel integrity after injection in situ. The gels were capable of quick and efficient self-healing after syringe extrusion, could span stiffnesses from 0.1 – 2 kPa, and supported NSC survival, growth, and neurogenesis. This work exemplifies novel and innovative material systems that are enabling improved NSC delivery for cell replacement therapies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wei Z, Yang JH, Liu ZQ, Xu F, Zhou JX, Zrinyi M, Osada Y, Chen YM. Novel biocompatible polysaccharide-based self-healing hydrogel. Adv Funct Mater. 2015;25:1352–1359. [Google Scholar]
- 74.Ding F, Wu S, Wang S, Xiong Y, Li Y, Li B, Deng H, Du Y, Xiao L, Shi X. A dynamic and self-crosslinked polysaccharide hydrogel with autonomous self-healing ability [Internet] Soft Matter. 2015;11:3971–3976. doi: 10.1039/c5sm00587f. [DOI] [PubMed] [Google Scholar]
- 75.Adil MM, Vazin T, Ananthanarayanan B, Rodrigues GMC, Rao AT, Kulkarni RU, Miller EW, Kumar S, Schaffer DV. Engineered hydrogels increase the post-transplantation survival of encapsulated hESC-derived midbrain dopaminergic neurons. Biomaterials. 2017;136:1–11. doi: 10.1016/j.biomaterials.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 76.Park JS, Chu JS, Tsou AD, Diop R, Wang A, Li S. The Effect of Matrix Stiffness on the Differentiation of Mesenchymal Stem Cells in Response to TGF-β. Biomaterials. 2012;32:3921–3930. doi: 10.1016/j.biomaterials.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wingate K, Floren M, Tan Y, Tseng PON, Tan W. Synergism of matrix stiffness and vascular endothelial growth factor on mesenchymal stem cells for vascular endothelial regeneration. [Internet] Tissue Eng Part A. 2014;20:2503–12. doi: 10.1089/ten.tea.2013.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rape AD, Zibinsky M, Murthy N, Kumar S. A synthetic hydrogel for the high-throughpu study of cell-ECM interactions. [Internet] Nat Commun. 2015;6:8129. doi: 10.1038/ncomms9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ranga A, Lutolf MP. High-throughput approaches for the analysis of extrinsic regulator of stem cell fate. Curr Opin Cell Biol. 2012;24:236–244. doi: 10.1016/j.ceb.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 80.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A Programmable Dual-RNA – Guided DNA Endonuclease in Adaptice Bacterial Immunity [Internet] Science. 2012;337:816–822. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. [Internet] Science. 2014;343:84–7. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]


