Abstract
Background
Exposure to airborne ultrafine particle (UFP; <100 nm in aerodynamic diameter) is an emerging public health problem. Nevertheless, the benefit of using high efficiency particulate arrestance (HEPA) filtration to reduce UFP concentrations in homes is not yet clear.
Methods
We conducted a randomized crossover study of HEPA filtration without a washout period in 23 homes of low-income Puerto Ricans in Boston and Chelsea, MA (USA). Most participants were female, older adults who were overweight or obese. Particle number concentrations (PNC, a proxy for UFP) were measured indoors and outdoors at each home continuously for six weeks. Homes received both HEPA filtration and sham filtration for three weeks each in random order.
Results
Median PNC under HEPA filtration was 50–85% lower compared to sham filtration in most homes, but we found no benefit in terms of reduced inflammation; associations between hsCRP, IL-6, or TNFRII in blood samples and indoor PNC were inverse and not statistically significant.
Conclusions
Limitations to our study design likely contributed to our findings. Limitations included carry-over effects, a population that may have been relatively unresponsive to UFP, reduction in PNC even during sham filtration that limited differences between HEPA and sham filtration, window opening by participants, and lack of fine-grained (room-specific) participant time-activity information. Our approach was similar to other recent HEPA intervention studies of particulate matter exposure and cardiovascular risk, suggesting that there is a need for better study designs.
Keywords: Urban air pollution, air filtration, HEPA, ultrafine particles, intervention, Puerto Rican, community-based
Background
While exposure to ambient airborne particulate matter <2.5 μm in aerodynamic diameter (PM2.5) is one of the top ten causes of morbidity and mortality worldwide [1], less is known about health effects from smaller particles, such as ultrafine particles (UFP; <0.1 μm in aerodynamic diameter), which are abundant in combustion emissions. In the U.S., PM2.5 is regulated by the EPA and is considered a regional pollutant because its concentration is relatively uniform over large distances (tens-to -hundreds of km). In contrast, UFP (that are primarily of traffic emission origin in urban areas) are quite variable over much shorter distances (tens-to-hundreds of m) [2, 3], are unregulated, and may represent an independent health burden. Furthermore, evidence from animal studies [4] and from observational epidemiology suggests that UFP are associated with indicators of CVD risk as well as adverse health outcomes [5–8].
While increasing outdoor air brought into buildings has traditionally been associated with improved health [9], there is convincing evidence that living close to ourdoor sources such as major roadways and highways is associated with elevated cardiovascular disease (CVD) and respiratory disease risk [10, 11]. UFP have been shown in many studies to also be elevated in these locations [3]. Accordingly, there is increasing interest in using air filtration to reduce exposure to urban UFP in both schools and homes. For example, recently a requirement for high-grade filtration in schools and homes near highways was enacted in Los Angeles [12]. While several studies have shown that filtration in mechanical air handling systems can reduce indoor UFP relative to outdoors [13, 14], it has been more difficult to reduce indoor UFP, especially in low-income households, that lack mechanical ventilation [2, 15]. To date, few studies have evaluated the health benefits of reducing indoor concentrations of urban UFP [16, 17].
We conducted a randomized crossover trial of air filtration in homes of low-income Puerto Rican residents in Boston and Chelsea, MA (USA). The intervention was a collaboration between the Community Assessment of Freeway Exposure and Health study (CAFEH; [18]) and the Boston Puerto Rican Health Study (BPRHS; [19]). In addition to the trial results, we conducted a meta-analysis with a second in-home HEPA intervention trial conducted in nearby Somerville, MA as part of CAFEH [17]. Our goals were to measure changes in cardiovascular health measures due to in-home air filtration and to provide guidance for emerging public health efforts that reduce exposure to urban pollution.
Methods
We hypothesized 1) that high efficiency particulate arrestance (HEPA) filtration in homes would reduce UFP concentrations indoors more than sham filtration and 2) that reduced UFP concentrations would lead to reductions in biomarkers of inflammation for residents. The study was a double-blind, randomized crossover trial in which each participant served as their own control, thereby greatly reducing the role of time-invariable confounders. Up to two homes were enrolled and randomized at a time, with one allocated to receive HEPA filtration and the other sham filtration first. At three weeks, the homes were switched from HEPA filtration to sham or vice versa. There was no washout period between sham and HEPA filtration. While field staff were aware of the type of filter in use, the participants and the lab that analyzed blood samples were not. The approach and methods were largely similar to another HEPA intervention we conducted in public housing in the City of Somerville, which was still in progress at the start of this study [17].
Participants were recruited from the BPRHS cohort. The parent study was in the process of follow-up at approximately five years since baseline with close to 1000 participants remaining. The cohort staff recommended non-smoking participants who they thought might be receptive to our intervention. Of the 25 participants enrolled, 23 (92%) completed the study and were included in the analysis. One home was removed due to the failure of the flow sensor, which identified indoor versus outdoor air, while the other was removed because the participant opted to end the study early. All participants lived in the cities of Boston or Chelsea. Data on demographics and health were obtained from surveys collected during longitudinal follow-up of the cohort. For the participants receiving the intervention, we collected additional surveys with information on recent exposures, and recent illnesses.
Participants signed consent forms for the parent study and a separate consent for the air filtration intervention. The studies were approved by the IRBs at Tufts Medical Center, Northeastern University, and the University of Massachusetts Lowell.
Window-mounted HEPAiRx air filtration units (Air Innovations, Inc., North Syracuse, NY, USA) equipped with MERV 17 filters (rated to remove ≥99.97% of particles ≥0.3 μm in diameter) were used. Previously, we determined that the HEPAiRx was able to reduce PNC (total particles 7–3,000 nm) by >99.9% under well-controlled conditions (i.e., from ~8x105 to <50 particles cm-3) [16]. These units can operate at ~10 exchanges/hour in a 28.3 m3 (103 ft3) room and have user-controlled air heating and cooling elements. The units were installed preferentially in living rooms of apartments (N=16), where participants spent much of their day. Eight units were installed in bedrooms due to space restrictions or because living room windows did not accommodate the HEPAiRx unit. To maximize particle removal, the HEPA units were operated at the highest possible fan speed and the vents were blocked so that there was no flow of outdoor air through the unit into the apartment. Also, participants were asked to keep windows closed as much as possible during the study period to minimize infiltration from outside. Filters were changed in each apartment after 21 days (HEPA for sham or vice versa). A new HEPA filter (MERV 17) was used in each apartment. The sham filter was an empty, perforated sheet metal box that was the same size and shape and had the same appearance as the metal frame around the HEPA filters. The HEPAiRx sounded the same regardless of sham or HEPA filtration. A sign written in English and Spanish was placed on the HEPA-unit cover asking participants not to tamper with or expose the filter. Participants were instructed not to tamper with the unit and to call if there was any problem with it.
Particle number concentrations (PNC) were measured continuously during the six-week trial in each apartment using water-based condensation particle counters (CPC; TSI Model 3783, d50 7 nm, maximum detectable particle >3 μm). The CPCs were installed in the same room as the HEPAiRx unit and recorded 30-second mean PNC (one-minute mean PNC in the first five homes). Both outdoor and indoor PNC were measured; a solenoid valve switched every 15 minutes between two, 1-m-long conductive silicon inlet tubes: one pulled from indoors and the other pulled from outdoors. An in-line flow sensor logged different voltages depending on whether a flow was detected in the line (2.49 V with flow, ~1.00 V with no flow); these were used to identify indoor and outdoor sampling periods. To minimize the possibility of measuring mixed indoor and outdoor air downstream of the solenoid valve when switching from one inlet to another, we removed the first and last data point within each 15 min sampling period (each data point was an average of 30–60 s of measurements). Six out of 13 homes in Boston and 6/10 homes in Chelsea had >87% data available for analysis; 6/13 homes in Boston and 4/10 homes in Chelsea required additional data be removed between switches but still had >77% data availability; one home in Boston (P03) had only 44% data availability due to a solenoid malfunction. Before the start of the intervention in each apartment, CPC flow rates were measured using a flow meter (TSI Model 4140) (no discrepancies were observed throughout the study). The CPC vacuum was also checked for leaks by placing a polyethersulfone membrane filter (rated at 99.96% removal efficiency for 0.45 μm particles) on the inlet to insure the CPC measured <100 particles/cm3. Sites were visited weekly for regular maintenance (flow checks, time resets, etc.) and to download data. Data flagged by the CPC as erroneous (typically <1% of all data per home) were removed from the data set. Consistent with manufacturer specifications, all CPCs performed within 10% of one another in laboratory side-by-side comparisons. Particle losses in the sampling lines were not accounted for because the sampling lines were relatively short and losses for particles >20 nm diameter were expected to be small [20].
The Somerville study, which we combined with the current study in a meta-analysis, followed the same study design and methods with the following differences: 1) there was no outdoor monitoring; 2) all study participants resided within 200 m of a highway; and 3) the study participants differed in their demographics (Table 1).
Table 1.
Participant demographics
| BPRHS | Somerville | Combined | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Category | Total (n = 23) | HEPA First (n = 11) | Sham First (n = 12) | Total (n = 20) | HEPA First (n = 10) | Sham First (n = 10) | Total (n = 43) | HEPA First (n =21) | Sham First (n =22) |
| Demographic Data | |||||||||
| Age, Mean (min-max), years | 64.1 (52–78) | 63.6 (52–73) | 64.5 (55–78) | 52.9 (42–79) | 55.3 (42–79) | 50.6 (42– 63) | 58.9 (42–79) | 59.6 (42–79) | 58.1 (42–78) |
| BMI, Median (Min-Max) | 31.6 (24.4–49.9) | 32.5 (24.4–42.6) | 29.9 (25.5–49.9) | 33.2 (20–72) | 33.6 (20–72) | 32.9 (25–51) | 32.9 (20–72) | 33.03 (20–72) | 32.9 (25–51) |
| Female | 18 (78%) | 8 (73%) | 10 (83%) | 16 (80%) | 7 (70%) | 9 (90%) | 34 (79%) | 15 (71%) | 19 (86%) |
| Hispanic | 23 (100%) | 11 (100%) | 12 (100%) | 7 (35%) | 3 (30%) | 4 (40%) | 30 (70%) | 14 (67%) | 16 (73%) |
| Annual Household Income <$24,999 | 19(83%) | 8 (73%) | 11 (92%) | 15 (75%) | 9 (90%) | 6 (60%) | 34 (79%) | 17 (81%) | 17 (77%) |
| Annual Household Income $25,000 – $74,999 | 1 (4%) | 1 (9%) | 0 (0%) | 3 (15%) | 1 (10%) | 2 (20%) | 4 (9%) | 2 (10%) | 2 (9%) |
| Eighth Grade Education | 9 (39%) | 4 (36%) | 5 (42 %) | 13 (65%) | 5 (50%) | 8 (80%) | 22 (51%) | 9 (43%) | 13 (31%) |
| Below Federal Poverty Threshold* | 12 (52%) | 4 (36%) | 8 (67%) | ||||||
| Employed | 0 (0%) | 0 (0%) | 0 (0%) | 9 (45%) | 3 (30%) | 6 (60%) | 9 (21%) | 3 (14%) | 6 (27%) |
| Distance to I-93: ≤100 m * | 10 (50%) | 6 (60%) | 4 (40%) | ||||||
| Distance to I-93: 101–200 m * | 10 (50%) | 4 (40%) | 6 (60%) | ||||||
| < 50 m to a major roadway * | 7 (32%) | 3 (30%) | 4 (33%) | ||||||
| Health data and Medicines used | |||||||||
| Total Cholesterol, mean (min- max), mg/dL | 207.3 (147–307) | 197.8 (147–307) | 220.4 (178–255) | 290.2 (100–450) | 263.6 (100–400) | 316.9 (141–450) | 249.8 (100–450) | 229.1 (100–400) | 274 (141–450) |
| Triglycerides, mean (min-max), mg/dL | 192 (75–610) | 187.3 (93–425) | 198.4 (75–610) | 211.4 (50–500) | 169 (50–375) | 253.9 (50–500) | 202 (50–610) | 178.6 (50–425) | 229.2 (50–610) |
| Previous Heart Attack | 8 (36%) | 5 (50%) | 3 (25%) | 1 (5%) | 1 (10%) | 0 (0%) | 9 (21%) | 6 (30%) | 3 (14%) |
| Diabetes | 12 (52%) | 8 (73%) | 4 (33%) | 2 (10%) | 0 (0%) | 2 (20%) | 14 (33%) | 8(38%) | 6 (27%) |
| High Blood Pressure or Hypertension | 18 (82%) | 8 (80%) | 10 (83%) | 11 (55%) | 8 (80%) | 3 (30%) | 29 (69%) | 16 (80%) | 13 (59%) |
| Anti-hypertension medicine | 18 (78%) | 9 (82%) | 9 (75%) | 10 (50%) | 7 (70%) | 3 (30%) | 28 (65%) | 16 (76%) | 12 (55%) |
| Anti-inflammatory medicine | 4 (17%) | 2 (18%) | 3 (25%) | 7 (35%) | 6 (60%) | 1 (10%) | 11 (26%) | 8(38%) | 4 (18%) |
| Anti-lipids medicine | 17 (74%) | 10 (91%) | 7 (58%) | 3 (15%) | 2 (20%) | 1 (10%) | 20 (47%) | 12 (57%) | 8 (36%) |
| Anti-diabetes medicine | 11(48%) | 8 (73%) | 3 (25%) | 3 (15%) | 1 (10%) | 2 (20%) | 14 (33%) | 9 (43%) | 5 (23%) |
| Window Opening | |||||||||
| Window opening during December to February | 8 (35%) | 6 (55%) | 2 (17%) | 9 (45%) | 4 (40%) | 5 (50%) | 17 (40%) | 10 (48%) | 7 (32%) |
| Window opening during June to August | 16 (70%) | 8 (73%) | 8 (67%) | 17 (85%) | 9 (90%) | 8 (80%) | 33 (77%) | 17 (81%) | 16 (73%) |
demographic data only recorded for one study
Indoor measurements reflect both outdoor UFP that infiltrate indoors and indoor-generated UFP – e.g., from cooking, cleaning, and candle, wood and incense burning [21–26]. Indoor sources result in large but variable magnitude spikes in indoor concentrations and further, the rate of decay of these spikes depends on several factors, such as source strength and duration, room volume and ventilation rate. It is thus challenging to completely separate the contributions from outdoor and indoor UFP sources based solely on indoor PNC measurements without continuous measurements of outdoor concentrations and characterization air exchange rates and infiltration rates in the homes. For example, to quantify indoor UFP contribution in seven homes during 3- to 9-day monitoring periods, Bhangar et al. performed continuous indoor and outdoor measurements, and characterized air exchange and infiltration rates based on occupancy, carbon dioxide concentrations and activities in the homes [21]. In addition, they characterized source emission and loss rates under controlled experiments. Bekö et al. used diary entries for occupancy and time-activity to identify indoor-origin UFP spikes in 45-h datasets in 56 homes [24]. Kearney et al. distinguished indoor-origin and outdoor-infiltrated fractions of UFP inside 50 homes using continuous indoor and outdoor measurements as well as tracers for infiltration (sulfur) and air exchange rates [25]. Distinguishing and quantifying particles of indoor- and outdoor-origin was beyond the scope of our study.
However, we generated a PNC time-series for each home in which indoor contributions were attenuated - if not completely separated, and from this we calculated the six-hour moving median for indoor PNC measurements. We then calculated the standard deviation for the three-week period corresponding to HEPA or sham filtration. Indoor measurements that were two standard deviations above the six-hour moving median were classified as spikes and replaced with the last indoor measurement that was not considered to be a spiked value. Even though our method did not completely remove the contribution from indoor sources, it significantly attenuated the contributions from spikes that skewed the three-week indoor average values used as exposure concentrations (see Supplemental Material). This attenuation is sufficient for our primary purpose which was to assess whether associations with biomarkers would be affected by partial removal of indoor sources.”
Indoor measurements reflect both outdoor UFP that infiltrate indoors and indoor-generated UFP – e.g., from cooking, cleaning, and candle, wood and incense burning [21–26]. Indoor sources result in large but variable magnitude spikes in indoor concentrations and further, the rate of decay of these spikes depends on several factors, such as source strength and duration, room volume and ventilation rate. It is thus challenging to completely separate the contributions from outdoor and indoor UFP sources based solely on indoor PNC measurements without continuous measurements of outdoor concentrations and characterization air exchange rates and infiltration rates in the homes. For example, to quantify indoor UFP contribution in seven homes during 3- to 9-day monitoring periods, Bhangar et al. performed continuous indoor and outdoor measurements, and characterized air exchange and infiltration rates based on occupancy, carbon dioxide concentrations and activities in the homes [21]. In addition, they characterized source emission and loss rates under controlled experiments. Bekö et al. used diary entries for occupancy and time-activity to identify indoor-origin UFP spikes in 45-h datasets in 56 homes [24]. Kearney et al. distinguished indoor-origin and outdoor-infiltrated fractions of UFP inside 50 homes using continuous indoor and outdoor measurements as well as tracers for infiltration (sulfur) and air exchange rates [25]. Distinguishing and quantifying particles of indoor- and outdoor-origin was beyond the scope of our study.
However, we generated a PNC time-series for each home in which indoor contributions were attenuated - if not completely separated, and from this we calculated the six-hour moving median for indoor PNC measurements. We then calculated the standard deviation for the three-week period corresponding to HEPA or sham filtration. Indoor measurements that were two standard deviations above the six-hour moving median were classified as spikes and replaced with the last indoor measurement that was not considered to be a spiked value. Even though our method did not completely remove the contribution from indoor sources, it significantly attenuated the contributions from spikes that skewed the three-week indoor average values used as exposure concentrations (see Supplemental Material). This attenuation is sufficient for our primary purpose which was to assess whether associations with biomarkers would be affected by partial removal of indoor sources.
A venous blood sample was collected at the start, at the change of filter types (end of week 3) and at end of the intervention (end of week 6). Samples were transported to the Human Nutrition Research Center on Aging (Tufts University, Boston campus), where they were processed to plasma and stored at minus 80 °C within 1–3 h of collection. Participants were instructed to fast overnight prior to the blood draws (79% confirming fasting), which occurred between 8 and 10 AM. Samples were assayed in batches using immunoassay kits for TNF-RII (Quantitative, R&D Systems, Minneapolis, MN, USA) and IL-6 (Quantitative HS, R&D Systems). High sensitivity CRP (hsCRP) was measured by a solid-phase, two-site chemiluminescent immunometric assay, (IMMULITE 2000, Siemens Healthcare Diagnostics, Los Angeles, CA 90045). These biomarkers are a measure of the levels of systemic inflammation.
The primary goal of the analysis was to evaluate the intervention (HEPA or sham filter) and carryover effects of the intervention, which may persist during the second intervention period. We used the PROC MIXED procedure with random subject-effects with compound symmetry covariance structure in SAS® 9.3 (SAS Institute Inc., Cary, NC, USA). Dependent variables (post-intervention blood biomarker levels) were natural log transformed. The independent variables (fixed-effect factors) included baseline biomarker level (natural log transformed), intervention (HEPA or sham filter), intervention period, interaction between intervention and period (carryover effect). We examined scatterplots of the relationships between PNC exposure and each of the blood markers. Assuming a linear relationship for these relationships seemed most reasonable. Post hoc subgroup analyses were conducted by removing the five homes (~20% of the homes, a number that retained enough homes for analysis of the remaining data) with least reductions in PNC (<43% PNC reduction) and, separately, by removing the five homes most heavily impacted by indoor sources (see following section).
Since the PROC MIXED procedure does not provide an option to report log-transformed analyses on the original scale, the beta coefficient (β) and its 95% confidence intervals for the intervention effect were obtained from the LSMEANS statement. This was converted to the ratio of percent change in blood biomarker levels from baseline using this following formula: 100% × (exp(β) − 1), which estimates the difference in the intervention effects comparing HEPA to sham filter.
Meta-analysis using the DerSimonian-Laird random-effects model was conducted to pool the results of the present trial and a second in-home HEPA intervention trial conducted as part of CAFEH [17]. The two trials were analyzed using the same statistical models as described earlier before pooling.
To test whether log-transformed PNC (with spikes attenuated) was associated with log-transformed levels of the blood biomarker concentrations, we used mixed-effects linear models with random intercepts for each participant in Stata 14.2. We also tested associations of log-transformed total PNC (with spikes) with log-transformed levels of the blood biomarker concentrations. For each of the models, we checked intraclass correlations to assess between-participant variation in comparison to within-participant variation. We also checked the normality and homoscedasticity of the residual errors in each model. Two-tailed p-values were used and were considered statistically significant at the 0.05 level.
Results
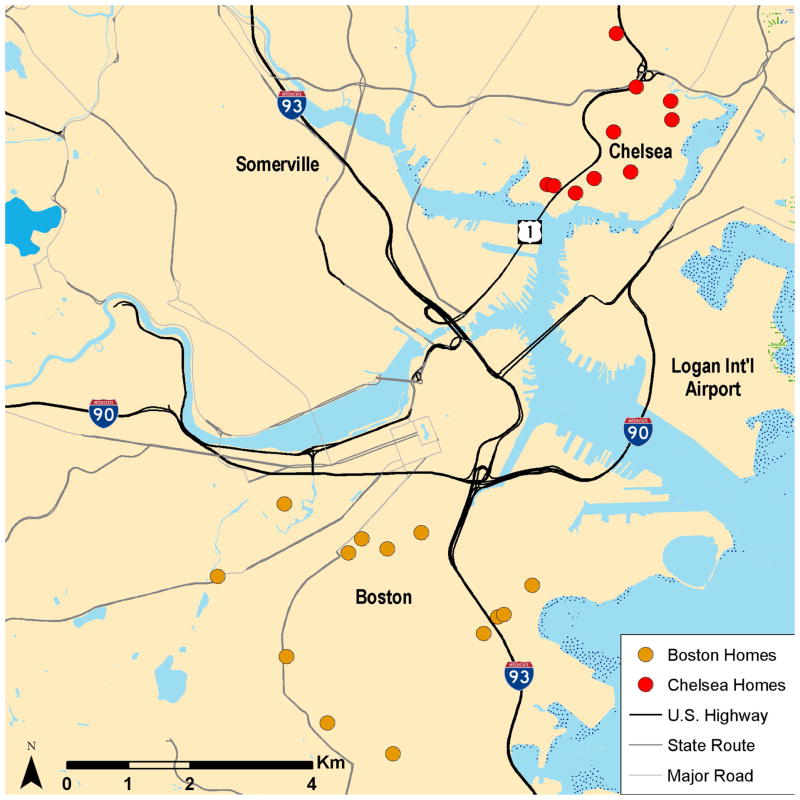
Table 1 presents demographic and health data for participants in the intervention. There were 23 participants in the analyses. Most were female, older adults who were overweight or obese (median BMI = 32.9). All were Hispanic and all lived in Boston or Chelsea (Figure 1).
Figure 1.
Map of study participant homes in Boston and Chelsea.
Most were low SES based on income, poverty level, and education. A majority had diabetes and/or hypertension. A third reported having had a heart attack. Participants had high blood lipid profiles and most took medications for these health problems. Window opening was common, and was reported for more apartments in the summer than in the winter. Only seven participants (32%) lived <50 m from a major roadway (≥20,000 vpd), one lived <50 m from a highway and the rest lived >100 m away from highways and major roadways. All participants lived in urban areas with substantial traffic.
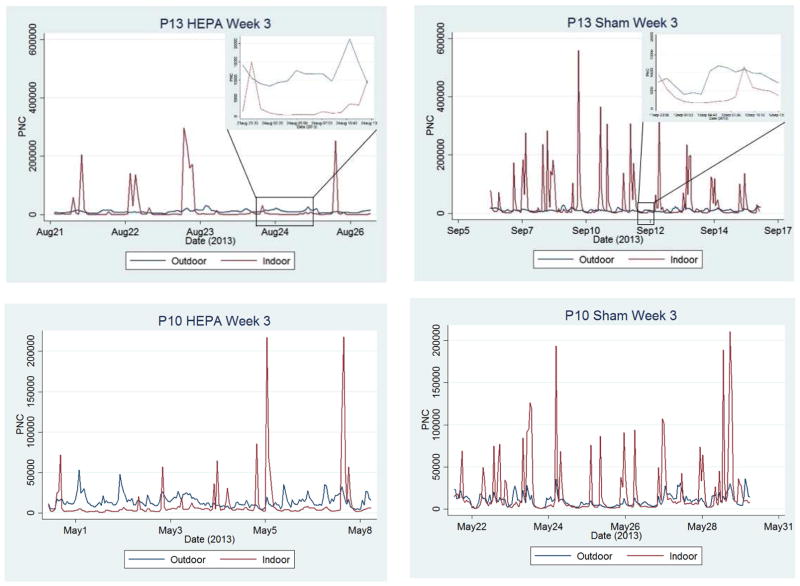
Summary data on PNC for each participant is presented in Table 2 and S1. The range of outdoor PNC measurements at the 23 homes (7,100–19,000 particles/cm3 in Boston and 6,000–17,000 particles/cm3 in Chelsea, medians, Table 2) is consistent with the findings of other geographically similar studies. For example, Wheeler et al. measured outdoor PNC at 48 and 45 homes in winter and summer, respectively, in Windsor, Ontario with median concentrations ranging from 7,600–13,400 particles/cm3 [27]. Weichenthal et al. measured outdoor PNC at 36 homes in Montreal, Quebec and Pembroke, Ontario and observed winter-time mean concentrations between 10,500 and 30,000 particles/cm3 [28]. Mean PNC tended to be higher than median PNC skewed by the PNC spikes of indoor-origin; up to 15% of measurements per home were classified as spikes. Outdoor mean and median PNC rarely exceeded 20,000 particles/cm3, which is consistent with most of the homes not being close to major roadways. Indoor concentrations tended to be lower than outdoor levels for both sham and HEPA periods, though concentrations were generally much lower in most homes during the HEPA intervention. We observed greater reductions and lower PNC concentrations during HEPA than during sham filtration, consistent with our hypothesis: reduction of PNC was 75%±20% (range: 6% to 92%) during HEPA filtration compared to 42%±20% (range: 0% to 70%) during sham filtration (Table 2 and Figure 2); Wilcoxian rank sum test on logit transformed I/O ratios (the natural log of [I/O]/[1-(I/O)]) supported our hypothesis (p-value <0.0001). Figure 2 shows PNC time series from two homes in which PNC reductions were larger with HEPA than with sham filtration along with examples of PNC spikes.
Table 2.
Summary of PNC measurements during HEPA and sham filtration
| HEPA | Sham | I/O Ratioa | Reduction in PNC during HEPA compared to shamb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Participant ID | Order | Exposure | Median PNC | Mean PNC | Median PNC | Mean PNC | Sham | HEPA | % | Abs. |
| P01 | Hepa-1st | Indoor | 4500 | 14000 | 7900 | 20000 | 0.88 | 0.38 | 43% | 3400 |
| Outdoor | 12000 | 13000 | 9000 | 11000 | ||||||
| P02 | Sham-1st | Indoor | 1300 | 17000 | 9100 | 34000 | 0.83 | 0.16 | 86% | 7800 |
| Outdoor | 7900 | 9400 | 11000 | 13000 | ||||||
| P03 | Hepa-1st | Indoor | 3700 | 11000 | 8400 | 31000 | 0.88 | 0.47 | 56% | 4700 |
| Outdoor | 7800 | 9100 | 9600 | 12000 | ||||||
| P04 | Sham-1st | Indoor | 2000 | 7200 | 5600 | 12000 | 0.62 | 0.27 | 64% | 3600 |
| Outdoor | 7400 | 8600 | 9000 | 11000 | ||||||
| P05 | Hepa-1st | Indoor | 1800 | 13000 | 7200 | 32000 | 0.60 | 0.18 | 75% | 5400 |
| Outdoor | 10000 | 14000 | 12000 | 17000 | ||||||
| P06 | Sham-1st | Indoor | 3700 | 38000 | 12000 | 68000 | 0.86 | 0.21 | 69% | 8300 |
| Outdoor | 18000 | 20000 | 14000 | 17000 | ||||||
| P07 | Sham-1st | Indoor | 7600 | 16000 | 15000 | 25000 | 1.00 | 0.94 | 49% | 7400 |
| Outdoor | 8100 | 13000 | 15000 | 19000 | ||||||
| P08 | Sham-1st | Indoor | 3600 | 9800 | 7900 | 17000 | 0.42 | 0.23 | 54% | 4300 |
| Outdoor | 16000 | 19000 | 19000 | 22000 | ||||||
| P10 | Hepa-1st | Indoor | 3400 | 11000 | 5800 | 16000 | 0.62 | 0.28 | 41% | 2400 |
| Outdoor | 12000 | 15000 | 9400 | 13000 | ||||||
| P11 | Hepa-1st | Indoor | 4500 | 17000 | 4300 | 10000 | 0.61 | 0.60 | −5% | −200 |
| Outdoor | 7500 | 9200 | 7100 | 9300 | ||||||
| P12 | Sham-1st | Indoor | 1400 | 5900 | 3200 | 10000 | 0.41 | 0.15 | 56% | 1800 |
| Outdoor | 9400 | 13000 | 7900 | 11000 | ||||||
| P13 | Hepa-1st | Indoor | 1500 | 24000 | 6500 | 40000 | 0.66 | 0.14 | 77% | 5000 |
| Outdoor | 11000 | 12000 | 9900 | 12000 | ||||||
| P14 | Sham-1st | Indoor | 2600 | 11000 | 5400 | 19000 | 0.36 | 0.17 | 52% | 2800 |
| Outdoor | 15000 | 17000 | 15000 | 18000 | ||||||
| P16 | Hepa-1st | Indoor | 1700 | 2600 | 5500 | 7300 | 0.42 | 0.11 | 69% | 3800 |
| Outdoor | 15000 | 16000 | 13000 | 15000 | ||||||
| P17 | Sham-1st | Indoor | 2200 | 12000 | 12000 | 19000 | 0.75 | 0.13 | 82% | 9800 |
| Outdoor | 17000 | 20000 | 16000 | 22000 | ||||||
| P19 | Sham-1st | Indoor | 1000 | 3200 | 3900 | 7100 | 0.35 | 0.11 | 74% | 2900 |
| Outdoor | 9400 | 13000 | 11000 | 15000 | ||||||
| P20 | Hepa-1st | Indoor | 1000 | 11000 | 3500 | 13000 | 0.35 | 0.08 | 71% | 2500 |
| Outdoor | 13000 | 18000 | 10000 | 12000 | ||||||
| P21 | Sham-1st | Indoor | 2000 | 15000 | 4900 | 14000 | 0.45 | 0.22 | 59% | 2900 |
| Outdoor | 9000 | 12000 | 11000 | 14000 | ||||||
| P22 | Sham-1st | Indoor | 500 | 4000 | 3300 | 16000 | 0.30 | 0.08 | 85% | 2800 |
| Outdoor | 6000 | 7600 | 11000 | 14000 | ||||||
| P23 | Hepa-1st | Indoor | 1000 | 8900 | 4300 | 17000 | 0.54 | 0.12 | 77% | 3300 |
| Outdoor | 8500 | 12000 | 8000 | 11000 | ||||||
| P24 | Hepa-1st | Indoor | 3600 | 9000 | 5400 | 8700 | 0.49 | 0.26 | 33% | 1800 |
| Outdoor | 14000 | 19000 | 11000 | 15000 | ||||||
| P25 | Sham-1st | Indoor | 1900 | 7800 | 4700 | 16000 | 0.49 | 0.17 | 60% | 2800 |
| Outdoor | 11000 | 14000 | 9600 | 14000 | ||||||
| P26 | Hepa-1st | Indoor | 3500 | 9500 | 5600 | 16000 | 0.43 | 0.25 | 38% | 2100 |
| Outdoor | 14000 | 18000 | 13000 | 16000 | ||||||
Ratio of median indoor measurement to median outdoor measurement.
(median indoor measurement during sham - median indoor measurement during HEPA)/median indoor measurement during sham.
Figure 2.
Time series of particle number concentration (PNC) from two participant homes.
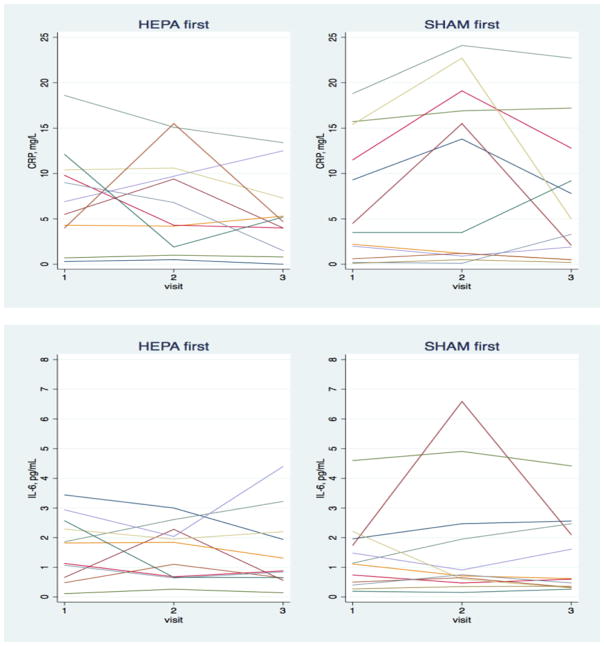
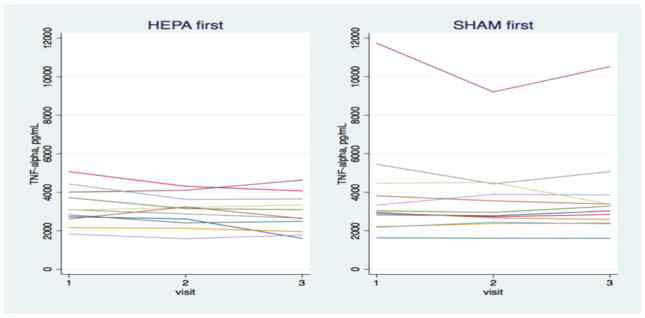
Figure 3 shows the change in hsCRP, TNFRII, and IL-6 for each participant stratified by HEPA- or sham-first randomization. Biomarker levels tended to be high, reflecting the poor health of most of the participants. Changes in biomarkers for individual participants varied considerably, with some changes consistent with benefits of HEPA filtration, others counter to a benefit, and still others changing little.
Figure 3.
Change in blood biomarkers over the intervention and sham filtration periods for individual participants.
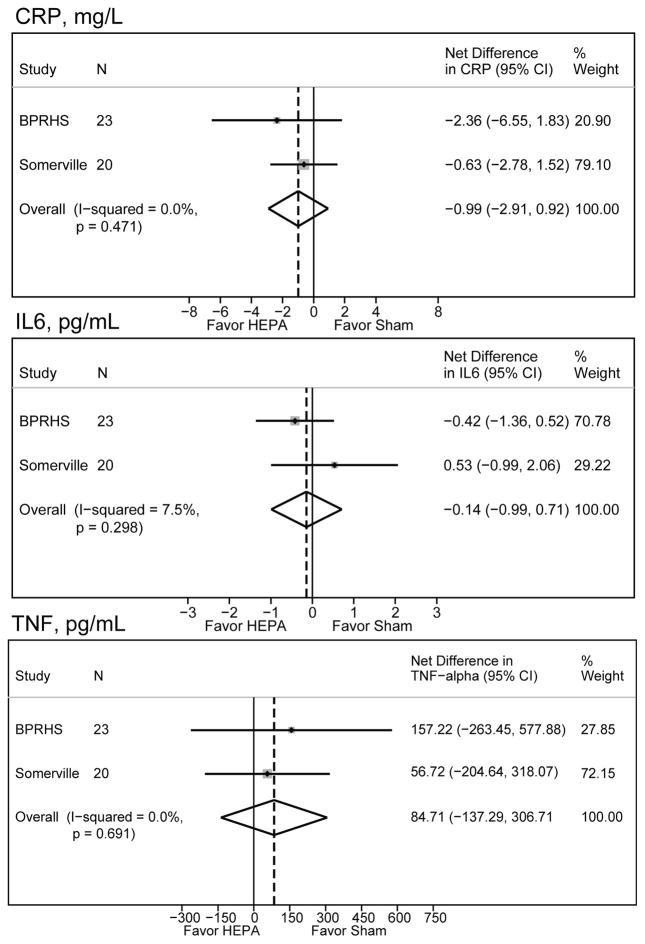
Table 3 presents the results of associations between HEPA and sham periods with percent changes in biomarkers. Percent changes in biomarkers (adjusted for baseline) were generally small and positive, counter to our hypothesis. The meta-analysis including our previous in-home air filtration study in Somerville did little to change the findings or suggest there was a benefit from HEPA filtration (Figure 4). Subgroup analyses were conducted by removing the five homes with the smallest reductions in PNC and, separately by removing the five homes with the most indoor spikes. This analysis suggested impacts on the mean effects on hsCRP but not IL-6 or TNFRII (Table 3).
Table 3.
HEPA vs. sham filtration comparison
| Outcome | % change* | Lower 95% CI | Upper 95% CI | Sample size | |
|---|---|---|---|---|---|
| Full analysis | hsCRP | 5.8% | −36.6% | 76.5% | 23 |
| IL-6 | 1.2% | −20.5% | 29.0% | ||
| TNFRII | 3.3% | −3.0% | 9.9% | ||
| Remove homes with least PNC reduction | hsCRP | 15.6% | −43.6% | 137.0% | 18** |
| IL-6 | 3.6% | −19.9 | 34.1% | ||
| TNFRII | 5.8% | −2.3% | 14.6% | ||
| Remove homes with largest number of indoor spikes | hsCRP | −8.9% | −37.9% | 33.7% | 18** |
| IL-6 | 0.3% | −25.8% | 35.5% | ||
| TNFRII | 2.0% | −3.8% | 8.3% |
Adjusted for baseline; percent change is positive for higher blood biomarkers during HEPA.
The subgroup analyses are not the same set of participants, but instead the 18 participants with the largest PNC reduction and the fewest spikes respectively (see Table 2).
Figure 4.
Meta-analysis of BPRHS findings with the in-home air filtration intervention in public housing in Somerville, MA.
Analysis for association between blood biomarkers and indoor PNC exposure (total and with indoor PNC spikes attenuated), resulted in inverse associations (lower biomarker levels for higher PNC) well within the bounds of CIs (Table 4).
Table 4.
Association of PNC with biomarkers
| Beta* (CI) | P-value (CI) | ||
|---|---|---|---|
| hsCRP | Ln mean indoor spike attenuated PNC | −0.25 (−0.67; 0.17) | 0.24 |
| Ln mean total PNC | −0.40 (−1.03; 0.24) | 0.22 | |
| IL-6 | Ln mean indoor spike attenuated PNC | −0.05 (−0.25; 0.14) | 0.59 |
| Ln mean total PNC | −0.12 (−0.39; 0.15) | 0.38 | |
| TNFRII | Ln mean indoor spike attenuated PNC | −0.05 (−0.12; 0.03) | 0.21 |
| Ln mean total PNC | −0.07 (−0.17; 0.03) | 0.18 |
Beta is negative for lower blood biomarker values with higher PNC.
Discussion
We conducted an in-home HEPA filtration intervention in 23 homes of participants in the BPRHS. Our intervention was moderately successful at reducing PNC indoors, but fell short of our goal of 80–90% reduction in all homes. Despite reducing PNC 50–85% in most homes, we saw no beneficial effect on biomarkers of inflammation for HEPA as compared to sham filtration periods. These findings are similar to another HEPA intervention we conducted with 20 participants in 19 homes in Somerville [17]. Pooling the two datasets in a meta-analysis did not appreciably alter our findings.
Because there are very few HEPA studies addressing UFP and cardiovascular disease, we also compare our findings with trials of PM more broadly. Brauner et al. saw no improvement in hsCRP, IL-6, or TNFRII in relation to reductions in UFP, although the intervention period was much shorter than ours and the ambient concentrations were lower as well [16]. In contrast, Allen et al. saw reductions in hsCRP and IL-6 in a crossover trial for wood smoke, though the exposure was PM2.5 rather than urban UFP [29]. Lin et al. found that for 60 healthy students, indoor PM2.5 was associated more strongly with blood pressure and heart rate when there was no air filtration [30]. Chen et al. reported that air filtration was significantly associated with decreases in monocyte chemoattractant protein-1, interleukin-1b, myeloperoxidase, and soluble CD40 ligand in 35 health college students. They also found reductions in blood pressure and exhaled nitrous oxide [31].
We think it is unlikely that our null findings were based on lack of toxicity of UFP as there is convincing evidence that UFP can drive inflammation [32, 33]. Therefore, we suspect that there are limitations to our study design that undermined our ability to see benefits from reducing PM in homes. We discuss next the lessons we learned from this research and make recommendations for future HEPA intervention trials.
A primary lesson is that randomized crossover designs have limitations for HEPA intervention trials. Critically, randomized crossover studies need a washout period, during which there is neither sham nor HEPA intervention, that exceeds the length of time that effects of filtration might exert on health outcome measures. In the absence of a sufficient washout period, effects could carry over into the subsequent sham period diluting the apparent impact on biomarkers. Observational panel studies suggest that hsCRP and IL-6 could have maximal changes in response to UFP at three to four weeks [34, 35]. We accounted for carryover effects in our statistical analysis, but it would have been preferable to eliminate them from affecting biomarker data. An alternative approach would be to enroll participants in longer intervention periods of many months or a year so that the intervention period exceeds the wash out period.
Washout periods have rarely been used in HEPA filtration randomized crossover trials. In our review of the literature, we found that only one [36] out of five [16, 17, 29, 37] recent HEPA intervention studies for CV outcomes had a washout period. Further, all but two of these studies had intervention periods that were shorter than we suspect is necessary (three weeks or more) based on observational studies [34, 35, 38, 39]. Given the logistical challenge of adding long washout periods to randomized crossover trials, it is worth considering standard randomized controlled trials, as have been used in some HEPA intervention studies for asthma [40]. An attention intervention, perhaps an educational module, could be included to reduce concerns about possible Hawthorn effects in a randomized controlled trial. These effects might arise due to study participants altering their behavior because they are engaged by the study independent of the intervention [41].
There is evidence from our indoor-outdoor monitoring that sham filtration reduces PNC; Table 2 and Figure 2 show reductions under both HEPA and sham filtration, although the reduction is considerably less during sham filtration. There is empirical evidence that air movement reduces PNC indoors [23, 42, 43]. While this effect could be due to tightness of the building envelop, which can reduce indoor pollution of outdoor origin depending on the infiltration rate rather than sham filtration, we doubt this is the case because most apartments opened windows (Table 1). Thus, not using a sham configuration for comparison might provide a better estimate of effect.
Window opening reduces the effectiveness of filtration by allowing ambient UFP into the home [44]. This may have affected our ability to reduce PNC indoors to our goal of 80–90% reduction. It is common for low-income residents who lack mechanical air handling systems and central air conditioning to open windows in hot weather to cool the interior space or to let out cooking or other fumes and excess heat in colder weather. Although we conducted our interventions year round, PNC are highest in colder weather [2], the same time period in which windows are more likely to stay closed. To us, this suggests that focusing HEPA interventions on the colder months could boost impact.
Indoor sources, primarily from cooking and cleaning, but also potentially from candles or incense (but not tobacco smoke since we used only non-smoking households), were evident as indoor PNC spikes in most of the homes. This finding is similar to other studies that have reported substantial PNC contributions from indoor sources [24, 26].
HEPA filtration may not attenuate indoor spikes readily because of the high concentration of UFP and rate of flow of air through filters relative to room volume. We assessed associations separately with the biomarkers for total indoor and spike-attenuated indoor exposures but differences in association were minimal (see Table 3).
We did not find positive associations between three-week PNC exposures and any of the biomarkers. One possibility is that the study population might be relatively immune to effects of PNC. Participants had high hsCRP levels, with a majority (69.6%) above 3 mg/L, the clinical cutoff for elevated inflammation. Also, about a third of our study population had a history of heart attack. Due to the small sample size and widespread use of medication (Table 1) we could not test for the effect of medications that influence inflammatory responses. Nevertheless, there is also countervailing evidence that people with pre-existing cardiovascular disease may be vulnerable to exposure to PM, including UFP [45].
Our finding of an inverse association between PNC and biomarkers, while not statistically significant, is unexpected and was consistent across multiple biomarkers and analyses. One possibility is that an unmeasured pollutant that is inversely associated with PNC affected the results. We did not measure additional pollutants, some of which could be of either indoor or outdoor origin, including PM2.5, black carbon and oxides of nitrogen. It seems, however, unlikely that any fractions of PM would be inversely associated with both PNC and our biomarkers they are all removed by HEPA filtration.
PNC measured in the room with the HEPA may not be an adequate indicator of personal exposure of study participants [46]. Accuracy and precision of affordable personal-PNC monitors is substantially lower than bench-grade monitors. Because of this, we used bench grade instruments placed in the room with the HEPA filter. More detailed time-activity information on participants would have allowed us to more accurately assign exposures. The approach we used for time activity was derived from our observational studies [47] and did not include time spent in different rooms within the house. Adherence to activity logs by study participants tends to be low; however, Bluetooth or beacon technology exists that could be deployed to record presence of participants in the room(s) with filtration [48].
Since filtration will reduce both PM mass and PNC, it is a limitation that we measured only PNC. Nevertheless, the reduction of PM mass, including PM2.5, would be expected to enhance the benefit of the HEPA filtration. Thus, the null finding for HEPA versus sham reflects overall reduction in PM, not only UFP. Data on size fractions of PM would help assess which fractions are being reduced and to what extent.
While our study had several limitations, it also had some strengths: 1) We succeeded in blinding participants to the filtration type; 2) indoor-outdoor monitoring allowed us to assess efficacy of filtration and to see evidence of PNC reductions during sham filtration; 3) based on observational research outcomes [34, 35] the length of our intervention and sham periods, three weeks, should have been long enough to see the influence of PNC exposure on the blood biomarkers; 4) the randomized crossover design, while problematic in other ways, effectively eliminated confounding as a concern; 5) having continuous monitoring in all homes for the entire (six-week) study period is rare in HEPA intervention trials and gave us greater confidence that we knew the PNC levels in each home; and 6) our ability to separate indoor-generated spikes in PNC from the rest of PNC exposure is innovative for intervention studies and needs to be replicated by others.
Conclusions
We succeeded in completing and analyzing a HEPA intervention trial for UFP. While we did not find benefits on blood biomarkers for CVD risk, we learned valuable lessons that could inform future trials.
Supplementary Material
Highlights.
A randomized intervention study of HEPA filtration in homes was conducted.
Our target pollutants were ultrafine particles from traffic.
We assessed whether residents had lower blood biomarkers during filtration.
We reduced ultrafine particle exposure, but not as much as we wanted.
Blood biomarkers were not reduced during filtration.
Acknowledgments
We would like to acknowledge the Boston Puerto Rican Health Study team, especially Katherine Tucker and Esther Carver. Alexis Soto, Nancy Figueroa, and Migdalia Tracy did recruitment and collected survey data from the participants. Alex Bob assisted with data collection in Boston. Flora Berklein assisted with preparing the manuscript. Funding for this work was provided by the National Heart, Lung, and Blood Institute (P01 AG023394 and P50 HL105185), the National Institute of Environmental Health Sciences (ES015462), the National Science Foundation (0966093) and the Department of Civil and Environmental Engineering at Tufts University.
Footnotes
Conflicts of Interest
The authors declare that they have no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012;380:2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Padró-Martínez LT, Patton AP, Trull JB, Zamore W, Brugge D, Durant JL. Mobile monitoring of particle number concentration and other traffic-related air pollutants in a near-highway neighborhood over the course of a year. Atmospheric Environ Oxf Engl 1994. 2012;61:253–64. doi: 10.1016/j.atmosenv.2012.06.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karner AA, Eisinger DS, Niemeier DA. Near-roadway air quality: synthesizing the findings from real-world data. Environ Sci Technol. 2010;44:5334–44. doi: 10.1021/es100008x. [DOI] [PubMed] [Google Scholar]
- 4.Araujo JA, Nel AE. Particulate matter and atherosclerosis: role of particle size, composition and oxidative stress. Part Fibre Toxicol. 2009;6:24. doi: 10.1186/1743-8977-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostro B, Hu J, Goldberg D, Reynolds P, Hertz A, Bernstein L, et al. Associations of Mortality with Long-Term Exposures to Fine and Ultrafine Particles, Species and Sources: Results from the California Teachers Study Cohort. Environ Health Perspect. 2015 doi: 10.1289/ehp.1408565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viehmann A, Hertel S, Fuks K, Eisele L, Moebus S, Möhlenkamp S, et al. Long-term residential exposure to urban air pollution, and repeated measures of systemic blood markers of inflammation and coagulation. Occup Environ Med. 2015;72:656–63. doi: 10.1136/oemed-2014-102800. [DOI] [PubMed] [Google Scholar]
- 7.Lane KJ, Levy JI, Scammell MK, Peters JL, Patton AP, Reisner E, et al. Association of modeled long-term personal exposure to ultrafine particles with inflammatory and coagulation biomarkers. Environ Int. 2016;92–93:173–82. doi: 10.1016/j.envint.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguilera I, Dratva J, Caviezel S, Burdet L, de Groot E, Ducret-Stich R. Particulate Matter and Subclinical Atherosclerosis: Associations between Different Particle Sizes and Sources with Carotid Intima-Media Thickness in the SAPALDIA Study. Environ Health Perspect. 2016:124. doi: 10.1289/EHP161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrer P, Wargocki P, Fanetti A, Bischof W, De Oliveira Fernandes E, Hartmann T, et al. What does the scientific literature tell us about the ventilation–health relationship in public and residential buildings? Build Environ. 2015;94(Part 1):273–86. [Google Scholar]
- 10.Georgetown Law Library; [Accessed 19 Jul 2016]. Traffic-related air pollution : a critical review of the literature on emissions, exposure, and health effects ; HEI panel on the health effects of traffic-related air pollution. http://cdm16064.contentdm.oclc.org/cdm/ref/collection/p266901coll4/id/2584. [Google Scholar]
- 11.Ghosh R, Lurmann F, Perez L, Penfold B, Brandt S, Wilson J, et al. Near-Roadway Air Pollution and Coronary Heart Disease: Burden of Disease and Potential Impact of a Greenhouse Gas Reduction Strategy in Southern California. Environ Health Perspect. 2015:124. doi: 10.1289/ehp.1408865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.15-1026_ORD_184245_6-4-16.pdf. [Accessed 19 Jul 2016]; http://clkrep.lacity.org/onlinedocs/2015/15-1026_ORD_184245_6-4-16.pdf.
- 13.Stephens B, Siegel JA. Ultrafine particle removal by residential heating, ventilating, and air-conditioning filters. Indoor Air. 2013;23:488–97. doi: 10.1111/ina.12045. [DOI] [PubMed] [Google Scholar]
- 14.Polidori A, Fine PM, White V, Kwon PS. Pilot study of high-performance air filtration for classroom applications. Indoor Air. 2013;23:185–95. doi: 10.1111/ina.12013. [DOI] [PubMed] [Google Scholar]
- 15.Batterman S, Du L, Mentz G, Mukherjee B, Parker E, Godwin C, et al. Particulate matter concentrations in residences: an intervention study evaluating stand-alone filters and air conditioners. Indoor Air. 2012;22:235–52. doi: 10.1111/j.1600-0668.2011.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bräuner EV, Forchhammer L, Møller P, Barregard L, Gunnarsen L, Afshari A, et al. Indoor Particles Affect Vascular Function in the Aged. Am J Respir Crit Care Med. 2008;177:419–25. doi: 10.1164/rccm.200704-632OC. [DOI] [PubMed] [Google Scholar]
- 17.Padró-Martínez LT, Owusu E, Reisner E, Zamore W, Simon MC, Mwamburi M, et al. A Randomized Cross-over Air Filtration Intervention Trial for Reducing Cardiovascular Health Risks in Residents of Public Housing near a Highway. Int J Environ Res Public Health. 2015;12:7814–38. doi: 10.3390/ijerph120707814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuller CH, Patton AP, Lane K, Laws MB, Marden A, Carrasco E, et al. A community participatory study of cardiovascular health and exposure to near-highway air pollution: study design and methods. Rev Environ Health. 2013;28:21–35. doi: 10.1515/reveh-2012-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tucker KL, Mattei J, Noel SE, Collado BM, Mendez J, Nelson J, et al. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10:107. doi: 10.1186/1471-2458-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar P, Fennell P, Symonds J, Britter R. Treatment of losses of ultrafine aerosol particles in long sampling tubes during ambient measurements. Atmos Environ. 2008;42:8819–26. [Google Scholar]
- 21.Bhangar S, Mullen NA, Hering SV, Kreisberg NM, Nazaroff WW. Ultrafine particle concentrations and exposures in seven residences in northern California. Indoor Air. 2011;21:132–44. doi: 10.1111/j.1600-0668.2010.00689.x. [DOI] [PubMed] [Google Scholar]
- 22.Rim D, Wallace L, Persily A. Infiltration of Outdoor Ultrafine Particles into a Test House. Environ Sci Technol. 2010;44:5908–13. doi: 10.1021/es101202a. [DOI] [PubMed] [Google Scholar]
- 23.Wallace L, Kindzierski W, Kearney J, MacNeill M, Héroux M-È, Wheeler AJ. Fine and Ultrafine Particle Decay Rates in Multiple Homes. Environ Sci Technol. 2013;47:12929–37. doi: 10.1021/es402580t. [DOI] [PubMed] [Google Scholar]
- 24.Bekö G, Weschler CJ, Wierzbicka A, Karottki DG, Toftum J, Loft S, et al. Ultrafine Particles: Exposure and Source Apportionment in 56 Danish Homes. Environ Sci Technol. 2013;47:10240–8. doi: 10.1021/es402429h. [DOI] [PubMed] [Google Scholar]
- 25.Kearney J, Wallace L, MacNeill M, Héroux M-È, Kindzierski W, Wheeler AJ. Residential infiltration of fine and ultrafine particles in Edmonton. Atmos Environ. 2014;94:793–805. [Google Scholar]
- 26.Kearney J, Wallace L, MacNeill M, Xu X, VanRyswyk K, You H, et al. Residential indoor and outdoor ultrafine particles in Windsor, Ontario. Atmos Environ. 2011;45:7583–93. [Google Scholar]
- 27.Wheeler AJ, Wallace LA, Kearney J, Ryswyk KV, You H, Kulka R, et al. Personal, Indoor, and Outdoor Concentrations of Fine and Ultrafine Particles Using Continuous Monitors in Multiple Residences. Aerosol Sci Technol. 2011;45:1078–89. [Google Scholar]
- 28.Weichenthal S, Dufresne A, Infante-Rivard C, Joseph L. Indoor ultrafine particle exposures and home heating systems: A cross-sectional survey of Canadian homes during the winter months. J Expo Sci Environ Epidemiol. 2007;17:288–97. doi: 10.1038/sj.jes.7500534. [DOI] [PubMed] [Google Scholar]
- 29.Allen RW, Carlsten C, Karlen B, Leckie S, van Eeden S, Vedal S, et al. An Air Filter Intervention Study of Endothelial Function among Healthy Adults in a Woodsmoke-impacted Community. Am J Respir Crit Care Med. 2011;183:1222–30. doi: 10.1164/rccm.201010-1572OC. [DOI] [PubMed] [Google Scholar]
- 30.Lin L-Y, Chen H-W, Su T-L, Hong G-B, Huang L-C, Chuang K-J. The effects of indoor particle exposure on blood pressure and heart rate among young adults: An air filtration-based intervention study. Atmos Environ. 2011;45:5540–4. [Google Scholar]
- 31.Chen R, Zhao A, Chen H, Zhao Z, Cai J, Wang C, et al. Cardiopulmonary benefits of reducing indoor particles of outdoor origin: a randomized, double-blind crossover trial of air purifiers. J Am Coll Cardiol. 2015;65:2279–87. doi: 10.1016/j.jacc.2015.03.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chin MT. Basic mechanisms for adverse cardiovascular events associated with air pollution. Heart. 2015:253–6. doi: 10.1136/heartjnl-2014-306379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Traboulsi H, Guerrina N, Iu M, Maysinger D, Ariya P, Baglole CJ. Inhaled Pollutants: The Molecular Scene behind Respiratory and Systemic Diseases Associated with Ultrafine Particulate Matter. Int J Mol Sci. 2017:18. doi: 10.3390/ijms18020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hertel S, Viehmann A, Moebus S, Mann K, Bröcker-Preuss M, Mohlenkamp S, et al. Influence of short-term exposure to ultrafine and fine particles on systemic inflation. Eur J Epidemiol. 2010;25:581–92. doi: 10.1007/s10654-010-9477-x. [DOI] [PubMed] [Google Scholar]
- 35.Fuller CH, Williams PL, Mittleman MA, Patton AP, Spengler JD, Brugge D. Response of biomarkers of inflammation and coagulation to short-term changes in central site, local, and predicted particle number concentrations. Ann Epidemiol. 2015;25:505–11. doi: 10.1016/j.annepidem.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weichenthal S, Mallach G, Kulka R, Black A, Wheeler A, You H, et al. A randomized double-blind crossover study of indoor air filtration and acute changes in cardiorespiratory health in a First Nations community. Indoor Air. 2013;23:175–84. doi: 10.1111/ina.12019. [DOI] [PubMed] [Google Scholar]
- 37.Kajbafzadeh M, Brauer M, Karlen B, Carlsten C, van Eeden S, Allen RW. The impacts of traffic-related and woodsmoke particulate matter on measures of cardiovascular health: a HEPA filter intervention study. Occup Environ Med. 2015;72:394–400. doi: 10.1136/oemed-2014-102696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delfino RJ, Staimer N, Tjoa T, Gillen DL, Polidori A, Arhami M, et al. Air pollution exposures and circulating biomarkers of effect in a susceptible population: Clues to potential causal component mixtures and mechanisms. Environ Health Perspect. 2009;117:1232–8. doi: 10.1289/ehp.0800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devlin RB, Smith CB, Schmitt MT, Rappold AG, Hinderliter A, Graff D, et al. Controlled exposure of humans with metabolic syndrome to concentrated ultrafine ambient particulate matter causes cardiovascular effects. Toxicol Sci. 2014;140:61–72. doi: 10.1093/toxsci/kfu063. [DOI] [PubMed] [Google Scholar]
- 40.Fisk W. Health benefits of particle filtration. Indoor Air. 2013;23:357–68. doi: 10.1111/ina.12036. [DOI] [PubMed] [Google Scholar]
- 41.McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: New concepts are needed to study research participation effects. J Clin Epidemiol. 2013;67:267–77. doi: 10.1016/j.jclinepi.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee W-C, Catalano PJ, Yoo JY, Park CJ, Koutrakis P. Validation and Application of the Mass Balance Model To Determine the Effectiveness of Portable Air Purifiers in Removing Ultrafine and Submicrometer Particles in an Apartment. Environ Sci Technol. 2015;49:9592–9. doi: 10.1021/acs.est.5b03126. [DOI] [PubMed] [Google Scholar]
- 43.Lee W-C, Wolfson JM, Catalano PJ, Rudnick SN, Koutrakis P. Size-Resolved Deposition Rates for Ultrafine and Submicrometer Particles in a Residential Housing Unit. Environ Sci Technol. 2014;48:10282–90. doi: 10.1021/es502278k. [DOI] [PubMed] [Google Scholar]
- 44.Jones RV, Fuertes A, Gregori E, Giretti A. Stochastic behavioural models of occupants’ main bedroom window operation for UK residential buildings. Build Environ. 2017;118(Supplement C):144–58. [Google Scholar]
- 45.Rückerl R, Ibald-Mulli A, Koenig W, Schneider A, Woelke G, Cyrys J, et al. Air Pollution and Markers of Inflammation and Coagulation in Patients with Coronary Heart Disease. Am J Respir Crit Care Med. 2006;173:432–41. doi: 10.1164/rccm.200507-1123OC. [DOI] [PubMed] [Google Scholar]
- 46.de Kluizenaar Y, Kuijpers E, Eekhout I, Voogt M, Vermeulen RCH, Hoek G, et al. Personal exposure to UFP in different micro-environments and time of day. Build Environ. 2017;122(Supplement C):237–46. [Google Scholar]
- 47.Lane KJ, Levy JI, Scammell MK, Patton AP, Durant JL, Mwamburi M, et al. Effect of time-activity adjustment on exposure assessment for traffic-related ultrafine particles. J Expo Sci Environ Epidemiol. 2015;25:506–16. doi: 10.1038/jes.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson D. [Accessed 11 Nov 2016];Guide to iBeacon Hardware. 2013 http://beekn.net/guide-to-ibeacons/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.