Abstract
Purpose: Following a motor incomplete spinal cord injury (iSCI), there is decreased volitional activation and changes in composition, architecture, and stiffness of affected muscles. We investigated the relationship between muscle properties and volitional activation. Methods: The relationship between central activation ratio (CAR), maximum volitional torque (MVT), and muscle properties was assessed in the gastrocnemius of 6 participants with iSCI. Results: No significant relationship was found between CAR and muscle properties, while a significant relationship was found between CAR and MVT. Conclusion: Our findings suggest there may be no relationship between volitional activation and muscle; thus, certain patients with iSCI may benefit from therapies targeted at neural control.
Keywords: central activation ratio, incomplete spinal cord injury, maximum volitional torque, passive stiffness, shear wave elastography
Following motor incomplete spinal cord injury (iSCI), individuals often have disrupted volitional activation from damaged neural pathways, resulting in a reduction in the maximum volitional torque (MVT) in affected muscles.1 Temporal changes in the structural properties of skeletal muscle are also observed and include atrophy,2 fiber type transformation,2 and increased passive stiffness.3 These changes in muscle properties are typically associated with decreased muscle strength such that all of these changes could have profound effects on the force-generating capacity of the affected musculature. While both motor control deficits and muscular changes have been well documented independently, there remains a lack of understanding of any direct relationship. An improved understanding could aid in the development and refinement of specific rehabilitation protocols, potentially improving the diagnostic, prognostic, and therapeutic landscape of patients with motor iSCI.
Inability to recruit all motor units because of damaged neuromuscular pathways results in decreased volitional activation.4 The severity of volitional activation deficits has previously been quantified in individuals with iSCI using supramaximal neuromuscular stimulation to determine the central activation ratio (CAR), which is the ratio between MVT and the force-generating capacity.1,5,6 Reductions in CAR have previously been reported in a discrete number of participants with chronic whiplash-associated disorder7 and iSCI.5
Muscle properties can be characterized by several imaging modalities. Decreased muscle size can be indicated by decreased muscle volume and cross-sectional area as determined by magnetic resonance imaging and muscle thickness as determined by B-mode ultrasound. Muscle composition, specifically fat and connective tissue, can be quantified by calculating echogenicity using B-mode ultrasound.8 Shear wave (SW) ultrasound elastography is a relatively recent method for in vivo quantification of muscle material properties, specifically stiffness, and has been applied to clinical populations such as stroke and cerebral palsy.9,10 SW velocity is related to shear modulus such that SWs travel faster in a stiffer material.11,12 Previously, in the paretic bicep brachii of individuals who have had a stroke, increased SW velocity was correlated with the time since stroke and the severity of impairment, measured by the Fugl-Meyer score.9 Based on previous findings, we hypothesize that a relationship exists between volitional activation (a measure of the severity of impairment) and muscle properties. However, to our knowledge, this relationship has not been investigated in neurologically impaired populations, including iSCI. Accordingly, the purpose of this preliminary study was to investigate the relationship between ultrasound measures of muscle properties, specifically stiffness, of the plantar flexor muscle, gastrocnemius, and biomechanical measures of plantar flexor MVT and CAR using burst superimposition.
Methods
Participants
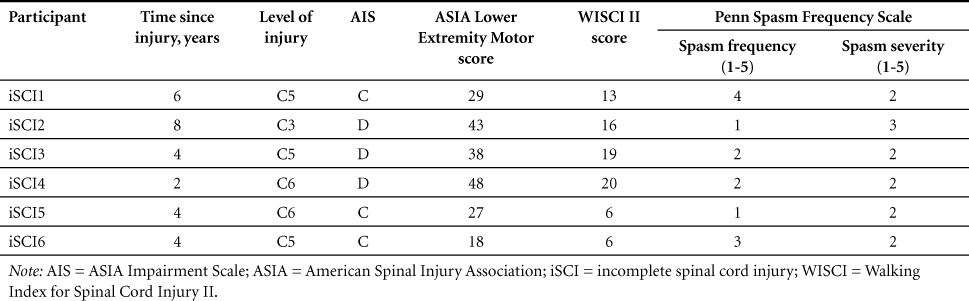
Six male participants with iSCI participated in this study (mean [SD] age, 41.3 [13.4] years; mass, 86.7 [15.0] kg; height, 1.82 [0.09] m; years since injury, 4.7 [2.1]). All subjects were American Spinal Injury Association (ASIA) Impairment Scale (AIS) C and D classification and could complete a 10-m walk test using an assistive device. All participants scored at least a 6 on the Walking Index for Spinal Cord Injury II (WISCI II) and scored greater than 15 on the ASIA Lower Extremity Motor score. See Table 1 for details on participant scores. All subjects gave informed consent prior to testing, and Northwestern University's Institutional Review Board approved all procedures.
Table 1.
Participant characteristics
Maximum voluntary torque and central activation ratio
Participants were seated upright in a Biodex chair (Biodex Medical System, Inc., Shirley, NY), with their foot secured to a plate, a hip angle of 75°, knees flexed at 10°, and ankle at 90°. MVT was assessed via 3 contractions of 3 to 4 seconds in duration while verbal encouragement was given. In separate trials, a brief train of supramaximal stimuli was applied to the triceps surae during MVT production once volitional torque plateaued (10 square-wave pulses, 600 μs pulse duration, 100 Hz, 135 V) (Grass S48, external isolation; Grass Technologies, West Warwick, RI), via 3 × 5 in. stimulation electrodes over the triceps surae, with the anode distal to the popliteal fossa and cathode distal to the soleus myotendinous junction.1,13 CAR is the ratio of the peak volitional torque over the sum of peak volitional and electrically stimulated torque.4 MVT was calculated from the mean torque produced 100 ms prior to stimulation.1 To prevent fatigue, participants were given at least 2 minutes of rest between trials. Additionally, pain was rated numerically before and after testing. CAR and MVT were evaluated bilaterally
Ultrasound
Subjects were seated as described above with their knee flexed at 10° and ankle at 90°. It has been well established that SW velocity14 and force are dependent on length; therefore, it was important that measurements of SW velocity, CAR, and MVT were taken in the same position and were consistent between subjects. Five images (SW and B-mode ultrasound simultaneously; Aixplorer SuperSonic Imagine, Aix en Provence, France) were collected bilaterally of the medial and lateral gastrocnemius while the participants were instructed to keep the muscle relaxed and at rest. Technical details of this imaging procedure have been described previously.12 Briefly, the transducer was positioned at the mid-belly region of the respective muscle and aligned with the fascicles as viewed from the B-mode image. The SW velocity map region of interest (ROI) was manually placed between the superficial and deep aponeurosis.
Data analysis
Custom-written software in MATLAB (Mathworks, Natick, MA) was used in this study. All the values from the ROI were used except for areas outside of the muscle belly, which were manually cropped. The spatial average of SW velocity was calculated across the cropped ROI and averaged across all trials for each respective muscle. To quantify echogenicity, a measure of the brightness of the B-mode images, the gain and power settings of the ultrasound system were kept the same for imaging of all muscles for all participants.9 The mean echogenicity was calculated from the same ROI within the muscle as the SW velocity. Echogenicity was calculated using the “regionprobs ‘Mean Intensity’” function in MATLAB, which determines the mean intensity values from a selected ROI. Average echogenicity was calculated between trials for each muscle respectively. Muscle thickness was measured by digitizing the distance between the superficial and deep aponeuroses in the medial and lateral gastrocnemius. An average thickness was calculated between trials for each muscle. The values for the medial and lateral gastrocnemius were averaged to get a gastrocnemius approximation similar to that obtained using CAR and MVT.
Statistical analysis
All statistical analyses were performed in Minitab (Version 17, State College, PA). Linear regression analyses with side as a categorical independent variable, in order to consider each limb as dependent of the other, were performed to evaluate the relationships between SW velocity, CAR, MVT, muscle thickness, and echogenicity. Significance was set at p ≤ .05.
Results
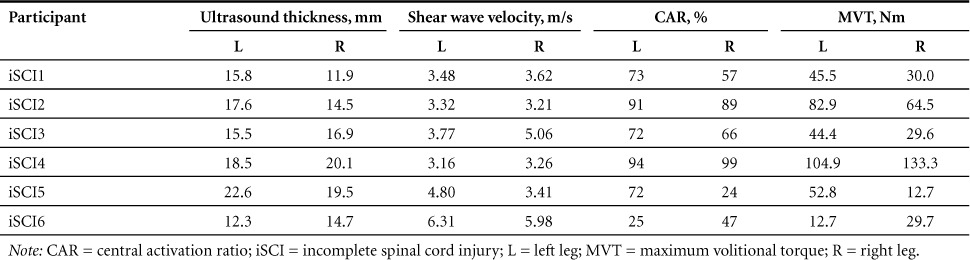
No significant regressions were found between the CAR and SW velocity (p = .12), muscle thickness (p = .56), and echogenicity (p = .31). No significant regressions were found between the MVT and SW velocity (p = .17), muscle thickness (p = .33), and echogenicity (p = .25). A significant regression was found between CAR and MVT (p = .001). See Table 2 for subject data.
Table 2.
Participant data
Discussion
Our key findings demonstrate that although a strong relationship exists between CAR and MVT in the gastrocnemius of patients with motor iSCI, volitional activation (CAR) and functional output (MVT) are not associated with muscle composition, size, or stiffness. While preliminary, and contrary to our hypothesis, these results suggest that changes in muscle properties do not account for decreased functional output and volitional activation in our patient population with motor iSCI. It is plausible that functional motor output is mechanistically aligned more with neural control factors. In other larger clinical investigations involving patients with stroke, the contribution of muscle to functional deficits seems variable. In some groups, muscle atrophy alone could not account for decreased force production,15 whereas in another stroke group, SW velocity was greater in the biceps brachii muscle on the paretic side compared to the contralateral nonparetic side indicating increased stiffness. This increased SW velocity was also correlated with the severity of impairment.9 Therefore, it is possible that in some iSCI patients, like the stroke population, impaired volitional activation contributes substantially to functional deficits.
Several important limitations need to be addressed. First, we acknowledge the relatively small sample size. This participant group is part of a larger heterogeneous participant pool.16 For the purpose of consistency, the subjects were prescreened to have similar clinical presentations of iSCI. It is also recognized that stimulation was applied to the entire triceps surea, whereas stiffness measurements were made only from the gastrocnemius; this study would benefit by the addition of stiffness measurements from the soleus. Last, it has been documented that SW velocity is sensitive to the level of activation14; however, in this experiment, electromyographical data were not monitored or collected.
Conclusion
Being able to determine the mechanism of impairment is important in developing effective, patient-specific rehabilitation protocols. In this group of motor iSCI patients, therapies primarily targeted at neural control may be more effective as opposed to therapies targeted at muscular changes, including atrophy.
Acknowledgments
The funding source, the NIH National Institute of Child Health and Human Development (grant nos. T32 HD057845, 5R01HD079076-03, K12HD073945), was not involved in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
James M. Elliott reports board membership, consultancy, other (35% ownership/investment interest in medical consultancy start-up: Pain ID LLC), and payment for lectures. The other authors declare no conflicts of interest related to this work.
REFERENCES
- 1. Hornby T, Lewek M, Thompson C, Heitz R.. Repeated maximal volitional effort contractions in human spinal cord injury: Initial torque increases and reduced fatigue. Neurorehabil Neural Repair. 2009; 23: 928– 938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biering-Sørensen B, Kristensen I, Kjaer M, Biering-Sørensen F.. Muscle after spinal cord injury. Muscle Nerve. 2009; 40 4: 499– 519. [DOI] [PubMed] [Google Scholar]
- 3. Diong J, Herbert R, Harvey L, . et al. Passive mechanical properties of the gastrocnemius after spinal cord injury. Muscle Nerve. 2012; 46 2: 237– 245. [DOI] [PubMed] [Google Scholar]
- 4. Kent-Braun J, Le Blanc R.. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve. 1996; 19 7: 861– 869. [DOI] [PubMed] [Google Scholar]
- 5. Smith A, Parrish T, Hoggrath M, . et al. Potential associations between chronic whiplash and incomplete spinal cord injury. Spinal Cord Ser Cases. 2015; 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim H, Thompson C, Hornby T.. Muscle activation varies with contraction mode in human spinal cord injury. Muscle Nerve. 2015; 51 2: 235– 245. [DOI] [PubMed] [Google Scholar]
- 7. Elliott J, Dewald J, Hornby T, Walton D, Parrish T.. Mechanisms underlying chronic whiplash: Contributions from an incomplete spinal cord injury? Pain Med. 2014; 15 11: 1983– 1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pillen S, Tak R, Zwarts M, . et al. Skeletal muscle ultrasound: Correlation between fibrous tissue and echo intensity. Ultrasound Med Biol. 2009; 35 3: 443– 446. [DOI] [PubMed] [Google Scholar]
- 9. Lee S, Spear S, Rymer W.. Quantifying changes in material properties of stroke-impaired muscle. Clin Biomechanics. 2015; 30 3: 269– 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee SSM, Gaebler-Spira D, Zhang LQ, Rymer WZ, Steele KM.. Use of shear wave ultrasound elastography to quantify muscle properties in cerebral palsy. Clin Biomechanics. 2016; 31: 20– 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brandenburg J, Eby S, Song P, . et al. Ultrasound elastography: The new frontier in direct measurement of muscle stiffness. Arch Phys Med Rehabil. 2014; 95 11: 2207– 2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bercoff J, Tanter M, Fink M.. Supersonic shear imaging: A new technique for soft tissue elasticity mapping. IEEE Trans Ultrasonics Ferroelectrics Frequency Control. 2004; 51 4: 396– 409. [DOI] [PubMed] [Google Scholar]
- 13. Scaglioni G, Martin A.. Assessment of plantar flexors activation capacity: Nerve versus muscle stimulation by single versus double pulse. Eur J Appl Physiol. 2009; 106: 563– 572. [DOI] [PubMed] [Google Scholar]
- 14. Chernak L, DeWall R, Lee K, Thelen D.. Length and activation dependent variations in muscle shear wave speed. Physiol Measure. 2013; 34 6: 713– 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knarr B, Ramsay J, Buchanan T, Higginson J.. Muscle volume as a predictor of maximum force generating ability in the plantar flexors post-stroke. Muscle Nerve. 2013; 48 6: 971– 976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith A, Weber K, Parrish T, . et al. Ambulatory function in motor incomplete spinal cord injury: A magnetic resonance imaging study of spinal cord edema and lower extremity muscle morphometry. Spinal Cord. 2017; 55: 672– 678. [DOI] [PMC free article] [PubMed] [Google Scholar]


