Abstract
Background and Purpose: Understanding the role and significance of trunk and upper extremity muscles in paraplegic gait can help in designing more effective assistive devices for these patients and also provides valuable information for improving muscle strengthening programs. Methods: In a patient with a spinal cord injury (SCI) who could walk independently (rating scale of ambulatory capacity, 9) with the aid of bilateral ankle-foot orthosis and a walker, the kinematics, kinetics and electromyographic (EMG) activities of 16 muscles from the trunk and upper and lower extremities were recorded during gait. The onset, cessation, and duration of the EMG signal were associated with the 4 phases of each step, distinguished based on the kinematics results. Results: It was found that the reciprocating activation pattern of the quadratus lumborum, latissimus dorsi, pectoralis major, and lower trapezius is responsible for trunk extension during the balance adjustment phase, leg unload and foot clearance creation during the leg raising phase, and propulsion force generation during the leg swing phase. Conclusion: The continuous activation of the rectus abdominis and erector spinae within the gait cycle helps stabilize the thorax and acts in reverse, that is, fixes the proximal joint and moves the distal limb. The shoulder girdle muscles contribute to the leg's unloading and then smooth landing during leg raising and leg swing phases, respectively.
Keywords: electromyography, gait analysis, paraplegic gait, spinal cord injury, trunk muscles, upper extremity muscles, walker
Standing and walking are reported to bring considerable advantages for spinal cord injury (SCI) patients such as decreasing the risks of bone osteoporosis, muscle spasm, and joint deformity. They can also improve the function of the bowel and bladder and the digestive, cardiovascular, and respiratory systems.1,2 Furthermore, walking, even with crutch or walker, has a significant impact on independence and quality of life of such individuals.
A wide range of assistive devices have been developed to facilitate the gait of SCI patients. However, paraplegic gait often causes a substantial energy expenditure, as high as 3 to 9 times of that of the normal population, which leads to early fatigue.3–5 This gait has also been reported to result in a higher prevalence of shoulder pain due to the necessity of transferring loads through crutches or walkers.6,7 Because the legs are paralyzed, the primary contributors to paraplegic locomotion are the muscles of the upper extremity and trunk. Understanding the role and significance of these muscles in the different phases of gait can help improve the efficiency of the gait pattern and contribute to the design of more effective assistive devices. It can also provide valuable information that can help physical therapists create more efficient muscle strengthening programs for SCI patients.
Several studies have investigated the excitation pattern of the muscles of the trunk and upper extremity of SCI patients during different activities, such as the compensatory role of trunk muscles in balance preservation,8 postural control,9 and forward reaching.10 Other studies have investigated the role and significance of the shoulder muscles of SCI patients during wheelchair propulsion11–13 and activities of daily living.14 Moreover, kinematics and kinetics of walker-assisted gait have been studied by some researchers.15,16
To the best of our knowledge, there are no studies in the literature on the activation of the trunk and upper extremity muscles of SCI patients during locomotion. The only previous electromyography (EMG) studies on the paraplegic gait examined the excitation pattern of the lower limb muscles,17–20 which is thought to originate from the H-reflex mechanisms and the spinal locomotor centers of paraplegic patients,21 with no major contribution to the gait.
The aim of this study was to investigate the activation pattern of the muscles of trunk and upper extremity of walker-assisted paraplegic gait (WAPG) to better understand the role and significance of each individual muscle. The kinematics and kinetics of the gait and the EMG data of 16 muscles of the trunk and upper and lower extremities were recorded for one individual with SCI. The EMG data were then associated with the 4 phases of the gait cycle, recognized based on the kinematics and kinetics patterns, to establish the role of muscle activation in the different tasks involved in the paraplegic gait.
Methods
A single individual (male; 30 years old; weight, 65 kg; height, 175 cm) with an SCI (level T7) from those receiving rehabilitation at Red Crescent Center in Tehran, Iran, participated in this investigation. The individual was injured 25 months prior to the study due to a car accident. He wore bilateral ankle-foot orthosis (AFO) and could walk independently with the aid of a walker after 22 months of training. The participant's ambulatory capacity was 9 (range, 0–20) based on the Walking Index for Spinal Cord Injury-II.22,23 Prior to the study, the individual was given a copy of the Bill of Rights of Human Subjects, and he read and signed an informed consent form that had been approved by Sharif University Ethical Committee.
Data collection was performed in the Gait Analysis Lab of Djavad Mowafaghian Research Center for Intelligent NeuroRehabilitaion Technologies (Tehran, Iran). Reflective markers were attached to the anatomical landmarks of the participant based on the guidelines described in Plug-in-Gait Marker Placement Protocol (Vicon Motion Systems, Oxford, UK). Pairs of Ag/AgCl surface electrodes were attached unilaterally in parallel to the fibers of 16 upper and lower limb muscles based on the guidelines described by McGill24 and others.25,26 The center-to-center distance of the electrodes was 25 mm, and they were placed in bipolar configuration. The muscles studied included G-Max (gluteus maximus), VM (vastus medialis), RF (rectus femuris), BF (biceps femuris), IO (internal oblique), EO (external oblique), RA (rectus abdominis), QL (quadratus lumborum), ES-IC (erector spinae illiocostalis), ES-LG (erector spinae longissimus), LT (lower trapezius), LD (latissimus dorsi), PM (pectoralis major), PD (posterior deltoid), TC-Lat (triceps lateral head), and TC-Long (triceps long head).
During the test, the participant was instructed to walk at his comfortable speed on a level surface along a 10-m walkway. Sixteen trials were recorded using an 8-camera motion analysis system (Vicon Motion Systems, Oxford, UK), 2 force plates (Kistler Instrument AG, Switzerland), and a WLAN telemetry electromyograph (Myon Ltd, Switzerland) at sample rates of 120, 1200, and 1200 Hz, respectively. Among the recorded trials, the 12 with the most complete tracking data were selected to be analyzed. The tracking data were filtered through a recursive low-pass digital Butterworth filter with a cut-off frequency of 4 Hz. The joints kinematics were obtained using Plug-in Gait Model.27,28 The pelvis and trunk rotations were determined with respect to the lab coordinate system and the hip and thorax rotations in relation to the pelvis. The trunk orientation was calculated once with respect to pelvis frame (relative angles) and once with respect to lab coordination system (absolute angles). The center of mass (CoM) was also obtained using kinematic centroid method in Nexus software (Vicon Motion Systems, Oxford, UK). The precision of this method has been compared to the double integration method, and an acceptable correlation has been reported except for in the vertical direction.29
The EMG signals were rectified and filtered by an IIR notch filter with 50 Hz cut-off frequency to eliminate the noise of power. The heart and other high frequency noises were also eliminated using a recursive band-pass FIR with 30 to 300 Hz cut-off frequencies.30 The root mean square of each signal, with a window size of 1 ms, was normalized by the highest 1 second of activity during maximum volunteer contraction at positions suggested by Kendall.31 The onset, cessation, and duration of the EMG signal were determined using Teager-Kaiser energy operator32 by applying the mean plus 3 standard deviation obtained from static EMG baseline as the threshold. The results were visually examined by an experienced examiner to ensure they reflected the true muscle activities.33 The data processing was performed using a custom-made code in Matlab (The Mathworks, Inc., Natick, MA).
Results
A 4-phase strategy was distinguished for each side during a WAPG step based on the kinematics and kinetics results (Figure 1): the balance adjustment, the walker propulsion, the leg raising, and the leg swing. Figure 2 illustrates the kinematics of the pelvis, trunk, and CoM trajectory during the 4 phases of the WAPG. In addition, the onset, duration, and cessation of muscle activation associated with these phases are illustrated in Figure 3.
Figure 1.
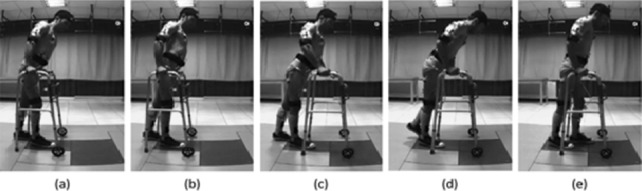
The gait phases for the left side of the paraplegic subject. (a&b) Balance adjustment: The trunk is extended and the pelvis tilted posteriorly to obtain an upright standing balance on both legs, less dependence upon the walker, and unload the upper extremities. (b&c) Walker propulsion: Having an upright balance over the legs, the walker is pushed forward. (c&d) Leg raising: After stabilizing the walker, the trunk is laterally bended and flexed, and the left pelvis is elevated and laterally tilted to transfer the weight to the upper extremity and unload and raise the left leg. Meanwhile, the pelvis is rotated to the left. (d&e) Leg swing: The pelvis is posteriorly tilted and rotated back to the right to throw the leg forward (note the distance between the hand and the hip joint).
Figure 2.
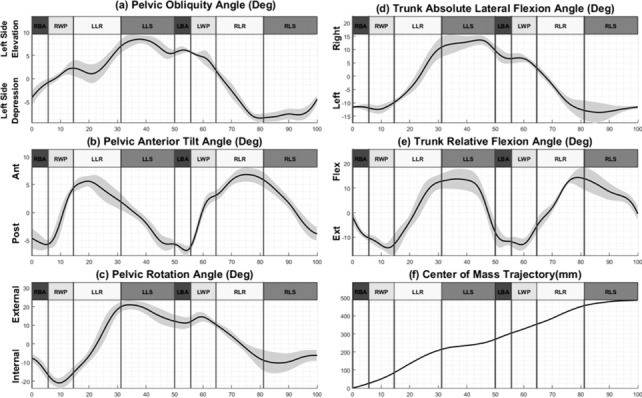
The pelvis and trunk kinematics and the center of mass trajectory during the 4 phases of the walker-assisted paraplegic gait (WAPG). (a) Pelvic lateral tilt, (b) pelvic anterior tilt, (c) pelvic rotation, (d) trunk absolute lateral flexion, (e) trunk relative flexion, and (f) center of mass trajectory in the direction of walking. The bold lines represent the means of 12 trails with the standard deviations shown as gray margins. RBA = right balance adjustment; RWP = right walker propulsion; LLR = left leg raising; LLS = left leg swing; LBA = left balance adjustment; LWP = left walker propulsion; RLR = right leg raising; RLS = right leg swing.
Figure 3.
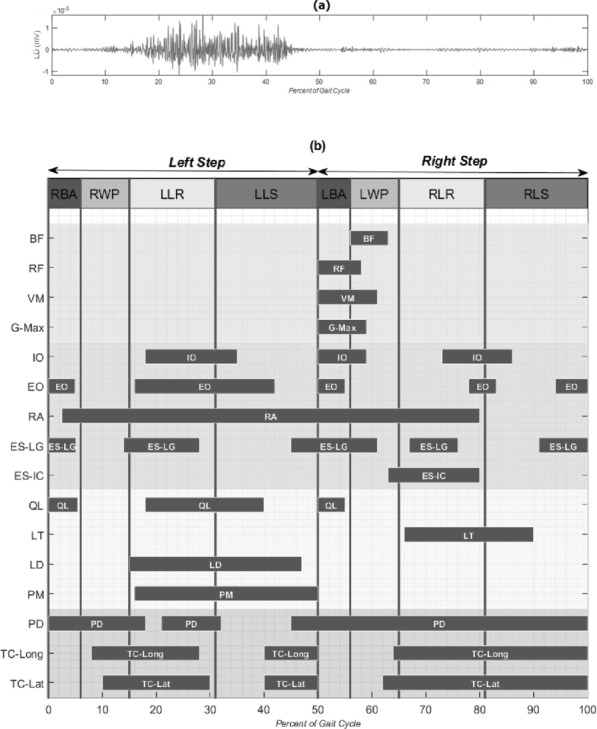
The EMG data of the trunk, upper and lower extremity muscles during the 4 phases of the walker-assisted paraplegic gait (WAPG). (a) The raw EMG signal of latissimus dorsi; (b) the onset, duration and cessation of the muscles. RBA = right balance adjustment; RWP = right walker propulsion; LLR = left leg raising; LLS = left leg swing; LBA = left balance adjustment; LWP = left walker propulsion; RLR = right leg raising; RLS = right leg swing; BF = biceps femuris; RF = rectus femuris; VM = vastus medialis; G-Max = gluteus maximus; IO = internal oblique; EO = external oblique; RA = rectus abdominis; ES-LG = erector spinae longissimus; ES-IC = erector spinae illiocostalis; QL = quadratus lumborum; LT = lower trapezius; LD = latissimus dorsi; PM = pectoralis major; PD = posterior deltoid; TC-Long = triceps long head; TC-Lat = triceps lateral head.
In the balance adjustment phase, which occupied about 6% of the gait cycle, the participant obtained a temporary upright standing balance on his legs and was less dependent upon the walker (Figure 1a and b). The major kinematic maneuvers involved in this phase were an 8° trunk extension relative to pelvis (Figure 2e), a 9° pelvis rotation to the right (Figure 2c) accompanied with a small posterior tilt (Figure 2b), and a lateral tilt to the right (Figure 2a). These movements shifted the CoM slightly forward (Figure 2f). The muscles contributing to this phase for each leg included RA, EO, IO, ES-LG, QL, and PD of the ipsilateral side and EO, ES-LG, QL, and PD of the contralateral side. In addition to these muscles, a very weak EMG signal (with an amplitude 2 orders of magnitude smaller than the other channels) was observed for ipsilateral RF, VM, and G-Max as the foot met the ground (Figure 3b).
In the walker propulsion phase, which formed about 9% of the gait cycle, the subject pushed the walker forward with his hands while enjoying a temporary upright balance over his legs. During this phase, the trunk underwent an absolute flexion (Figure 1c), mainly due to a relatively large (about 10°) anterior tilt of the pelvis (Figure 2b). Early in this phase, all muscles of the trunk and upper extremity, except for RA and PD, were relatively relaxed, considering the baseline activity. However, in the middle of this phase, the shoulder muscles group, for example, bilateral TC-Long and TC-Lat, started contraction (Figure 3b).
In the leg raising phase, which included about 16% of the gait cycle, the participant raised his left leg to make it ready for swing in the next phase. As illustrated in Figure 2d, the trunk experienced a large absolute lateral flexion to the right (about 21°) accompanied by a 24° relative flexion (Figure 2e). Also, the pelvis rotated 35° to the left and, later on, tilted laterally to the right by about 6° (Figure 2a). The longest CoM displacement in walking direction took place in this phase (Figure 2f). Most of the ipsilateral trunk and abdominal muscles, such as IO, EO, RA, ES-LG, QL, LD, and PM, as well as the contralateral ES-IC and LT were active during this phase. Another group of active muscles during leg raising were the upper extremity muscles PD, TC-Long, and TC-Lat.
During the leg swing phase, which occupied about 19% of the gait cycle, the participant threw his left leg forward to accomplish a gait step. The pelvis experienced an 8° posterior tilt (Figure 2b) accompanied by an 8° internal rotation (Figure 2c) and, late in the phase, a slight lateral tilt to the left (Figure 2a). Also, late in this phase, the trunk extended relative to the pelvis by about 21° (Figure 2e). Early in this phase, the abdominal oblique muscles continued their activity from the previous phase. While the ipsilateral LD and PM were active throughout the phase, the ipsilateral QL and the TC and PD were only active during its first and second halves, respectively.
Discussion
In this case report, the activation patterns of the trunk and lower and upper extremity muscles of an individual with SCI were studied during walker-assisted locomotion, and these patterns were associated with the kinematics and kinetics of the gait cycle. To describe the participant's maneuvers during locomotion, a simple 4-phase model, consisting of balance adjustment, walker propulsion, leg raising, and leg swing phases, was proposed to represent the major events in each step of the gait. This new phasing was necessary in view of the fact that the classical phases of normal gait cycle are not applicable to the substantially altered gait pattern of the walker-assisted paraplegic locomotion. Our methodology for gait phasing was adopted from the work of Perry,34 who suggested that the basic functional objective of each event be considered when trying to describe and interpret a pathological gait.
Second, our kinematics description of the WAPG was based mainly on the movements of the trunk and pelvis. In normal gait, the lower extremities are considered as the locomotor and the segments above the pelvis as the passenger.34 As a result, the kinematics of the gait is always described based on the movements at the lower extremity. However, in paraplegic walking, the locomotor and passenger sections are mixed; the trunk and upper extremities are also functioning as the main sources of movement of the lower extremities. In particular, the pelvis plays a critical role in paraplegic gait; it connects the 2 legs, as important members of the locomotor section, and provides a linkage between them and the trunk and upper extremities as the passenger-locomotor section. Hence, the pelvic movements are of high importance in paraplegic gait and have been studied in detail (in 3 anatomical planes) in our investigation.
The results of our study indicate that each of the 4 phases of the WAPG is realized through a complicated series of muscle synergies and joint movements. In the balance adjustment phase, the participant intended to obtain a temporary upright standing balance on his legs, with less dependence upon the walker, to unload his upper extremities and prepare them for moving into the next phase (Figure 1a and b). A major maneuver of this phase was the trunk extension relative to the pelvis (Figure 2e), which was achieved by activating the bilateral QL and ES-LG muscles (Figure 3b). This movement was accompanied with a pelvis posterior tilt and rotation to the right (Figure 2b and c) to shift the CoM slightly forward (Figure 2f). This helped to keep the weight vector in front of the knees and to lock the joints at full extension in absence of the quadriceps functioning. The small posterior tilt (Figure 2b) and rotation to the right (Figure 2c) of the pelvis were realized by right EO and left EO activation (Figure 3b) and the bilateral RA synergy (Figure 3b), respectively. The former muscles also acted as an antagonistic for ES-LG to stabilize the trunk and pelvis. Finally, a small lateral tilt of the pelvis to the right, into neutral position, helped in lowering the right hip joint and loading the 2 legs equally.
In the walker propulsion phase, the participant experienced a temporary upright balance over his legs, allowing him to push the walker forward with his hands. As a result, the trunk underwent an absolute flexion (Figure 1c), mainly due to a relatively large anterior tilt of pelvis (Figure 2b). The main muscles contributing in this phase were the shoulder muscles group (bilateral TC-Long and TC-Lat). After the trunk flexed and the CoM passed the toe tip and was outside the support polygon, these muscles started activation to contribute to the balance and weight bearing (Figure 3b).
The aim of the leg raising phase was to prepare the left leg for a gravity-assisted swing, similar to a passive pendulum, in the next phase. Three main maneuvers of this phase included unloading the left leg, creating foot clearance by hip hiking, and positioning the CoM in front of the toe tip. As a result, the longest CoM displacement in walking direction took place in this phase (Figure 2f). Right after stabilizing the walker on the ground, the participant started transferring his weight gradually to the upper extremity and unloading the left leg. While activation of the shoulder girdle muscles allowed for holding the trunk upward and accepting the weight, the trunk muscles contributed to elevating the pelvis unilaterally to unload the left leg. The increased activity of TC-Long and TC-Lat, especially in the contralateral side (shown in Figure 3b) is a result of this strategy. The activation of the contralateral LT helped transfer the load from the left lower extremity to the right shoulder and the ipsilateral LD and PM worked in synergy to push up the pelvis (Figure 3b). In addition, the ES-IC contributed to the trunk lateral flexion to the right (Figure 2d).
As illustrated in Figure 2a and Figure 3b, late in the leg raising phase, the ipsilateral QL was activated and produced a right lateral tilt in the pelvis to increase the height of the hip joint from the ground and create foot clearance. At the same time, the participant moved his CoM forward, by flexing the trunk, and rotated his pelvis to the left to achieve a posterior hip joint position for the next phase. These movements were realized by contracting the bilateral RA, EO, and IO muscles. The left EO had a reverse action in this phase and rotated the pelvis to the left while the trunk was fixed by contraction of LT and shoulder girdle muscle group.
During the leg swing phase, the participant attempted leg propulsion by throwing his leg forward using the pelvic movements. The initial posterior position of the hip joint, due to the pelvis left rotation in previous phase, allowed it to move anteriorly by rotating the pelvis to the right (Figure 2c), which threw the leg forward. Another major contribution to the propulsion force was provided by the posterior tilt of the pelvis (Figure 2b) that accompanied this rotation. These movements were realized by activation of the RA, EO, LD, and PM muscles. The transfer of part of the body weight to the upper extremity and the contralateral leg was continued in this phase. As a result, the bilateral shoulder girdle muscles (PD, TC-Lat, and TC-Long) and the contralateral LT remained active to support the force path to the shoulder.
The leg swing phase ended with a lateral tilt of the pelvis to the left (Figure 2a) to enable a smooth landing of the leg on the ground and prepare it for weight bearing in the next phase (ie, the balance adjustment for the contralateral leg step). The contralateral ES-IC was responsible for this maneuver. A gradual load bearing by the leg was also helped by the eccentric activation of the ipsilateral TC and PD that contributed to bear the weight load.
Our results suggest that the trunk muscles play a critical role in several phases of the WAPG, which is different from their minor action in normal gait.35 The activation pattern of some of these muscles (eg, QL, LD, PM, and LT) was reciprocating; they were active in 1 or 2 phases of the gait and were relaxed in the other phases. The QL muscles were responsible for extending the trunk in the balance adjustment phase and facilitated generating the foot clearance in leg raising phase. The LD and PM synergy during the leg raising and leg swing phases was the main contributor to the foot clearance creation and then the propulsion force production for leg swing. The LT muscles helped stabilize the spine and transfer the load from the lower extremity to the shoulder during the leg raising phase.
For some other trunk muscles, such as RA and ES, a continuous activation pattern was observed in the whole gait cycle. The main responsibility of these muscles is thought to be stabilizing the thorax and acting in reverse, that is, fixing the proximal joint and moving the distal limb. The bilateral ES-LG muscles helped extend the trunk during the balance adjustment phase and the ES-IC muscles contributed to the foot clearance generation and leg's smooth landing by lateral flexion of the trunk in the leg raising and leg swing phases. The RA muscle, on the other hand, was an important stabilizer of pelvis in the whole gait cycle and a contributor to propulsion force generation in the leg swing phase.
In the upper extremity, we found considerable muscle activity for the shoulder girdle muscles, such as PD, TC-Long, and TC-Lat, as expected. The TC muscles were responsible for bearing a part of body weight and unloading the legs, especially at the contralateral side, during the leg raising and leg swing phases. Also, the ipsilateral TC and PD helped the smooth landing and gradual load bearing by the swing leg, just before foot contact. An excessive extension torque in the elbow joint has been reported by Bachschmidt et al16 in their study of walker-assisted gait.
In the lower extremity muscles, very weak but distinguishable EMG signals were recorded during the balance adjustment phase. Similar abnormal spontaneous muscle activity has been reported by previous studies in limb pelagic muscles,36 however, its underlying mechanism is not well understood. We think this response might be an indication of the leg's increasing external loading in the balance adjustment phase. Nevertheless, such low intensity signals are insufficient for a real contribution to the paraplegic gait maneuvers, including the knee joint locking.21
Considering the fact that early fatigue has been reported to be a major concern in the paraplegic locomotion,37 our results suggest that special muscle strengthening programs are necessary for paraplegic individuals. In particular, selective strengthening of the TC, PM, LD, and LT muscles is important to facilitate the gait maneuvers associated with the trunk and pelvis movements. Similarly, for RA, ES, EO, and IO muscles that have a stabilizer role and are active for most of the whole gait cycle, selective strengthening can provide improved gait performance and postpone the occurrence of fatigue.
Our results (Figure 3) suggest that the leg raising phase of the walker-assisted gait contains the most intense muscular effort, in comparison with the other phases, due to the complicated maneuvers required to achieve foot clearance. Previous studies examining the development of more effective mechanical gait orthoses have often focused on the leg swing phase and have tried to facilitate the generation of the propulsion force using reciprocating mechanisms.37–39 Our results, however, suggest that providing the foot clearance might be of even more importance than the propulsion force in order to reduce the energy expenditure and avoid muscular fatigue. It is recommended that implementation of appropriate mechanisms for facilitating the foot clearance should be taken into consideration in the future designs of the mechanical gait orthoses.
There are limitations in this study that make generalization of the results difficult. First, our results were obtained in a case study from a single paraplegic subject. Moreover, the EMG data measured in our study might have been affected by measurement errors. The EMG signals were recorded using surface electrodes. For deep muscles, such electrodes can reflect the cross-talk from adjacent superficial muscles. Furthermore, the EMG signals were recorded unilaterally due to limited number of available channels while the asymmetrical nature of motor and sensory deficits might influence the gait. Future study should consider bilateral recordings with a larger number of participants. Finally, there are other muscles that seem to have a major contribution in walker-assisted gait, such as serratus anterior, infraspinatus, and rhomboid, that were not investigated in our study due to the limitation in the available EMG channels. These limitations should be addressed in detail in the future investigations.
Conclusion
A 4-phase strategy can be distinguished for each step of paraplegic externally assisted gait, including the balance adjustment, the walker propulsion, the leg raising, and the leg swing. The trunk and upper extremity muscles play a critical role in several of these phases, which is much different from their minor action in normal gait. Considering the fact that early fatigue has been reported to be a major concern in the paraplegic locomotion, it is concluded that special muscle strengthening programs are necessary for selective strengthening of the trunk and upper extremity muscles to provide improved gait performance and postpone the occurrence of fatigue. Moreover, the leg raising phase of the gait requires the most intense muscular effort due to the complicated maneuvers needed to achieve the foot clearance. This suggests that implementation of appropriate mechanisms for facilitating foot clearance should be taken into consideration in the future designs of the mechanical gait orthoses.
Acknowledgments
The authors declare no conflicts of interest. The authors thank the participant in this study for his patience in the tests. The valuable support of the administration and staff of the Red Crescent Rehabilitation Division of Tehran, especially physical therapist Mohsen Ghassami, and the Gait Analysis Lab of Djavad Mowafaghian Research Center for Intelligent NeuroRehabilitation Technologies, is also appreciated.
REFERENCES
- 1. Karimi MT. Evidence-based evaluation of physiological effects of standing and walking in individuals with spinal cord injury. Iranian J Med Sci. 2011; 36: 242– 253. [PMC free article] [PubMed] [Google Scholar]
- 2. Massucci M, Brunetti G, Piperno R, Betti L, Franceschini M.. Walking with the advanced reciprocating gait orthosis (ARGO) in thoracic paraplegic patients: Energy expenditure and cardiorespiratory performance. Spinal Cord. 1998; 36: 223– 227. [DOI] [PubMed] [Google Scholar]
- 3. Bernardi M, Canale I, Castellano V, Di Filippo L, Felici F, Marchetti M.. The efficiency of walking of paraplegic patients using a reciprocating gait orthosis. Spinal Cord. 1995; 33: 409– 415. [DOI] [PubMed] [Google Scholar]
- 4. Cerny K, Perry J, Walker J.. Effect of an unrestricted knee-ankle-foot orthosis on the stance phase of gait in healthy persons. Orthopedics. 1990; 13: 1121– 1127. [DOI] [PubMed] [Google Scholar]
- 5. Nakhaee K, Farahmand F, Salarieh H.. Studying the effect of kinematical pattern on the mechanical performance of paraplegic gait with reciprocating orthosis. Proc Inst Mech Eng H. 2012; 226: 600– 611. [DOI] [PubMed] [Google Scholar]
- 6. Mulroy SJ, Farrokhi S, Newsam CJ, Perry J.. Effects of spinal cord injury level on the activity of shoulder muscles during wheelchair propulsion: An electromyographic study. Arch Phys Med Rehabil. 2004; 85: 925– 934. [DOI] [PubMed] [Google Scholar]
- 7. Gellman H, Sib I, Waters RL.. Late complications of the weight-bearing upper extremity in the paraplegic patient. Clin Orthop Rel Res. 1988; 233: 132– 135. [PubMed] [Google Scholar]
- 8. Bjerkefors A, Carpenter MG, Cresswell AG, Thorstensson A.. Trunk muscle activation in a person with clinically complete thoracic spinal cord injury. J Rehabil Med. 2009; 41: 390– 392. [DOI] [PubMed] [Google Scholar]
- 9. Seelen H, Potten Y, Drukker J, Reulen J, Pons C.. Development of new muscle synergies in postural control in spinal cord injured subjects. J Electromyogr Kinesiol. 1998; 8: 23– 34. [DOI] [PubMed] [Google Scholar]
- 10. Potten Y, Seelen H, Drukker J, Reulen J, Drost M.. Postural muscle responses in the spinal cord injured persons during forward reaching. Ergonomics. 1999; 42: 1200– 1215. [DOI] [PubMed] [Google Scholar]
- 11. van Drongelen S, van der Woude LH, Janssen TW, Angenot EL, Chadwick EK, Veeger DH.. Mechanical load on the upper extremity during wheelchair activities. Arch Phys Med Rehabil., 2005; 86: 1214– 1220. [DOI] [PubMed] [Google Scholar]
- 12. van Drongelen S, van der Woude LH, Janssen TW, Angenot EL, Chadwick EK, Veeger DH.. Glenohumeral contact forces and muscle forces evaluated in wheelchair-related activities of daily living in able-bodied subjects versus subjects with paraplegia and tetraplegia. Arch Phys Med Rehabil. 2005; 86: 1434– 1440. [DOI] [PubMed] [Google Scholar]
- 13. Collinger JL, Boninger ML, Koontz AM, . et al. Shoulder biomechanics during the push phase of wheelchair propulsion: A multisite study of persons with paraplegia. Arch Phys Med Rehabil. 2008; 89: 667– 676. [DOI] [PubMed] [Google Scholar]
- 14. Gronley J, Newsam CJ, Mulroy SJ, Rao SS, Perry J, Helm M.. Electromyographic and kinematic analysis of the shoulder during four activities of daily living in men with tetraplegia. J Rehabil Res Dev. 2000; 37: 423– 432. [PubMed] [Google Scholar]
- 15. Kopf M, Jahanian O, Schnorenberg AJ, . et al. Quantitative assessment of walker-assisted gait in transtibial amputees. https://www.resna.org/sites/default/files/conference/2016/wheelchair_seating/kopf.html
- 16. Bachschmidt RA, Harris GF, Simoneau GG.. Walker-assisted gait in rehabilitation: A study of biomechanics and instrumentation. IEEE Trans Neural Syst Rehabil Eng. 2001; 9: 96– 105. [DOI] [PubMed] [Google Scholar]
- 17. Steele KM, Seth A, Hicks JL, Schwartz MH, Delp SL.. Muscle contributions to vertical and fore-aft accelerations are altered in subjects with crouch gait [online ahead of print November 27, 2012]. Gait Posture. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fenuta AM, Hicks AL.. Muscle activation during body weight-supported locomotion while using the ZeroG. J Rehabil Res Dev. 2014; 51: 51– 58. [DOI] [PubMed] [Google Scholar]
- 19. Colombo G, Wirz M, Dietz V.. Driven gait orthosis for improvement of locomotor training in paraplegic patients. Spinal Cord. 2001; 39: 252– 255. [DOI] [PubMed] [Google Scholar]
- 20. Kojima N, Nakazawa K, Yamamoto S-I, Yano H.. Phase-dependent electromyographic activity of the lower-limb muscles of a patient with clinically complete spinal cord injury during orthotic gait. Exp Brain Res. 1998; 120: 139– 142. [DOI] [PubMed] [Google Scholar]
- 21. Dietz V, Colombo G, Jensen L, Baumgartner L.. Locomotor capacity of spinal cord in paraplegic patients. Ann Neurol. 1995; 37: 574– 582. [DOI] [PubMed] [Google Scholar]
- 22. Kim CM, Eng JJ, Whittaker MW.. Level walking and ambulatory capacity in persons with incomplete spinal cord injury: Relationship with muscle strength. Spinal Cord. 2004; 42: 156– 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ditunno P, Dittuno J.. Walking Index for Spinal Cord Injury (WISCI II): Scale revision. Spinal Cord. 2001; 39: 654– 656. [DOI] [PubMed] [Google Scholar]
- 24. McGill S, Juker D, Kropf P.. Appropriately placed surface EMG electrodes reflect deep muscle activity (psoas, quadratus lumborum, abdominal wall) in the lumbar spine. J Biomech. 1996; 29: 1503– 1507. [DOI] [PubMed] [Google Scholar]
- 25. Louis N, Gorce P.. Surface electromyography activity of upper limb muscle during wheelchair propulsion: Influence of wheelchair configuration. Clin Biomech. 2010; 25: 879– 885. [DOI] [PubMed] [Google Scholar]
- 26. Vera-Garcia FJ, Moreside JM, McGill SM.. MVC techniques to normalize trunk muscle EMG in healthy women. J Electromyogr Kinesiol. 201; 20: 10– 16. [DOI] [PubMed] [Google Scholar]
- 27. Kadaba MP, Ramakrishnan H, Wootten M.. Measurement of lower extremity kinematics during level walking. J Orthop Res. 1990; 8: 383– 392. [DOI] [PubMed] [Google Scholar]
- 28. Davis RB, Ounpuu S, Tyburski D, Gage JR.. A gait analysis data collection and reduction technique. Human Movement Sci. 1991; 10: 575– 587. [Google Scholar]
- 29. Gutierrez-Farewik EM, Bartonek Å, Saraste H.. Comparison and evaluation of two common methods to measure center of mass displacement in three dimensions during gait. Human Movement Sci. 2006; 25: 238– 256. [DOI] [PubMed] [Google Scholar]
- 30. Redfern MS, Hughes RE, Chaffin DB.. High-pass filtering to remove electrocardiographic interference from torso EMG recordings. Clin Biomech. 1993; 8: 44– 48. [DOI] [PubMed] [Google Scholar]
- 31. Kendall FP, McCreary EK, Provance PG, Rodgers MM, Romani WA.. Muscles: Testing and Function, with Posture and Pain. Baltimore, MD: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 32. Solnik S, Rider P, Steinweg K, DeVita P, Hortobágyi T.. Teager–Kaiser energy operator signal conditioning improves EMG onset detection. Eur J Appl Physiol. 2010; 110: 489– 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hodges PW, Bui BH.. A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr Clin Neurophysiol Electromyogr Motor Control. 1996; 101: 511– 519. [DOI] [PubMed] [Google Scholar]
- 34. Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: Slack; 1992. [Google Scholar]
- 35. Kagawa T, Fukuda H, Uno Y.. Analysis of trunk movement in orthotic gait of paraplegics. In: 27th Annual International Conference of Engineering in Medicine and Biology Society; 2005: 6904– 6907. [DOI] [PubMed] [Google Scholar]
- 36. Thomas C, Dididze M, Martinez A, Morris R.. Identification and classification of involuntary leg muscle contractions in electromyographic records from individuals with spinal cord injury. J Electromyogr Kinesiol. 2014; 24: 747– 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arazpour M, Samadian M, Bahramizadeh M, . et al. The efficiency of orthotic interventions on energy consumption in paraplegic patients: A literature review [online ahead of print January 20, 2015]. Spinal Cord. [DOI] [PubMed] [Google Scholar]
- 38. Arazpour M, Bani MA, Hutchins SW.. Reciprocal gait orthoses and powered gait orthoses for walking by spinal cord injury patients. Prosthet Orthotics Int. 2013; 37: 14– 21. [DOI] [PubMed] [Google Scholar]
- 39. Ahmadi Bani M, Arazpour M, Farahmand F, Mousavi ME, Hutchins SW.. The efficiency of mechanical orthoses in affecting parameters associated with daily living in spinal cord injury patients: A literature review. Disabil Rehabil Assist Technol. 2015: 10 3: 183– 190. [DOI] [PubMed] [Google Scholar]


