Abstract
Background: Physical therapists frequently use neuromuscular electrical stimulation (NMES) therapy in an effort to increase the voluntary strength of partially paralyzed muscles in people with spinal cord injury (SCI), but it is not clear whether this treatment is effective. Objective: To determine the effectiveness of NMES for increasing voluntary strength in the partially paralyzed muscles of people with SCI. Methods: A systematic review of scientific literature was conducted in MEDLINE, CINAHL, PEDro, ScienceDirect, and Embase. Inclusion criteria were randomized controlled trials and controlled trials that compared NMES aimed at increasing strength in partially paralyzed muscles versus placebo/nothing or versus a nonstrengthening intervention or versus any other type of strengthening intervention in adults with SCI. Results: Five studies were included. Two studies found an increase in strength measured by peak force and manual muscle force test after an NMES protocol. One study found a between-group difference in favor of the NMES associated with progressive resistance training, and the other study showed an increase in the number of muscles improved by at least 1 degree of strength after NMES in combination with a cycle ergometer. The other 3 studies made several comparisons and found no differences between groups that received NMES and the controls. Conclusions: There is some suggestion that NMES increases voluntary strength in partially paralyzed muscle following SCI. However, there is no strong evidence to affirm the superiority of NMES over other treatment strategies used to gain strength in partially paralyzed muscles after SCI. These findings need replicating in large high-quality randomized controlled trials.
Keywords: electrical stimulation, randomized control trials, spinal cord injury, strength, systematic review
Spinal cord injury (SCI) is a neurological condition that disrupts the neural inputs between the upper and lower regions of the spinal cord, leading to a pronounced muscular pareses/paralyses caudal to the lesion level. After SCI, a progressive deterioration of skeletal muscles may occurs due to a reduction in muscle activation, leading to systemic disorders and muscle composition changes, increased intramuscular fat, and a reduction in muscle trophism. These morphological changes promote a reduction in the capacity to generate torque and strength, a common problem in people with SCI.1–3 The ability to generate strength is an important prerequisite for an individual to carry out his/her daily living activities (DLA), maintaining his/her function and independence.4–6
There are currently a range of different treatments used in clinical practice to increase strength in people with SCI, such as progressive resistance training (PRT), robotic gait training, cycle ergometer, and neuromuscular electrical stimulation (NMES).7,8 Although these treatments are widely used for increasing voluntary strength in the partially paralyzed muscles of people with SCI, scientific evidence only supports therapeutic intervention with PRT,9 but this is hard training to be performed on very weak muscles.
The use of an electric current with the purpose of increasing voluntary strength is well established for other neurological conditions such as stroke, advanced progressive diseases, cerebral palsy, and critically ill patients.10–13 Although current standard of care for partially paralyzed muscles after an SCI14 is basically physical therapy, there is no strong scientific evidence to support the application of an electric current in this specific population in order to improve muscle strength.
A previous review that discusses NMES and strength in SCI was published in 2007, however it included studies of several neurological disorders, such as hemiparesis, peripheral nerve injury, cerebral palsy, spina bifida, and SCI. Specifically in the case of SCI, no conclusion could be reached concerning the effect of NMES on muscle strength due to the limited number of studies available in the literature to that date and the poor methodological quality.15 Therefore the purpose of the present systematic review was to examine the efficacy of NMES for increasing the voluntary strength of partially paralyzed muscles in the SCI population and to carry out an update of the literature on this matter.
Methods
Searches
Searches were made in MEDLINE, CINAHL, PEDro, ScienceDirect, and Embase for relevant studies in English, without date restrictions. The search terms included words related to SCI, words related to muscle strength, and words related to electrical stimulation (see Appendix A). In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA),16 the titles and abstracts were displayed and screened by 2 reviewers in order to identify relevant studies. The methods sections of the retrieved studies were extracted and reviewed independently by 2 reviewers using predetermined inclusion criteria. Both reviewers were blinded to the authors, journals, and results, and disagreements or ambiguities were resolved by consensus after discussion with a third reviewer.
Inclusion criteria
Studies were included if they were randomized or controlled trials of adults (>16 years old) with a diagnosis of SCI with muscle weakness due to partial paralysis (including zone of partial preservation [ZPP] in motor-complete injuries) were included. Intervention was electrical stimulation aimed at increasing strength in partially paralyzed muscles (the clearly stated aim of the intervention was to increase strength or strength was an outcome measure). The electrical stimulation could be provided alone or in combination with other therapeutic interventions. If another intervention was also present in the control group, it was considered as a co-intervention.
The primary outcome was strength measurement. It had to be reported as the peak force/torque generation and be representative of the maximum voluntary contraction (example, manual muscle test or dynamometry). When more than one strength measurement was reported, only the one reflecting the trained muscles was used.
The accepted control interventions were (1) nothing, (2) placebo, (3) any other non-strengthening intervention, or (4) any other strengthening intervention.
Assessment of the trial characteristics
The quality of the studies included was evaluated by extracting scores from the Physiotherapy Evidence Database (PEDro). The PEDro scale is an 11-item scale designed for rating the methodological quality of randomized trials. Each item, except item 1, contributes 1 point to the total PEDro score range of 0 to 10. All the studies included were evaluated by 2 reviewers who had completed the PEDro Scale Training Tutorial.
The studies were classified according to their score on the PEDro scale, and their methodological qualities were stratified. Studies scoring below 4 were considered to be of “poor” quality, studies between 4 and 5 were considered to be of “fair” quality, studies with scores ranging from 6 to 8 were considered to be of “good” quality, and studies scoring from 9 to 10 were considered to be of “excellent” quality.17
Data collection and analysis
Two authors (G.R. and C.S.) independently extracted data from the included trials. Data extraction was made in order to record the following details for each study: participants (number of participants, age, American Spinal Injury Association Impairment Scale [AIS], SCI level, and time from SCI); intervention (group comparisons, period, NMES dosage); and results (primary outcomes).
The data were compiled in an Excel table and were checked for agreement between authors, with a third author (J.I.) arbitrating when consensus was not reached.
Results
Flow of trials through the review
The electronic search strategy identified 1,836 articles, excluding duplicates. After screening the titles, abstracts, and reference lists, 80 potentially relevant full articles were retrieved. Seventy-five of these articles failed to meet the inclusion criteria; hence 5 articles were included in the systematic review. Figure 1 shows the flow of these studies.
Figure 1.
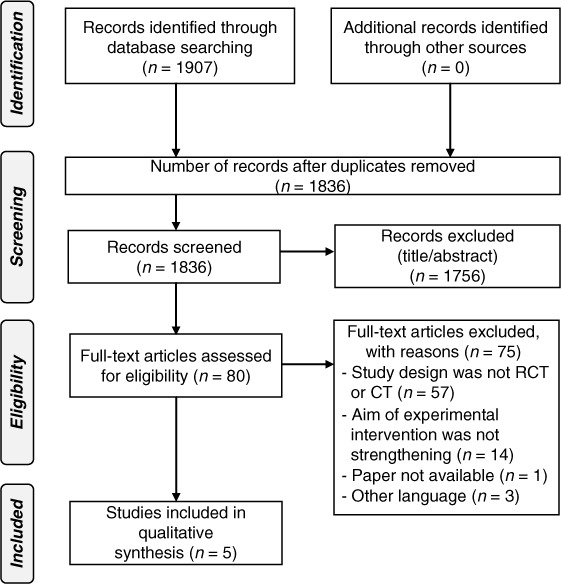
Flow of studies throughout the systematic review.
Characteristics of the trials included
The 5 trials included in the systematic review involved 170 participants and investigated the efficacy of NMES in improving muscle strength after SCI. Table 1 shows the details of the individual trials. One study compared NMES with nothing/placebo.18 One study compared NMES in combination with arm cycle ergometric exercise with arm cycle ergometric exercise alone.19 One study compared NMES and PRT with nothing/placebo.20 Two trials compared NMES with other/conventional strengthening interventions.21,22
Table 1.
PEDro criteria and scores for studies included (N = 5)
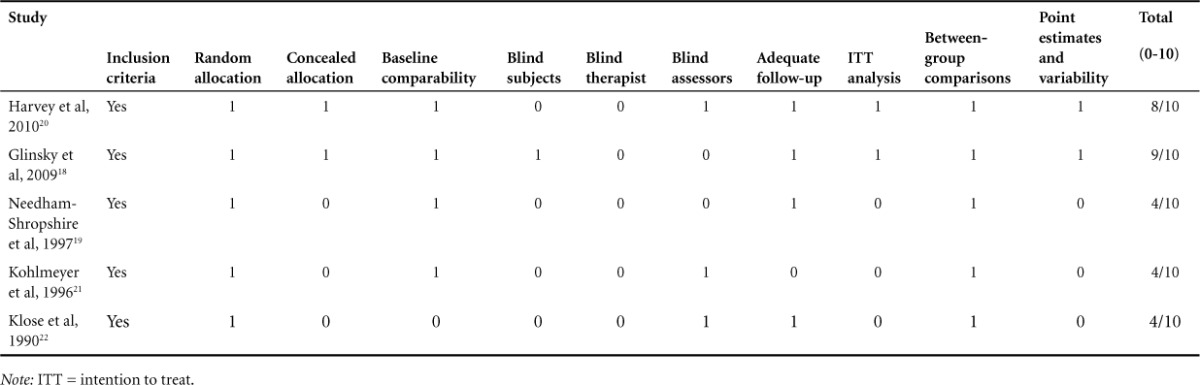
Quality
The mean PEDro score of the articles was 5.8 (range, 4–9). All the trials had randomly allocated participants, 2 trials had concealed allocation, 3 trials had similar groups at the baseline, 2 studies had blinded participants, 1 study had a blinded therapist, 3 studies had blinded assessors, 2 studies had intention-to-treat analysis, all the studies reported between-group differences, and 2 studies reported point estimates and variability (see Table 1). According to the stratification made using the PEDro Scale scores, 3 articles were considered as “fair” methodology,19,21,22 1 study showed “good” methodology,20 and 1 study showed “excellent” methodology.18
Participants
The mean age of the participants in all the studies ranged from 18 to 61 years old. In the trials of subacute participants, the mean time after SCI ranged from 5 to 6 months18,21; in the trials involving chronic participants, it ranged from 1 to 11 years.19,20,22 Participants with SCI levels from C3 to C8 were included in 4 trials,18,19,21,22 and 1 trial included all SCI levels.20
Intervention
Considering the interesting interventions of this study, 3 studies applied NMES as single therapy intervention group,18,21,22 1 study applied NMES with PRT as the experimental intervention,20 and 1 study applied NMES-assisted arm cycle ergometric exercise as intervention therapy.19
Outcome measurements
All the studies included muscle strength as the main outcome measure and evaluated it with the maximum voluntary strength. For the evaluation of strength, 3 studies used the manual muscle test,19,21,22 and 2 studies used the generation of peak force in newtons.18,20 The studies also evaluated measurements other than muscle strength, such as mobility,22 functional score,21 fatigue ratio,18 and participant perception of treatment effectiveness.20
Effect of electrical stimulation on muscle strength
The overall effect of electrical stimulation on muscle strength after intervention was examined in all the studies included in this review, and the results are shown in Table 2.
Table 2.
Characteristic and results of studies included (N = 5)(CONT.)
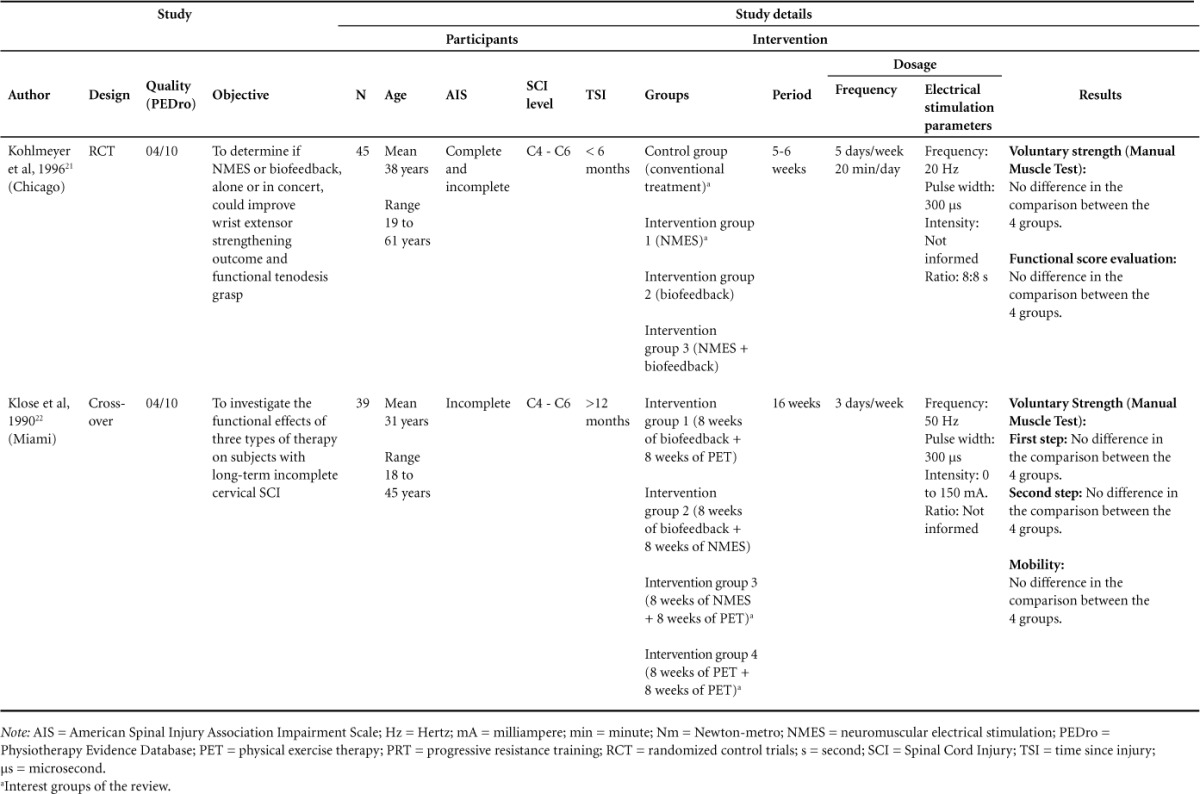
Table 2.
Characteristic and results of studies included (N = 5)
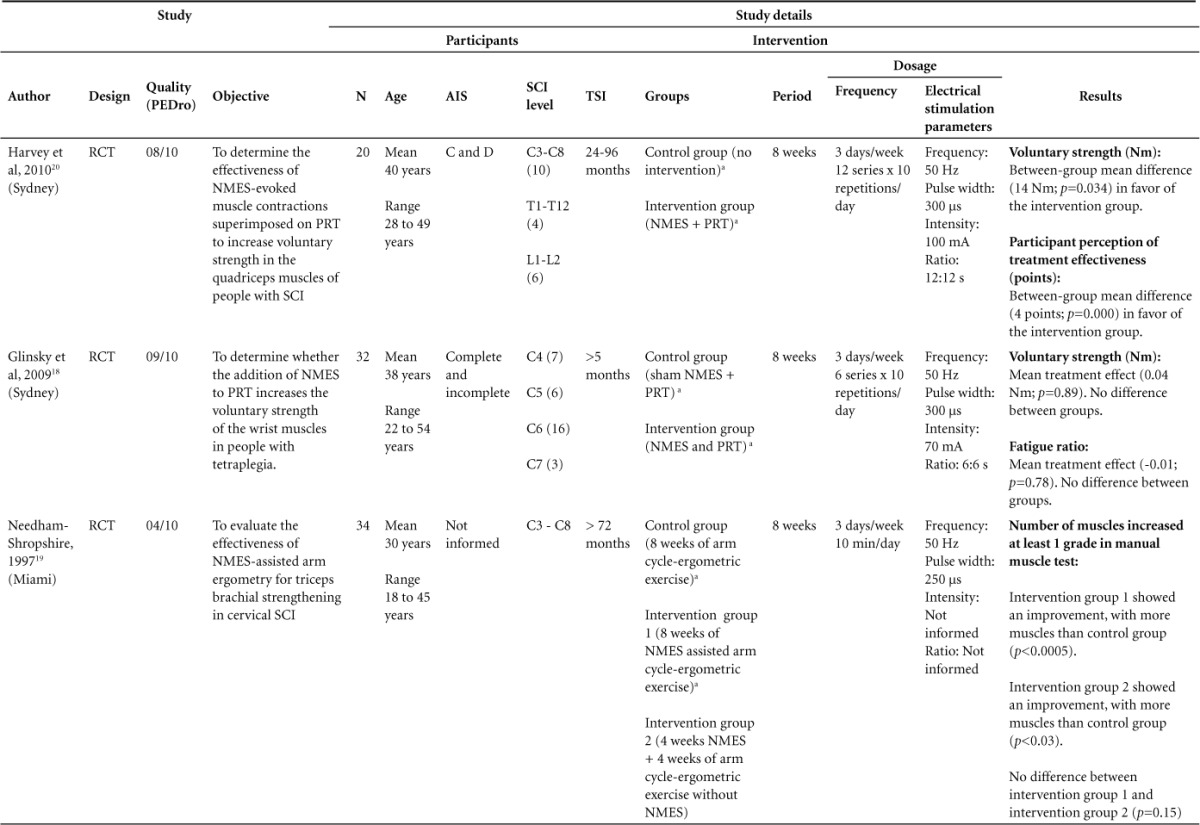
Effect of electrical stimulation versus nothing on muscle strength
Two studies included in this review make the comparison of electric stimulation versus nothing or placebo treatment. One study compared the control group that received no intervention with that receiving electrical stimulation plus resistance training in quadriceps muscle. It showed a mean between-group mean difference of the 14 Nm with p = .034 in favor of the intervention group after 8 weeks of treatment.20 The other study compared the control group with electric stimulation group in wrist extensor and wrist flexor muscles in people with tetraplegia and found no differences between-group means (0.04 Nm; p = .89) after 8 weeks of treatment.18
Effect of electrical stimulation versus other strengthening intervention on muscle strength
Only one study shown favorable results comparing NMES-assisted arm cycle ergometric exercise with NMES applied in triceps muscle and compared with arm cycle ergometer exercise alone.19 In this study, the triceps muscle strength was assessed by Manual Muscle Test as for the AIS. The posttraining test was scored as “improved” if the AIS motor score for the triceps muscle was higher than the baseline in at least 1 degree, or “not improved” if the motor score remained the same or decreased. The analysis was performed for the number of the muscles that were improved or not for groups after training, showing a significant increased number of muscles in the intervention group when compared to the control group (p = .003) after 4 weeks of intervention.19
The other 2 studies made several comparisons but found no differences between the group that received electrical stimulation and the group receiving conventional treatment or other treatment that was not electrical stimulation. One study compared electrical stimulation applied in wrist extensor muscles with a conventional treatment of the wrist extensor muscles and found no between-group differences after 6 weeks of treatment.21 The last study made a comparison in the first step of the study of 8 weeks of NMES alone compared to 8 weeks of physical exercise therapy (PET) (in the first step of a cross-over design) on muscle strength of the upper limbs and found no differences between groups.22 However, in both studies, no differences between groups were found.
Discussion
To our knowledge, there is only one previous systematic review, published in 2007, about the effects of NMES on strength, but that study included several neurological disorders. At the time of that review, there were only 3 specific studies available on adults with SCI with methodological quality of randomized clinical trials, and it was not possible to reach a conclusion about the effect of NMES on muscle strength due to the limited number of studies available in the literature and poor methodological quality.15 The present systematic review is an update of the effects of NMES on the muscle strength of partially paralyzed muscles in adult people with SCI. To our knowledge, it is the first systematic review carried out on this specific research topic.
Only 5 articles that met the inclusion criteria were included in this systematic review. The first important study of the topic, with reasonable methodological quality, was carried out in 1990 by Klose et al.22 This was a clinical trial with a cross-over design with 3 different therapies (biofeedback, physical therapy exercises, and NMES), combined 2 × 2 in 4 different groups, aimed at strengthening the partially paralyzed muscles of the upper extremities. In the first step of the cross-over design, the authors found no differences in the comparisons of 8 weeks of NMES applied in upper extremities muscles and 8 weeks of PET, concluding that both treatments were able to increase muscle strength and mobility when compared across time for the intragroup intervention, without the superiority of any specific intervention with respect to the gain.
The second relevant study was carried out by Kohlmeyer et al in 1996; it compared the conventional treatment as the control with NMES in wrist extensors muscles.21 Despite the high dosage (5 days a week of treatment for 6 weeks), they found no significant differences in the between-group comparisons with respect to the muscle strength analysis, again showing no superiority of one therapy against other.
In contrast, the study of Needham-Shropshire et al, carried out in 1997, compared the arm cycle ergometric exercise as the control with triceps muscles NMES-assisted arm cycle ergometric exercise.19 They found an improvement in the number of muscles, which increased at least one grade in Manual Muscle Test - AIS motor score, in the group that received NMES. As the arm cycle ergometric exercise is a co-intervention given to both groups, this study was able to provide the superiority of NMES, answering the second question of this review. The same positive result was not found by other studies using similar NMES parameters.18,21,22 This difference may have been due to the time gap after SCI; they carried out the study in people who were long time chronic patients, whereas other studies, which did not have favorable results, were performed with patients in the subacute phase to the chronic phase, without the elimination of bias by spontaneous recovery that can occur in this period.
In another study, no differences were found between the groups with respect to the voluntary strength of the wrists after 8 weeks of NMES in wrist flexor and extensor muscles in comparison with a placebo treatment.18 Meanwhile some positive results were found in one study testing 8 weeks of NMES in quadriceps muscle with PRT in comparison to control group. They found a significant between-group difference (14 Nm, p = .034) in voluntary strength. Both aforementioned studies used a treatment dose of 3 times per week with the same parameters of NMES frequency (50 Hz) and pulse width of 300 μs,18,20 which are the parameters widely used in the literature and in clinical practice to generate muscle contraction.10,12,13,23–25
The studies that apply NMES in SCI population seem to follow the same pattern of electrical stimulation application; the 5 studies18–22 included in this systematic review administer low frequency electric current with parameters between 20 Hz and 50 Hz and lower pulse width between 250 μs and 300 μs. The frequency of treatment seems to have 2 different types of therapy – one used more in clinical therapy with 3 times per week with prolonged protocol during at least 8 to 16 weeks,18,19,20,22 and another type of therapy more intensive with 5 times per week for at least 6 weeks.21
Two studies that showed differences between control and intervention groups applied a similar dose therapy (frequency and parameters of NMES)19,20 in contrast with the studies that did not find between-group differences.18,21,22 This 2 studies conducted the therapy in very chronic SCI population with more than 24 months since injury.19,20 As previously showed, people in acute or subacute phases after SCI have the greatest potential of therapeutic gain in functional recovery.26–29
An important limitation of the studies included in this review may be the fact that they did not take into consideration the effects of the spontaneous recovery. In this context, both chronicity and AIS injury classification could influence the results of the studies in this review.26,27 Two studies included participants in the acute or subacute phase less than 12 months after injury. Furthermore, these studies included subjects with SCI classified as complete and/or incomplete injury by AIS.18,21 On the other hand, 2 studies included participants with incomplete injury20,22; the study of Harvey et al20 covered only the levels C and D.
After the SCI, mainly in acute and subacute phase, until 1 year post injury, spontaneous recovery may occur in a proportion of the SCI population, particularly in incomplete injuries when there is some degree of function below the level of injury.28,29 The spontaneous recovery is significantly higher in incomplete injuries classified as AIS B or AIS C when compared to AIS A.28 In the period of 1 year post injury, when the neurologic recovery is more marked, the average of recovery varied, but the functional gain from AIS B to AIS C was between 15% and 40% and the increase from AIS C to AIS D was between 60% and 80%.28 Even in motor-complete SCI, considerable motor recovery could be evidenced in the zone of partial preservation over 12 months after injury.26,27 All these points have a great importance when therapeutic clinical trials are conducted and need to be considered in further studies.28
Although there has been an increase in the number of randomized clinical trials in the neurorehabilitation field,9 very few articles have been published concerning muscle strength in SCI. Furthermore, the low methodological qualities of the studies, which were mostly considered as “fair,”19,21,22 one as “good,”20 and only one as “excellent,”18 in combination with the lack of the sample size calculation or of an adequate sample size and no consensus in the assessment tools used to measure the muscle strength, made it impossible to conclude whether NMES really did have superior beneficial effects on improving strength in partially paralyzed muscle after SCI. An important point to be considered about the quality of the studies is the assessment conducted in a blind manner. Two18,19 of the 518–22 included studies did not have a blind assessor, which made these studies susceptive to a bias that involves the expectations of the assessor/researcher about the therapeutic intervention.
Perhaps the lack of results concerning the benefit of NMES on muscle strength in people with SCI may also be due to the fact that the studies did not divide the participants according to their degrees of strength (very weak, weak, or moderate). As shown by other neurological populations, individuals with a weaker muscle strength may reap more benefit from NMES than individuals with greater muscle strength.12 In a review carried out about stroke patients, when the trials were grouped according to the initial strength level of the subjects, NMES was able to increase the muscle strength in studies involving only weak or very weak participants.12 Thus the degree of muscle strength may have influenced the results of the studies included in the present review, because none of the studies selected carried out a classification of the initial degrees of muscle strength.
Conclusion
In the studies included in our review, there are some indications that NMES may be beneficial in improving muscle strength after SCI. However, due to the small number of studies on the topic and the low methodological quality, even close to a decade after the first review carried out on this topic,15 there are still not sufficient data to affirm the superiority of intervention with NMES over other treatment strategies clinically used to gain strength in partially paralyzed muscles after SCI. Further studies are required that use more rigorous methodology, an appropriate sample size, and stratification of the degrees of muscle strength and time after injury in order to more accurately define the effects of NMES on muscle strength after SCI.
Additionally, the fact the literature search was restricted to English and the scarcity of the randomized clinical trials must be considered the major limitations of this review.
Appendix A
Search Strategy
MEDLINE
“Spinal Cord Injury”.
Spinal injury.
Paraplegia.
Paraplegic.
Paraplegi$.
Tetraplegia.
Tetraplegic.
Tetraplegi$.
OR / 1 – 8.
Strength.
Muscle.
“Muscle strength”.
OR / 10 – 12.
“Electrical Stimulation”.
“Transcutaneous electrical Stimulation”.
“Functional electrical stimulation”.
“Electric stimulation”.
“Cyclical Electrical Stimulation”.
“Neuromuscular electrical Stimulation”.
Electric$.
“Electric$ Stimulation”.
OR / 14 – 21.
9 AND 13 AND 22.
CINAHL
Spinal Cord Injury
Spinal Injury
Paraplegia
Paraplegic
Tetraplegia
Tetraplegic
OR / 1 – 6.
Electrical Stimulation
Transcutaneous electrical Stimulation.
Functional electrical stimulation
Electric stimulation
Cyclical Electrical Stimulation
Neuromuscular Electrical Stimulation
OR / 8 – 13.
Strength
Muscle strength
15 OR 16.
7 AND 10 AND 17.
PEDro
Spinal Cord Injury
Electrical Stimulation
Strength
Embase
Spinal Cord Injury (Key word, Title and Abstract)
Electrical Stimulation (Key word, Title and Abstract)
Strength (Key word, Title and Abstract)
Science Direct
“Spinal Cord Injury” OR (Spinal Cord Injury).
Spinal injury.
Paraplegia.
Paraplegic.
Paraplegi*.
Tetraplegia.
Tetraplegic.
Tetraplegi*.
OR / 1 – 8.
Strength.
Muscle.
“Muscle strength” OR (Muscle Strength).
OR / 10 – 13.
“Electrical Stimulation” OR (Electrical Stimulation).
“Transcutaneous electrical Stimulation” OR (Transcutaneous electrical Stimulation).
“Functional electrical stimulation” OR (Functional electrical stimulation).
“Electric stimulation” OR (Electric stimulation).
“CyclicalElectricalStimulation”OR(Cyclical Electrical stimulation).
“Neuromuscular electrical Stimulation” OR (Neuromuscular electrical Stimulation).
Electric*.
“Electric* Stimulation” OR (Electric* Stimulation).
OR / 14 – 21.
Humans
9 AND 13 AND 22 AND 24.
REFERENCES
- 1. DiPiro ND, Embry AE, Fritz SL, Middleton A, Krause JS, Gregory CM.. Effects of aerobic exercise training on fitness and walking-related outcomes in ambulatory individuals with chronic incomplete spinal cord injury. Spinal Cord. 2016; 54 9: 675– 681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moore CD, Craven BC, Thabane L, . et al. Lower-extremity muscle atrophy and fat infiltration after chronic spinal cord injury. J Musculoskelet Neuronal Interact. 2015; 15 1: 32– 41. [PMC free article] [PubMed] [Google Scholar]
- 3. Jayaraman A, Gregory CM, Bowden M, . et al. Lower extremity skeletal muscle function in persons with incomplete spinal cord injury. Spinal Cord. 2006; 44 11: 680– 687. [DOI] [PubMed] [Google Scholar]
- 4. Stevens SL, Fuller DK, Morgan DW.. Leg strength, preferred walking speed, and daily step activity in adults with incomplete spinal cord injuries. Top Spinal Cord Inj Rehabil. 2013; 19 1: 47– 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Devillard X, Rimaud D, Roche F, Calmels P.. Effects of training programs for spinal cord injury. Annales de Réadaptation et de Médecine Physique. 2007; 50 6: 490– 498. [DOI] [PubMed] [Google Scholar]
- 6. Kapadia NM, Zivanovic V, Furlan JC, Craven BC, McGillivray C, Popovic MR.. Functional electrical stimulation therapy for grasping in traumatic incomplete spinal cord injury: Randomized control trial. Artif Organs. 2011; 35 3: 212– 216. [DOI] [PubMed] [Google Scholar]
- 7. Gorgey AS, Dolbow DR, Cifu DX, Gater DR.. Neuromuscular electrical stimulation attenuates thigh skeletal muscles atrophy but not trunk muscles after spinal cord injury. J Electromyogr Kinesiol. 2013; 23 4: 977– 984. [DOI] [PubMed] [Google Scholar]
- 8. Jones ML, Evans N, Tefertiller C, . et al. Activity-based therapy for recovery of walking in chronic spinal cord injury: Results from a secondary analysis to determine responsiveness to therapy. Arch Phys Med Rehabil. 2014; 95 12: 2247– 2252. [DOI] [PubMed] [Google Scholar]
- 9. Harvey LA. Physiotherapy rehabilitation for people with spinal cord injuries. J Physiother. 2016; 62 1: 4– 11. [DOI] [PubMed] [Google Scholar]
- 10. Wright PA, Durham S, Ewins DJ, Swain ID.. Neuromuscular electrical stimulation for children with cerebral palsy: A review. Arch Dis Child. 2012; 97 4: 364– 371. [DOI] [PubMed] [Google Scholar]
- 11. Maddocks M, Gao W, Higginson IJ, Wilcock A.. Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease. Cochrane Database Syst Rev. 2013. 1: CD009419. [DOI] [PubMed] [Google Scholar]
- 12. Nascimento LR, Michaelsen SM, Ada L, Polese JC, Teixeira-Salmela LF.. Cyclical electrical stimulation increases strength and improves activity after stroke: A systematic review. J Physiother. 2014; 60 1: 22– 30. [DOI] [PubMed] [Google Scholar]
- 13. Maffiuletti NA, Roig M, Karatzanos E, Nanas S.. Neuromuscular electrical stimulation for preventing skeletal-muscle weakness and wasting in critically ill patients: A systematic review. BMC Med. 2013; 11: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gorgey AS, Khalil RE.. Neuromuscular electrical stimulation training increases intermuscular fascial length but not tendon cross-sectional area after spinal cord injury. Top Spinal Cord Inj Rehabil. 2015; 21 1: 87– 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glinsky J, Harvey L, Van Es P.. Efficacy of electrical stimulation to increase muscle strength in people with neurological conditions: A systematic review. Physiother Res Int. 2007; 12 3: 175– 194. [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Shamseer L, Clarke M, . et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Foley NC, Teasell RW, Bhogal SK, Speechley MR.. Stroke rehabilitation evidence-based review: Methodology Top Stroke Rehabil. 2003; 10 1: 1– 7. [PubMed] [Google Scholar]
- 18. Glinsky J, Harvey L, van Es P, Chee S, Gandevia SC.. The addition of electrical stimulation to progressive resistance training does not enhance the wrist strength of people with tetraplegia: A randomized controlled trial. Clin Rehabil. 2009; 23 8: 696– 704. [DOI] [PubMed] [Google Scholar]
- 19. Needham-Shropshire BM, Broton JG, Cameron TL, Klose KJ.. Improved motor function in tetraplegics following neuromuscular stimulation-assisted arm ergometry. J Spinal Cord Med. 1997; 20 1: 49– 55. [DOI] [PubMed] [Google Scholar]
- 20. Harvey LA, Fornusek C, Bowden JL, . et al. Electrical stimulation plus progressive resistance training for leg strength in spinal cord injury: A randomized controlled trial. Spinal Cord. 2010; 48 7: 570– 575. [DOI] [PubMed] [Google Scholar]
- 21. Kohlmeyer KM, Hill JP, Yarkony GM, Jaeger RJ.. Electrical stimulation and biofeedback effect on recovery of tenodesis grasp: A controlled study. Arch Phys Med Rehabil. 1996; 77 7: 702– 706. [DOI] [PubMed] [Google Scholar]
- 22. Klose KJ, Schmidt DL, Needham BM, Brucker BS, Green BA, Ayyar DR.. Rehabilitation therapy for patients with long-term spinal cord injuries. Arch Phys Med Rehabil. 1990; 71 9: 659– 662. [PubMed] [Google Scholar]
- 23. Fornusek C, Davis GM, Russold MF.. Pilot study of the effect of low-cadence functional electrical stimulation cycling after spinal cord injury on thigh girth and strength. Arch Phys Med Rehabil. 2013; 94 5: 990– 993. [DOI] [PubMed] [Google Scholar]
- 24. Popovic MR, Kapadia N, Zivanovic V, Furlan JC, Craven BC, McGillivray C.. Functional electrical stimulation therapy of voluntary grasping versus only conventional rehabilitation for patients with subacute incomplete tetraplegia: A randomized clinical trial. Neurorehabil Neural Repair. 2011; 25 5: 433– 442. [DOI] [PubMed] [Google Scholar]
- 25. Ratchford JN, Shore W, Hammond ER, . et al. A pilot study of functional electrical stimulation cycling in progressive multiple sclerosis. NeuroRehabilitation. 2010; 27 2: 121– 128. [DOI] [PubMed] [Google Scholar]
- 26. Mange KC, Ditunno JF Jr, Herbison GJ, Jaweed MM.. Recovery of strength at the zone of injury in motor complete and motor incomplete cervical spinal cord injured patients. Arch Phys Med Rehabil. 1990; 71 8: 562– 565. [PubMed] [Google Scholar]
- 27. Wu L, Marino RJ, Herbison GJ, Ditunno JF Jr.. Recovery of zero-grade muscles in the zone of partial preservation in motor complete quadriplegia. Arch Phys Med Rehabil. 1992; 73 1: 40– 43. [PubMed] [Google Scholar]
- 28. Fawcett W, Curt A, Steeves JD, . et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: Spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007; 45: 190– 205. [DOI] [PubMed] [Google Scholar]
- 29. Onifer SM, Smith GM, Fouad K.. Plasticity after spinal cord injury: Relevance to recovery and approaches to facilitate it. Exp NeuroTherapeut. 211; 8: 283– 293. [DOI] [PMC free article] [PubMed] [Google Scholar]


