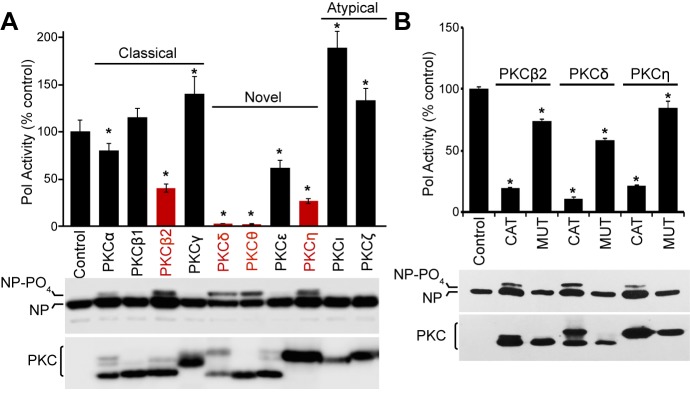
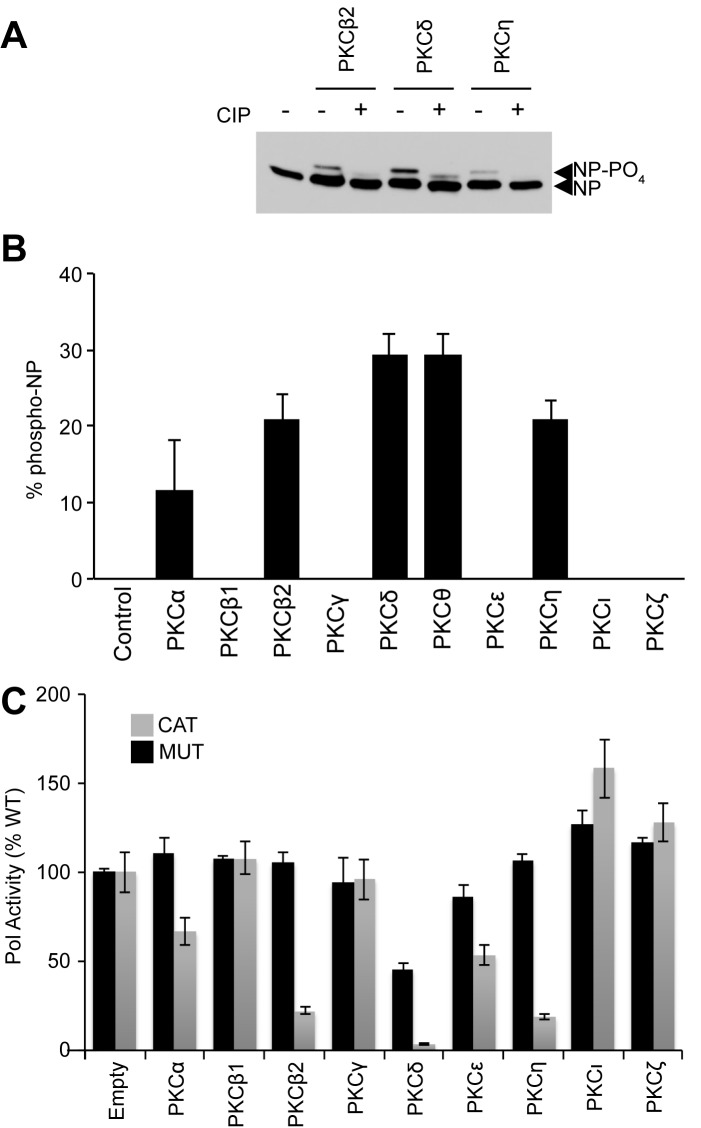
Figure 1. Constitutively active PKC phosphorylates NP leading to impaired influenza virus polymerase activity.
(A) Expression of constitutively active PKC impairs influenza virus polymerase activity. Polymerase activity assays were performed in 293T cells in the presence or absence of the catalytic domains from classical, novel or atypical PKC isoforms. Data were averaged and normalized to the empty vector control. NP and PKC were detected by western blotting whole cell lysate. A hyper-phosphorylated form of NP was detected in some conditions. (n=3 ± standard deviation, *p<0.05 one-way ANOVA when compared to the empty vector control). (B) Polymerase activity assays were performed in the presence of PKC catalytic domains, catalytically inactive mutants, or empty vector controls. Polymerase activity and protein expression were analyzed as in (A).
Figure 1—figure supplement 1. Polymerase activity assays.