Abstract
Background
Osteoarthritis (OA) is the most common form of arthritis associated with an increased prevalence of type 2 diabetes mellitus (T2DM), however their impact on decreasing joint replacement surgery has yet to be elucidated. This study aimed to investigate if the combination of COX-2 inhibitor and metformin therapy in OA with T2DM were associated with lower the rate of joint replacement surgery than COX-2 inhibitor alone.
Methods
In total, 968 subjects with OA and T2DM under COX-2 inhibitor and metformin therapy (case group) between 1 January to 31 December 2000 were selected from the National Health Insurance Research Database of Taiwan, along with 1936 patients were the 1:2 gender-, age-, and index year-controls matched without metformin therapy (control group) in this study. Cox proportional hazards analysis was used to compare the rate of receiving joint replacement surgery during 10 years of follow-up.
Results
At the end of follow-up, 438 of all enrolled subjects (15.08%) had received the joint replacement surgery, including 124 in the case group (12.81%) and 314 in the control group (16.22%). The case group tended to be associated with lower rate of receiving the joint replacement surgery at the end of follow-up than the control group (p = 0.003). Cox proportional hazards regression (HR) analysis revealed that study subjects under combination therapy with metformin had lower rate of joint replacement surgery (adjusted HR 0.742 (95% CI = 0.601–0.915, p = 0.005)). In the subgroups, study subjects in the combination metformin therapy who were female, good adherence (>80%), lived in the highest urbanization levels of residence, treatment in the hospital center and lower monthly insurance premiums were associated with a lower risk of joint replacement surgery than those without.
Conclusions
Patients who have OA and T2DM receiving combination COX-2 inhibitors and metformin therapy associated with lower joint replacement surgery rates than those without and this may be attributable to combination therapy much more decrease pro-inflammatory factors associated than those without metformin therapy.
Introduction
Osteoarthritis(OA) is the most common form of arthritis and possesses marked variability of disease expression. The incidence of OA is rising because of the ageing population and the epidemic of obesity. [1] OA has a predilection for the hand, knee, hip, and spine, and less commonly affects the shoulder, elbow, wrist, and ankle. OA may be diagnosed without the use of radiography and/or laboratory investigations in the presence of typical symptoms and signs in the at-risk age group. [2] One recent hypothesis has suggested a new classification for phenotyping OA that includes ageing, metabolic syndrome(Mets) and post-traumatic events and genetic-related OA. [3]
OA is also associated with an increased prevalence of Mets studied in NHANES III data, [4] the other components of Mets, such as type 2 diabetes mellitus(T2DM), hypertension or dyslipidemia may cause OA pathophysiology. [5] The first paper describing an association between OA and diabetes was published in 1961. [6] Insulin resistance(IR) and T2DM seemed to be associated with OA in the Ulm OA and ROAD studies. [7, 8]http://rmdopen.bmj.com/content/1/1/e000077-ref-8 In addition, the link between the two diseases may be supported by the accumulation of advanced glycation end products, oxidative stress and promotion of systemic inflammation. [9, 10] Moreover, one recent meta-analysis highlights a high frequency of OA in patients with T2DM and an association between both diseases. [11]
The goals of OA management are to minimize pain, optimize function, and beneficially modify the process of joint damage. Pain and loss of function are the main clinical features that lead to treatment, including biomechanical interventions, exercise (land-based and water-based), self-management and education, strength training, and weight management, pharmacological, and surgical approaches. [12, 13] Clinical trial data show that the traditional nonsteroidal anti-inflammatory drugs (NSAIDs) are more effective than acetaminophen in the treatment of patients with symptoms and signs of OA. [14, 15] In patients with comorbidities such as T2DM, hypertension, previous gastrointestinal bleeding and advanced age, a cyclooxygenase (COX)-2 selective NSAID should be better than NSAIDs. One meta-analysis enhanced that OA treatment with celecoxib was significantly improved than that with placebo. [16] However, surgical treatment is dominated in patients with advanced knee and hip OA when conservative therapies have failed to provide adequate pain relief. [17, 18]
Metformin is the preferred initial pharmacologic agent for the treatment of T2DM [19] that has been shown to reduce chronic inflammation indirectly through reduction of hyperglycemia, or directly acting as anti-inflammatory drug. [20] As described in detail previously that additional effect of metformin and celecoxib against adipose tissue inflammation that resulted in a reduction in adipose tissue macrophage infiltration and decreases in levels of adipose tissue TNF-α, MCP-1, and leptin levels in high-fat fed rats. [21] No previous study reported combination metformin and COX-2 inhibitor therapy in OA comorbid with T2DM associated with lower joint replacement surgery rate than used COX-2 inhibitor only. Therefore, the aim of this study was to clarify this association using data from a nationwide health insurance database, the Taiwan National Health Insurance Research Database (NHIRD).
Materials and methods
Data sources
In this study, we used data from the NHIRD to investigate combination metformin and COX-2 inhibitor therapy in OA comorbid with T2DM could lower joint replacement surgery rate than used COX-2 inhibitor only over a 10-year period, from the outpatient Longitudinal Health Insurance Database (LHID) in Taiwan (2000–2010). As described in detail previously, [22] the National Health Insurance (NHI) Program was launched in Taiwan in 1995, and as of June 2009 it included contracts with 97% of the medical providers in Taiwan with approximately 23 million beneficiaries, or more than 99% of the entire population in Taiwan. [23] The NHIRD uses International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes to record diagnoses. [24] All diagnoses of T2DM were made by board-certified medical specialist, and OA were confirmed by orthopedic specialist. The Bureau of NHI randomly reviews the records of 1 in 100 ambulatory care visits and 1 in 20 in-patient claims to verify the accuracy of the diagnoses. [25] Several studies have demonstrated the accuracy and validity of the diagnoses in the NHIRD. [26, 27]
Study design and sampled participants
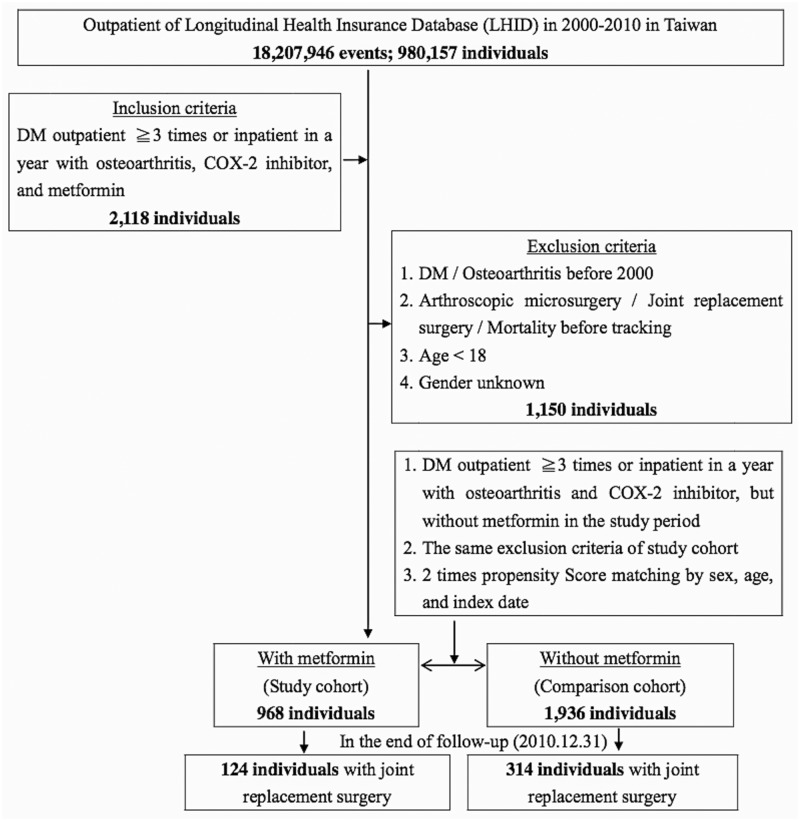
This study was a retrospective matched-cohort design. Patients with diagnosed OA and T2DM were selected from 1 January 2000 to 31 December 2010 according to ICD-9-CM 715.XX (OA) and ICD-9-CM 250.XX (T2DM). In addition, each enrolled patient was required to have made at least 3 outpatient visits within the study period according to these ICD-9-CM codes under COX-2 inhibitors therapy with or without metformin therapy. The patients with OA and/or T2DM before 2000 were excluded. In addition, the patients received joint replacement surgery before tracking were also excluded. All patients aged <18 years were also excluded. A total of 2118 enrolled patients that excluded 1150 patients then the 968 subjects with OA and T2DM under COX-2 inhibitor and metformin therapy (case group) along with 1936 patients were the 1:2 sex-, age-, and index year-controls matched without metformin therapy (control group) in this study. (Fig 1)
Fig 1. The flowchart of study sample selection from National Health Insurance Research Database in Taiwan.
DM = Diabetes mellitus: ICD-9-CM 250; Osteoarthritis: ICD-9-CM 715; COX-2 inhibitor / Metformin: ≧ 90 days. Arthroscopic microsurgery was including synovectomy or/and capsulotomy (NHIRD order code 64054B-64057B), partial meniscectomy (NHIRD order code 64218B), and arthroscopic surgery (NHIRD order code 64243B-64244B). Joint replacement surgery was including osteosynthesis (NHIRD order code 64038B-64040B), total hip replacement (NHIRD order code 64162B-64168B), partial joint replacement (NHIRD order code 64169B-64170B), arthroplasty (NHIRD order code 64171B-64177B), arthrodesis (NHIRD order code 64178B-64183B), removal of prosthesis (NHIRD order code 64198B-64200B), revision replacement (64201B-64202B), and girdle stone procedure of hip (NHIRD order code 64203B).
The covariates included gender, age, Charlson Comorbidity Index(CCI) removed T2DM, geographical area of residence (north, center, south, east of Taiwan, and outlets islands), urbanization level of residence (level 1 the highest; level 3 the lowest) and monthly income (in New Taiwan Dollars [NTD]; <10,000, 10,000–14,999, ≥15,000). The urbanization level of residence was defined according to the population and various indicators of the level of development. Level 1 was defined as a population >1,250,000, and a specific designation as political, economic, cultural and metropolitan development. Level 2 was defined as a population between 500,000 and 1249,999, and as playing an important role in the political system, economy, and culture. Urbanization levels 3 was defined as a population <500,000. [28]
Outcome measures
All of the study participants were followed from the index date until the onset of receiving joint replacement surgery from the NHI program before the end of 2010.
Statistical analysis
All analyses were performed using SPSS software version 22 (SPSS Inc., Chicago, Illinois, USA). Conditional logistic regression was used to evaluate the distributions of study and control groups. Multivariable Cox proportional hazards regression analysis was used to determine the risk of receiving joint surgical replacement, and the results were present as adjusted hazard ratio with 95% confidence interval (CI). The difference in the risk of receiving joint surgical replacement between the study and control groups was estimated using the Kaplan-Meier method with the log-rank test. A 2-tailed p value <0.05 was considered to indicate statistical significance.
Ethics
This study was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). The Institutional Review Board of Tri-Service General Hospital approved this study and waived the need for individual written informed consent (TSGH IRB No. 2-105-05-082).
Results
Table 1 shows the gender, age, comorbidities, location, urbanization, level of care and income of the study subjects and controls. Compared to the controls, the study subjects much more tended to receive therapy in hospital center and have more lived in higher urbanized areas, northern areas of Taiwan (p < 0.001).
Table 1. Characteristics of study in the baseline.
| Metformin | Total | With | Without | P | |||
|---|---|---|---|---|---|---|---|
| Variables | n | % | n | % | n | % | |
| Total | 2,904 | 968 | 33.33 | 1,936 | 66.67 | ||
| Gender | |||||||
| Male | 1,245 | 42.87 | 415 | 42.87 | 830 | 42.87 | Reference |
| Female | 1,659 | 57.13 | 553 | 57.13 | 1,106 | 57.13 | 0.852 |
| Age (years) | 70.55±10.66 | 70.17±10.85 | 70.74±10.56 | 0.070 | |||
| CCI removed DM | 0.71±1.16 | 0.80±1.26 | 0.67±1.10 | 0.008 | |||
| Location | |||||||
| Northern Taiwan | 1,013 | 34.88 | 439 | 45.35 | 574 | 29.65 | Reference |
| Middle Taiwan | 867 | 29.86 | 204 | 21.07 | 663 | 34.25 | <0.001 |
| Southern Taiwan | 775 | 26.69 | 244 | 25.21 | 531 | 27.43 | <0.001 |
| Eastern Taiwan | 224 | 7.71 | 74 | 7.64 | 150 | 7.75 | 0.378 |
| Outlets islands | 25 | 0.86 | 7 | 0.72 | 18 | 0.93 | 0.589 |
| Urbanization level | |||||||
| 1 (The highest) | 817 | 28.13 | 362 | 37.40 | 455 | 23.50 | <0.001 |
| 2 | 1,187 | 40.87 | 393 | 40.60 | 794 | 41.01 | 0.011 |
| 3 (The lowest) | 900 | 40.00 | 213 | 22.00 | 687 | 35.49 | Reference |
| Level of care | |||||||
| Hospital center | 925 | 31.85 | 368 | 38.02 | 557 | 28.77 | 0.274 |
| Regional hospital | 1,164 | 40.08 | 367 | 37.91 | 797 | 41.17 | 0.035 |
| Local hospital | 815 | 28.06 | 233 | 24.07 | 582 | 30.06 | Reference |
| Insured premium (NT$) | |||||||
| <10,000 | 1,469 | 50.58 | 488 | 50.41 | 981 | 50.67 | Reference |
| 10,000–14,999 | 730 | 25.14 | 251 | 25.93 | 479 | 24.74 | 0.375 |
| ≧15,000 | 705 | 24.28 | 229 | 23.66 | 476 | 24.59 | 0.362 |
CCI = Charlson Comorbidity Index
P: Conditional logistic regression
Table 2 shows that at the end of follow-up, 438 of all enrolled subjects (15.08%) had received the joint replacement surgery, including 124 in the case group (12.81%) and 314 in the control group (16.22%). The case group tended to be associated with a lower rate of receiving the joint replacement surgery at the end of follow-up than the control group (p = 0.003).
Table 2. Characteristics of study in the endpoint.
| Metformin | Total | With | Without | P | |||
|---|---|---|---|---|---|---|---|
| Variables | n | % | n | % | n | % | |
| Total | 2,904 | 968 | 33.33 | 1,936 | 66.67 | ||
| Joint replacement surgery | |||||||
| Without | 2,466 | 84.92 | 844 | 87.19 | 1,622 | 83.78 | Reference |
| With | 438 | 15.08 | 124 | 12.81 | 314 | 16.22 | 0.003 |
| Gender | |||||||
| Male | 1,245 | 42.87 | 415 | 42.87 | 830 | 42.87 | 0.653 |
| Female | 1,659 | 57.13 | 553 | 57.13 | 1,106 | 57.13 | Reference |
| Age (years) | 72.32±10.68 | 71.90±10.86 | 72.53±10.59 | 0.066 | |||
| CCI removed DM | 1.10±1.99 | 1.19±1.94 | 1.06±2.02 | 0.183 | |||
| Location | |||||||
| Northern Taiwan | 1,021 | 35.16 | 429 | 44.32 | 592 | 30.58 | Reference |
| Middle Taiwan | 856 | 29.48 | 206 | 21.28 | 650 | 33.57 | <0.001 |
| Southern Taiwan | 771 | 26.55 | 244 | 25.21 | 527 | 27.22 | <0.001 |
| Eastern Taiwan | 227 | 7.82 | 80 | 8.26 | 147 | 7.59 | 0.229 |
| Outlets islands | 29 | 1.00 | 9 | 0.93 | 20 | 1.03 | 0.369 |
| Urbanization level | |||||||
| 1 (The highest) | 825 | 28.41 | 331 | 34.19 | 494 | 25.52 | 0.020 |
| 2 | 1,210 | 41.67 | 402 | 41.53 | 808 | 41.74 | 0.034 |
| 3 (The lowest) | 869 | 29.92 | 235 | 24.28 | 634 | 32.74 | Reference |
| Level of care | |||||||
| Hospital center | 922 | 31.75 | 338 | 34.92 | 584 | 30.17 | 0.393 |
| Regional hospital | 1,187 | 40.87 | 374 | 38.64 | 813 | 41.99 | 0.419 |
| Local hospital | 795 | 27.38 | 256 | 26.45 | 539 | 27.84 | Reference |
| Insured premium (NT$) | |||||||
| <10,000 | 1,469 | 50.58 | 488 | 50.41 | 981 | 50.67 | Reference |
| 10,000–14,999 | 730 | 25.14 | 251 | 25.93 | 479 | 24.74 | 0.375 |
| ≧15,000 | 705 | 24.28 | 229 | 23.66 | 476 | 24.59 | 0.362 |
CCI = Charlson Comorbidity Index
P: Conditional logistic regression
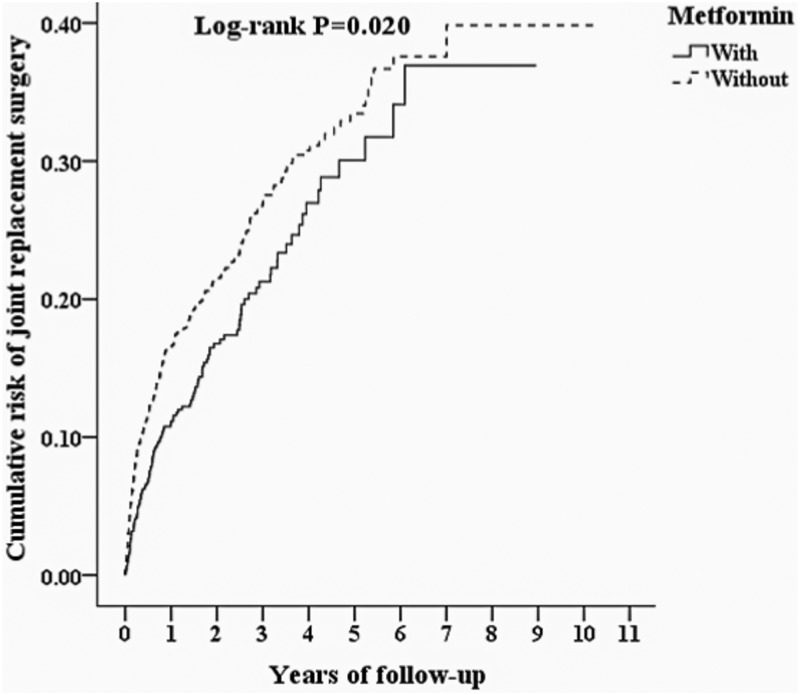
Fig 2 shows the Kaplan-Meier analysis for the cumulative risk of joint replacement surgery in the case and control groups with difference statistically significant (log-rank, p = 0.02). In addition, at the first year of follow-up, the difference between the two groups became significant (log-rank test <0.001).
Fig 2. Kaplan-Meier for cumulative risk of joint replacement surgery among DM with osteoarthritis and COX-2 inhibitor patients aged 18 and over stratified by metformin with log-rank test.
Table 3 shows the results of Cox regression analysis of the factors associated with the rate of joint replacement surgery. Cox proportional hazards regression (HR) analysis revealed that study subjects under metformin therapy were associated with lower rate of joint replacement surgery (adjusted HR 0.742 (95% CI = 0.601–0.915, p = 0.005)). The study subjects with increasing aged, medication possession ratio (MPR) with good adherence (>80%), high comorbidity (CCI scores) who removed diabetic patients and lived in non-Northern areas of Taiwan were associated with lower rate receiving joint replacement surgery. Otherwise, the study subjects lived in higher urbanized areas and receive therapy in hospital center were associated with higher rate receiving joint replacement surgery.
Table 3. Factors of joint replacement surgery by using Cox regression.
| Variables | Crude HR | 95% CI Lower limit |
95% CI Upper limit |
P | Adjusted HR | 95% CI Lower limit |
95% CI Upper limit |
P |
|---|---|---|---|---|---|---|---|---|
| Metformin | ||||||||
| Without | Reference | Reference | ||||||
| With | 0.781 | 0.634 | 0.962 | 0.020 | 0.742 | 0.601 | 0.915 | 0.005 |
| MPR of metformin | ||||||||
| Without | Reference | |||||||
| <40% | 0.885 | 0.629 | 1.162 | 0.316 | ||||
| 40–80% | 0.797 | 0.598 | 1.061 | 0.120 | ||||
| >80% | 0.634 | 0.412 | 0.977 | 0.039 | ||||
| Gender | ||||||||
| Male | 0.850 | 0.701 | 1.029 | 0.095 | 0.920 | 0.757 | 1.117 | 0.399 |
| Female | Reference | Reference | ||||||
| Age (years) | 0.969 | 0.962 | 0.977 | <0.001 | 0.971 | 0.964 | 0.979 | <0.001 |
| CCI removed DM | 0.612 | 0.544 | 0.688 | <0.001 | 0.647 | 0.578 | 0.725 | <0.001 |
| Location | Had collinearity with urbanization level | |||||||
| Northern Taiwan | Reference | |||||||
| Middle Taiwan | 0.613 | 0.484 | 0.775 | <0.001 | ||||
| Southern Taiwan | 0.705 | 0.555 | 0.895 | 0.004 | ||||
| Eastern Taiwan | 0.628 | 0.429 | 0.918 | 0.016 | ||||
| Outlets islands | 0.817 | 0.303 | 2.200 | 0.689 | ||||
| Urbanization level | ||||||||
| 1 (The highest) | 1.773 | 1.452 | 2.457 | 0.006 | 1.734 | 1.401 | 2.001 | 0.013 |
| 2 | 1.245 | 1.126 | 2.118 | 0.012 | 1.201 | 1.096 | 1.978 | 0.027 |
| 3 (The lowest) | Reference | Reference | ||||||
| Level of care | ||||||||
| Hospital center | 1.995 | 1.554 | 2.561 | <0.001 | 1.673 | 1.265 | 2.214 | <0.001 |
| Regional hospital | 1.258 | 0.978 | 1.618 | 0.074 | 1.113 | 0.862 | 1.437 | 0.410 |
| Local hospital | Reference | Reference | ||||||
| Insured premium (NT$) | ||||||||
| <10,000 | Reference | Reference | ||||||
| 10,000–14,999 | 1.459 | 0.875 | 3.742 | 0.305 | 1.269 | 0.855 | 3.307 | 0.256 |
| ≧15,000 | 1.546 | 0.903 | 3.886 | 0.496 | 1.334 | 0.894 | 3.521 | 0.452 |
HR = hazard ratio; CI = confidence interval; Adjusted HR: Adjusted variables listed in the table
In the subgroups stratified by the gender, urbanization, level of care and monthly income, the study subjects who were female, lived in the highest urbanization levels of residence, treatment in the hospital center and monthly insurance premiums of NT$ <10,000 were associated with a lower risk of joint replacement surgery in the combination metformin therapy group than those without metformin which respectively adjusted HR as 0.687 (p = 0.008), 0.549 (p = 0.003), 0.625 (p = 0.005), and 0.740 (p = 0.003) (Table 4). The patients stratified by MPR, in those with good adherence (>80%) with a trend that associated lower adjusted HR 0.566 (95% CI = 0.366–0.875, p = 0.01) (Table 5).
Table 4. Factors of joint replacement surgery stratified by variables listed in the table by using Cox regression.
| Metformin | With | Without | Ratio | Adjusted HR | 95%CI Lower limit |
95%CI Upper limit |
P | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stratified | Event | PYs | Rate (per 105 PYs) | Event | PYs | Rate (per 105 PYs) | |||||
| Total | 124 | 5,014.97 | 2,472.60 | 314 | 10,402.46 | 3,018.52 | 0.819 | 0.742 | 0.601 | 0.915 | 0.005 |
| Gender | |||||||||||
| Male | 53 | 2,179.67 | 2,431.56 | 121 | 4,404.22 | 2,747.37 | 0.885 | 0.855 | 0.616 | 1.189 | 0.352 |
| Female | 71 | 2,835.30 | 2,504.14 | 193 | 5,998.24 | 3,217.61 | 0.778 | 0.687 | 0.522 | 0.905 | 0.008 |
| Urbanization level | |||||||||||
| 1 (The highest) | 37 | 1,449.69 | 2,552.27 | 96 | 2,294.72 | 4,183.52 | 0.610 | 0.549 | 0.371 | 0.811 | 0.003 |
| 2 | 66 | 2,255.01 | 2,926.82 | 140 | 4,402.77 | 3,179.82 | 0.920 | 0.881 | 0.656 | 1.184 | 0.401 |
| 3 (The lowest) | 21 | 1,310.27 | 1,602.72 | 78 | 3,704.97 | 2,105.28 | 0.761 | 0.668 | 0.454 | 1.752 | 0.164 |
| Level of care | |||||||||||
| Hospital center | 49 | 1,535.77 | 3,190.58 | 127 | 2,580.49 | 4,921.55 | 0.648 | 0.625 | 0.449 | 0.871 | 0.005 |
| Regional hospital | 44 | 1,971.72 | 2,231.55 | 123 | 4,569.93 | 2,691.51 | 0.829 | 0.809 | 0.567 | 1.153 | 0.241 |
| Local hospital | 31 | 1,507.48 | 2,056.41 | 64 | 3,252.04 | 1,968.00 | 1.045 | 0.920 | 0.695 | 1.421 | 0.706 |
| Insured premium (NT$) | |||||||||||
| <10,000 | 113 | 2,511.24 | 4,499.77 | 292 | 5,301.70 | 5,507.67 | 0.817 | 0.740 | 0.599 | 0.913 | 0.003 |
| 10,000–14,999 | 7 | 1,413.39 | 495.26 | 13 | 2,577.24 | 504.42 | 0.982 | 0.889 | 0.720 | 1.097 | 0.129 |
| ≧15,000 | 4 | 1,090.34 | 366.86 | 9 | 2,523.52 | 356.64 | 1.029 | 0.932 | 0.755 | 1.149 | 0.337 |
PYs = Person-years; Adjusted HR = Adjusted Hazard ratio: Adjusted for the variables listed in Table 3.; CI = confidence interval
Table 5. Factors of joint replacement surgery stratified by MPR of metformin by using Cox regression.
| Metformin | With | Without | Ratio | Adjusted HR | 95%CI Lower limit |
95%CI Upper limit |
P | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MPR | Event | PYs | Rate (per 105 PYs) | Event | PYs | Rate (per 105 PYs) | |||||
| Total | 124 | 5,014.97 | 2,472.60 | 314 | 10,402.46 | 3,018.52 | 0.819 | 0.742 | 0.601 | 0.915 | 0.005 |
| <40% | 47 | 1,700.27 | 2,764.27 | 0.916 | 0.828 | 0.608 | 1.126 | 0.916 | |||
| 40–80% | 55 | 2,150.56 | 2,557.47 | 0.847 | 0.757 | 0.567 | 1.009 | 0.847 | |||
| >80% | 22 | 1,164.14 | 1,889.81 | 0.626 | 0.566 | 0.366 | 0.875 | 0.626 | |||
PYs = Person-years; Adjusted HR = Adjusted Hazard ratio: Adjusted for the variables listed in Table 3.; CI = confidence interval
Discussion
Previous studies have reported that a high frequency of OA in patients with T2DM and COX-2 inhibitors were significantly improved joint pain. [11, 16] Furthermore, some studies addressing the influence of T2DM on OA and its therapeutic outcomes suggests that DM may augment the development and severity of OA and that T2DM increases risks associated with joint replacement surgery. However, no studies discussed that combination COX-2 inhibitors and metformin therapy in OA patients with T2DM were associated with lower joint replacement surgery rates.
We found that the OA patients with T2DM under COX-2 inhibitors and metformin therapy were associated with lower joint replacement surgery rates than COX-2 inhibitors only. Even after adjusting for comorbidities and other covariates, the over-all adjusted HR was 0.742 (95% CI 0.601–0.915, P = 0.005). Kaplan-Meier analysis revealed that the study subjects were associated with a significantly lower 10-year risk of joint replacement surgery than the controls. In addition, it took just only 1 year to achieve a significantly adjusted HR. Our study is the first to indicate that OA patients with T2DM under COX-2 inhibitors combined metformin therapy were associated with lower joint replacement surgery risk in a nationwide, population-based study.
OA is a heterogeneous disorder that metabolic OA is wider than obesity-related OA since metabolic syndrome and OA are epidemiologically linked. [4, 12] In addition, one study showed that OA and T2DM were significantly associated that overall risk of OA in the T2DM population was 1.46 (1.08 to 1.96) and that of T2DM in the OA population was 1.41 (1.21 to 1.65). [11] Moreover, previous studies showed that T2DM independently alters the prognosis by increasing the risk of total joint replacement [29] and could be a specific OA risk factor. [30, 31] Long-standing T2DM is independently associated with advanced OA of knee and hip joints. The mechanisms responsible for the T2DM is a predictor for severe OA remain unclear. Schett et al have shown that impact of T2DM on symptoms or on OA structural lesions were more severe in T2DM than those without T2DM. [29] Moreover, the inflammatory aspect in imaging corroborates with the higher release of inflammatory mediators in OA cartilage explants from T2DM than those without T2DM. [32]
This finding adds to the yet short list of risk predictors for OA established in prospective evaluations. [33–35] After controlling the analysis for age, BMI, and other potential confounders, T2DM comprised a twofold risk of severe OA necessitating arthroplasty. These data suggest that hypertension, hypercholesterolemia, and blood glucose are associated with both unilateral and bilateral knee OA independent of obesity, and support the concept that OA has an important systemic and metabolic component in its etiology.
High glucose in T2DM may participate in IL-1β–induced inflammation via oxidative stress and the polyol pathway that increased inflammation in OA. [32] Much more increased IL-1β–induced IL-6 and PGE2 production in OA cartilage from T2DM than non-diabetes group which associated with IL-6 and COX2 mRNA expression, IL-6 and PGE2 release, and ROS and NO production in cultured chondrocytes. The traditional NSAIDs or selective COX-2 inhibitors are effective in the treatment of patients with symptoms and signs of OA. [14–16] When conservative treatment options are limited, and surgical replacement of damaged joints may be necessary in severe OA patients.
Our previous study showed that additional effect of metformin and celecoxib against lipid dysregulation and adipose tissue inflammation in high-fat fed rats with IR and fatty liver. [21] Combination therapy with celecoxib and metformin resulted in a reduction in adipose tissue macrophage infiltration and decreases in levels of adipose tissue TNF-α, MCP-1, and leptin levels in high-fat fed rats. We therefore hypothesize that combination therapy with COX-2 inhibitors and metformin may decrease these inflammatory factors resulting in OA patients with T2DM were associated with lower joint replacement surgery rates than COX-2 inhibitors only. It may be associated with additional effect of combination therapy in against inflammatory factors of OA that lower joint replacement surgery rates.
In our study, the subjects shows the factors associated with lower rate of joint replacement surgery stratified by MPR in those with good adherence. Treatment adherence is critical to effective management of T2DM or other chronic systemic diseases. [36, 37] Patient with well treatment adherence is important that poor adherence contributes to disease progression and increased morbidity and mortality. [38] In Taiwan, the average life expectancy is 76.0 years in males and 82.5 years in females, [39] and it is important for the elderly with much higher CCI with much more risk for anesthesia and surgery then caused a lower rate for joint replacement surgery. However, the reasons why the subgroups lived in higher urbanized areas, received therapy in hospital center, lower monthly insurance premiums and the female patients associated with a lower rate for joint replacement surgery are unknown, and further studies are needed to clarify this issue.
Limitations
There are several limitations to this study. First, patients with OA or T2DM could be identified using the insurance claims data, however data on the severity, disease duration and impact on diabetes control as HbA1c level were not available. Second, medical treatment may be effective in symptom improvement by decreasing inflammatory factors, however details regarding OA assessment scores were not available in the NHIRD. Finally, a longer follow-up period may be necessary to clarify risk for patients receiving joint replacement surgery.
Conclusion
Patients with T2DM are at a higher risk of developing OA than those without T2DM. Combination COX-2 inhibitors and metformin therapy in OA patients with T2DM are associated with lower joint replacement surgery rates than COX-2 inhibitor alone. Although the mechanisms responsible for this association are still unclear, inflammatory factors may contribute to the decreasing surgical rate. Further studies on the effects in reducing the joint replacement surgery risk of OA with T2DM are warranted.
Acknowledgments
This study was funded by Tri-Service General Hospital Research and Medical Affairs Bureau, Ministry of National Defense Foundation (TSGH-C104-122, MAB-105-07, TSGH- C106-002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
Data are from the National Health Institute Research Database (NHIRD), which are available to researchers in Taiwan and has been extensively used in epidemiologic studies. It is allowed to use for academic purpose only after proof by National Health Research Institute. Thus, the data cannot be made publicly available. Data requests may be sent to National Health Institute Research Database (http://nhird.nhri.org.tw/) at nhird@nhri.org.tw.
Funding Statement
This study was funded by Tri-Service General Hospital Research and Medical Affairs Bureau, Ministry of National Defense Foundation (TSGH-C104-122, MAB-105-07, TSGH- C106-002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann Rheum Dis. 2005;64(5):682–7. doi: 10.1136/ard.2004.023564 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim C, Nevitt MC, Niu J, Clancy MM, Lane NE, Link TM, et al. Association of hip pain with radiographic evidence of hip osteoarthritis: diagnostic test study. BMJ. 2015;351:h5983 doi: 10.1136/bmj.h5983 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yusuf E, Nelissen RG, Ioan-Facsinay A, Stojanovic-Susulic V, DeGroot J, van Osch G, et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis. 2010;69(4):761–5. doi: 10.1136/ard.2008.106930 . [DOI] [PubMed] [Google Scholar]
- 4.Puenpatom RA, Victor TW. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgrad Med. 2009;121(6):9–20. doi: 10.3810/pgm.2009.11.2073 . [DOI] [PubMed] [Google Scholar]
- 5.Alberti KG, Zimmet P, Shaw J, Group IDFETFC. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366(9491):1059–62. doi: 10.1016/S0140-6736(05)67402-8 . [DOI] [PubMed] [Google Scholar]
- 6.Waine H, Nevinny D, Rosenthal J, Joffe IB. Association of osteoarthritis and diabetes mellitus. Tufts Folia Med. 1961;7:13–9. . [PubMed] [Google Scholar]
- 7.Sturmer T, Brenner H, Brenner RE, Gunther KP. Non-insulin dependent diabetes mellitus (NIDDM) and patterns of osteoarthritis. The Ulm osteoarthritis study. Scand J Rheumatol. 2001;30(3):169–71. . [DOI] [PubMed] [Google Scholar]
- 8.Yoshimura N, Muraki S, Oka H, Kawaguchi H, Nakamura K, Akune T. Association of knee osteoarthritis with the accumulation of metabolic risk factors such as overweight, hypertension, dyslipidemia, and impaired glucose tolerance in Japanese men and women: the ROAD study. J Rheumatol. 2011;38(5):921–30. doi: 10.3899/jrheum.100569 . [DOI] [PubMed] [Google Scholar]
- 9.Berenbaum F. Diabetes-induced osteoarthritis: from a new paradigm to a new phenotype. Ann Rheum Dis. 2011;70(8):1354–6. doi: 10.1136/ard.2010.146399 . [DOI] [PubMed] [Google Scholar]
- 10.Mobasheri A. Glucose: an energy currency and structural precursor in articular cartilage and bone with emerging roles as an extracellular signaling molecule and metabolic regulator. Front Endocrinol (Lausanne). 2012;3:153 doi: 10.3389/fendo.2012.00153 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louati K, Vidal C, Berenbaum F, Sellam J. Association between diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD Open. 2015;1(1):e000077 doi: 10.1136/rmdopen-2015-000077 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115–26. doi: 10.1016/S0140-6736(11)60243-2 . [DOI] [PubMed] [Google Scholar]
- 13.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363–88. doi: 10.1016/j.joca.2014.01.003 . [DOI] [PubMed] [Google Scholar]
- 14.Derry S, Moore RA, Rabbie R. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2012; (9):CD007400 doi: 10.1002/14651858.CD007400.pub2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfe F, Zhao S, Lane N. Preference for nonsteroidal antiinflammatory drugs over acetaminophen by rheumatic disease patients: a survey of 1,799 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Arthritis Rheum. 2000;43(2):378–85. doi: 10.1002/1529-0131(200002)43:2<378::AID-ANR18>3.0.CO;2-2 . [DOI] [PubMed] [Google Scholar]
- 16.Xu C, Gu K, Yasen Y, Hou Y. Efficacy and Safety of Celecoxib Therapy in Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore). 2016;95(20):e3585 doi: 10.1097/MD.0000000000003585 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skou ST, Roos EM, Laursen MB, Rathleff MS, Arendt-Nielsen L, Simonsen O, et al. A Randomized, Controlled Trial of Total Knee Replacement. N Engl J Med. 2015;373(17):1597–606. doi: 10.1056/NEJMoa1505467 . [DOI] [PubMed] [Google Scholar]
- 18.Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. 2012;2(1):e000435 doi: 10.1136/bmjopen-2011-000435 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chamberlain JJ, Herman WH, Leal S, Rhinehart AS, Shubrook JH, Skolnik N, et al. Pharmacologic Therapy for Type 2 Diabetes: Synopsis of the 2017 American Diabetes Association Standards of Medical Care in Diabetes. Ann Intern Med. 2017;166(8):572–8. doi: 10.7326/M16-2937 . [DOI] [PubMed] [Google Scholar]
- 20.Diaz A, Romero M, Vazquez T, Lechner S, Blomberg BB, Frasca D. Metformin improves in vivo and in vitro B cell function in individuals with obesity and Type-2 Diabetes. Vaccine. 2017;35(20):2694–700. doi: 10.1016/j.vaccine.2017.03.078 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu CH, Hung YJ, Hsieh PS. Additional effect of metformin and celecoxib against lipid dysregulation and adipose tissue inflammation in high-fat fed rats with insulin resistance and fatty liver. Eur J Pharmacol. 2016;789:60–7. doi: 10.1016/j.ejphar.2016.07.012 . [DOI] [PubMed] [Google Scholar]
- 22.Tzeng NS, Chung CH, Yeh CB, Huang RY, Yuh DY, Huang SY, et al. Are Chronic Periodontitis and Gingivitis Associated with Dementia? A Nationwide, Retrospective, Matched-Cohort Study in Taiwan. Neuroepidemiology. 2016;47(2):82–93. doi: 10.1159/000449166 . [DOI] [PubMed] [Google Scholar]
- 23.Ho Chan WS. Taiwan's healthcare report 2010. EPMA J. 2010;1(4):563–85. doi: 10.1007/s13167-010-0056-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Association CH: ICD-9-CM English-Chinese Dictionary. Taipei, Taiwan: Chinese Hospital Association Press; 2000. [Google Scholar]
- 25.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nature reviews Molecular cell biology. 2008;9(5):367–77. doi: 10.1038/nrm2391 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang CH, Toh S, Lin JW, Chen ST, Kuo CW, Chuang LM, et al. Cancer risk associated with insulin glargine among adult type 2 diabetes patients—a nationwide cohort study. PLoS One. 2011;6(6):e21368 doi: 10.1371/journal.pone.0021368 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rau HH, Hsu CY, Lin YA, Atique S, Fuad A, Wei LM, et al. Development of a web-based liver cancer prediction model for type II diabetes patients by using an artificial neural network. Comput Methods Programs Biomed. 2016;125:58–65. doi: 10.1016/j.cmpb.2015.11.009 . [DOI] [PubMed] [Google Scholar]
- 28.Chang CY, Chen WL, Liou YF, Ke CC, Lee HC, Huang HL, et al. Increased risk of major depression in the three years following a femoral neck fracture—a national population-based follow-up study. PLoS One. 2014;9(3):e89867 doi: 10.1371/journal.pone.0089867 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schett G, Kleyer A, Perricone C, Sahinbegovic E, Iagnocco A, Zwerina J, et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes Care. 2013;36(2):403–9. doi: 10.2337/dc12-0924 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hart DJ, Doyle DV, Spector TD. Association between metabolic factors and knee osteoarthritis in women: the Chingford Study. J Rheumatol. 1995;22(6):1118–23. . [PubMed] [Google Scholar]
- 31.Berenbaum F. Diabetes-induced osteoarthritis: from a new paradigm to a new phenotype. Postgrad Med J. 2012;88(1038):240–2. doi: 10.1136/pgmj.2010.146399rep . [DOI] [PubMed] [Google Scholar]
- 32.Laiguillon MC, Courties A, Houard X, Auclair M, Sautet A, Capeau J, et al. Characterization of diabetic osteoarthritic cartilage and role of high glucose environment on chondrocyte activation: toward pathophysiological delineation of diabetes mellitus-related osteoarthritis. Osteoarthritis Cartilage. 2015;23(9):1513–22. doi: 10.1016/j.joca.2015.04.026 . [DOI] [PubMed] [Google Scholar]
- 33.Hawker GA, Guan J, Croxford R, Coyte PC, Glazier RH, Harvey BJ, et al. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54(10):3212–20. doi: 10.1002/art.22146 . [DOI] [PubMed] [Google Scholar]
- 34.Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109(1):18–24. . [DOI] [PubMed] [Google Scholar]
- 35.Schett G, Kiechl S, Bonora E, Zwerina J, Mayr A, Axmann R, et al. Vascular cell adhesion molecule 1 as a predictor of severe osteoarthritis of the hip and knee joints. Arthritis Rheum. 2009;60(8):2381–9. doi: 10.1002/art.24757 . [DOI] [PubMed] [Google Scholar]
- 36.Stolpe S, Kroes MA, Webb N, Wisniewski T. A Systematic Review of Insulin Adherence Measures in Patients with Diabetes. J Manag Care Spec Pharm. 2016;22(11):1224–46. doi: 10.18553/jmcp.2016.22.11.1224 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mikyas Y, Agodoa I, Yurgin N. A systematic review of osteoporosis medication adherence and osteoporosis-related fracture costs in men. Appl Health Econ Health Policy. 2014;12(3):267–77. doi: 10.1007/s40258-013-0078-1 . [DOI] [PubMed] [Google Scholar]
- 38.Brunton SA, Polonsky WH. Hot Topics in Primary Care: Medication Adherence in Type 2 Diabetes Mellitus: Real-World Strategies for Addressing a Common Problem. J Fam Pract. 2017;66(4 Suppl):S46–S51. . [PubMed] [Google Scholar]
- 39.Ministry of Interior: Life table in Taiwan. 2015, 2016(April, 7).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are from the National Health Institute Research Database (NHIRD), which are available to researchers in Taiwan and has been extensively used in epidemiologic studies. It is allowed to use for academic purpose only after proof by National Health Research Institute. Thus, the data cannot be made publicly available. Data requests may be sent to National Health Institute Research Database (http://nhird.nhri.org.tw/) at nhird@nhri.org.tw.