Abstract
Background
Using an organotypic culture system termed human Fetal Testis Assay (hFeTA) we previously showed that 0.01 μM BPA decreases basal, but not LH-stimulated, testosterone secreted by the first trimester human fetal testis. The present study was conducted to determine the potential for a long-term antiandrogenic effect of BPA using a xenograft model, and also to study the effect of BPA on germ cell development using both the hFETA and xenograft models.
Methods
Using the hFeTA system, first trimester testes were cultured for 3 days with 0.01 to 10 μM BPA. For xenografts, adult castrate male nude mice were injected with hCG and grafted with first trimester testes. Host mice received 10 μM BPA (~ 500 μg/kg/day) in their drinking water for 5 weeks. Plasma levels of total and unconjugated BPA were 0.10 μM and 0.038 μM respectively. Mice grafted with second trimester testes received 0.5 and 50 μg/kg/day BPA by oral gavage for 5 weeks.
Results
With first trimester human testes, using the hFeTA model, 10 μM BPA increased germ cell apoptosis. In xenografts, germ cell density was also reduced by BPA exposure. Importantly, BPA exposure significantly decreased the percentage of germ cells expressing the pluripotency marker AP-2γ, whilst the percentage of those expressing the pre-spermatogonial marker MAGE-A4 significantly increased. BPA exposure did not affect hCG-stimulated androgen production in first and second trimester xenografts as evaluated by both plasma testosterone level and seminal vesicle weight in host mice.
Conclusions
Exposure to BPA at environmentally relevant concentrations impairs germ cell development in first trimester human fetal testis, whilst gonadotrophin-stimulated testosterone production was unaffected in both first and second trimester testis. Studies using first trimester human fetal testis demonstrate the complementarity of the FeTA and xenograft models for determining the respective short-term and long term effects of environmental exposures.
Introduction
Over recent decades, the incidence of male reproductive disorders has been steadily increasing [1–4]. These disorders such as cryptorchidism, hypospadias, low sperm count and quality, and testicular cancer are hypothesized to arise from abnormal development of the fetal testis. These associated disorders have been collectively described as a testicular dysgenesis syndrome (TDS) [5–8].
In 1993, Sharpe and Skakkebaek hypothesized that endocrine disruptors (EDs), particularly EDs with an estrogenic effect, could be an explanation for the increase in male reproductive disorders [9] initiating a large number of studies in reproductive toxicology [4,10,11]. Among such EDs, bisphenol A (BPA; 4,4'-dihydroxy-2,2-diphenylpropane) has been the focus of considerable research [12–15].
BPA is one of the most frequently produced synthetic chemicals worldwide, with approximately 70% used to produce polycarbonate plastics for a variety of products, including housewares and appliances, opticals, construction materials and medical, packaging. A further 20% of BPA is used as an essential component of epoxy resins that are mainly used to coat the inner surface of metallic food and beverage cans. Finally, BPA is used as antioxidant or inhibitor of polymerization in some plasticizers, polyvinyl chloride, and thermal cash register paper.
Many studies have shown that BPA exposure of rodents during intrauterine life can induce a range of adverse effects in adult testes. It has been shown that in utero or perinatal BPA exposure induces a decrease in sperm quality and production and testosterone secretion in adults [14,16–21]. These results suggest that BPA potentially disturbs fetal testis development and future function.
However, there is limited data and conflicting results concerning the direct immediate effect of in vivo BPA exposure on fetal testis development and function. In pregnant rats, exposure to high doses of BPA (876 μM i.e. calculated to be approximately 20,000 μg/kg/day, based on an adult rat weight of 300 g and a fluid intake of about 30 mL/day) in drinking water reduced plasma testosterone in the offspring at birth [22]. Gavage of pregnant rats with 1–200 mg/kg/day BPA increased the mRNA level but not the protein levels of Raf1 in the Leydig cells at postnatal day 3 [23]. Administration of 250 μg/kg/day BPA reduced the anogenital distance in male rat pups, which suggests a reduction in fetal androgen exposure, whereas lower BPA doses did not have any effect [24]. On the contrary, three other studies investigating potential effects of BPA exposure, found no difference in AGD between BPA and vehicle exposed pups following gestational gavage with doses equal or higher than 1 mg/kg/day and reaching 200 to 50000 mg/kg/day [25–27].
Furthermore, recent ex vivo analyses have demonstrated the complexity of the potential effect of BPA on Leydig cell function and development. Using an organotypic culture system termed “Fetal Testis Assay” (FeTA) developed for rat fetal testis in 1990's [28] and extended for mouse and human fetal testes thereafter [29,30], we demonstrated that BPA concentrations as low as 0.01 μM (i.e. 2.28 μg/L) reduced basal testosterone secretion and Insl-3 expression by the human fetal testis, but not by mouse and rat fetal testes for which concentrations equal or higher to 1 μM BPA were required in order to induce similar effects [31]. This was confirmed for human fetal testes subsequently by another laboratory using the same FeTA approach [32]. In the continuous presence of LH or hCG, 1 or 10 μM BPA was necessary to reduce testosterone secretion in human, mouse and rat [15,32].
To date, no study has investigated the effect of BPA on male germ cell (GC) development during fetal life in either rodent or human models. Fetal primordial GC migrate into the embryonic genital ridge. By 7 weeks of gestation in human they are enclosed in the seminiferous cords at which point they are termed gonocytes [33–36]. Gonocytes express the pluripotency markers such as Activating Enhancer Binding Protein 2 Gamma (AP-2y), a transcription factor associated with an undifferentiated state and encoded by the TFAP2C gene. As GC differentiate from gonocytes to pre-spermatogonia the expression of AP-2y is reduced and the cells begin to express Melanoma Associated Antigen A4 (MAGE-A4) a differentiation marker also expressed in adult spermatogonia [37–42]. In rodents, this transition from gonocyte to pre-spermatogonia occurs synchronously during late gestation, whereas in humans this transition occurs asynchronously over the remainder of fetal and into early postnatal life [37,38,40,42–44]. During the first trimester of gestation, we have previously shown that the rate of GC proliferation is ~25–30%, and ~1–3% of GC are apoptotic [36]. In the second trimester ~20–25% of gonocytes are proliferative, compared with only ~3% of pre-spermatogonia at this stage [41].
The present study was designed to investigate the effects of BPA on both endocrine function and GC development in the human fetal testis. We combined two experimental models: we used the ‘short term’ organotypic culture system (FeTA) and the ‘long term’ xenograft system [41,45,46], both of which have been validated for assessment of effects of exposures to chemicals on human fetal testis development.
The specific aims were 1) to investigate the potential for an antiandrogenic effects of BPA in human fetal testes from the first and the second trimesters using the xenograft system 2) to study the effect of BPA on GC development in first trimester testes using both FeTA and xenograft systems, 3) to compare the results of the two experimental models using first trimester testis tissue.
Materials and methods
First trimester human Fetal Testis Assay (hFeTA) and xenograft studies
Collection of human fetal testis
Human fetal testes (5–12 Gestational Weeks; GW) were obtained from pregnant women referred to the Department of Gynecology and Obstetrics at the Antoine Béclère hospital (Clamart, France) for legally induced abortions in the first trimester of pregnancy as previously described [30]. All women provided written informed consent for scientific use of the fetal tissues. None of the abortions were due to fetal abnormality. The fetal age was determined by measuring the length of limbs and feet [47]. Gonads within the abortive material were retrieved in 50% of cases. Testes were identified based on their size and their morphology. The project was approved by the local Medical Ethics Committee and by the French Biomedicine Agency (reference number PFS 12–002).
Human Fetal Testis Assay (hFeTA)
Human Fetal Testis Assay (hFeTA) is the organotypic culture system developed in our laboratory and largely described previously [28–30,48,49]. Briefly, human testes were cut into small pieces (12 to 30 pieces depending on the age of the fetus) and 3–4 pieces were randomly placed on Millicell-CM Biopore membranes (pore size 0.4 μm, Millipore, Billerica, MA) floating on 320 μL culture medium in tissue culture dishes. Cultures were performed at 37°C in a humidified atmosphere containing 95% air/ 5% CO2. The culture medium was phenol-red free Dulbecco modified Eagle medium/Ham F12 (1:1; Gibco, Grand Island, NY), supplemented with 80 μg/mL gentamicin (Sigma, St. Louis, MO). BPA was purchased from Sigma (CAS no. 80-09-1; Sigma, St. Louis, MO) and diluted in ethanol.
After 24 hours in control medium, explants were cultured with 100 ng/mL LH from human pituitary (≥ 5,000 IU/mg; Sigma) in the presence of ethanol vehicle (control explants) or BPA at concentrations ranging from 0.01 to 10 μM for three subsequent days. Control and treated explants were from the same testis. The whole culture medium was changed every 24h. At the end of the culture, explants were fixed in Bouin’s and formaldehyde for analysis of cell apoptosis and proliferation of germ cells as previously described [30,48].
Host mice for xenografting
Nude mice (NMRI-Foxnlnu/foxnlnu) from Janvier (Saint Berthevin, France) were received at 4 weeks-old and housed in our animal facility under controlled photoperiod conditions (lights on 08:00 am to 08:00 pm). They were supplied with estrogen-free commercial food and tap water ad libitum. Mice received water via glass water bottles. The animal facility is licensed by the French Ministry of Agriculture (agreement n° B92-032-02). All efforts were made to avoid or minimize animal suffering.
Xenografting procedure
Pieces of human fetal testis were xenografted into the muscle of the back as previously described for ovarian xenografts [50]. Briefly, both human fetal testes from 9 fetuses (9.1 to 11.3 GW) were cut into pieces (~1 mm3 approx.). Male Nude mice (aged 5–8 weeks) were anesthetized by isoflurane inhalation. All pieces from one testis were grafted in one mouse which was then exposed to BPA whilst all the pieces from the other testis of the same human fetus were grafted into another mouse which will serve as a control. The testicular pieces were inserted in the left back muscle of the host mice. Mice were then immediately castrated and injected subcutaneously with 20 IU hCG (Human Chorionic Gonadotrophin, MSD France). Mice received analgesia (Carprofen, 25μg/mL, Santa Cruz, sc 205621) in the drinking water for one recovery week post-surgery.
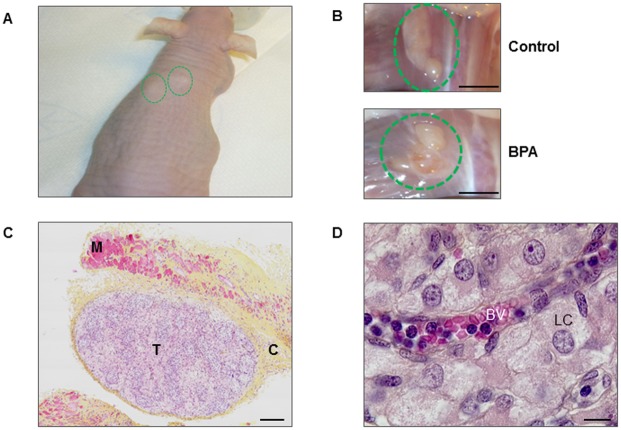
Six weeks post-xenografting, the xenografted testes were visible as subcutaneous swellings of the host mouse indicating growth of the xenografts in this in vivo model (Fig 1A). Xenografts were approximately five times larger than they were when grafted. After euthanasia the xenografts were visible in the back muscle by their white color and their large size in both control and BPA-treated host mice, (Fig 1B).
Fig 1. Xenografting first trimester human fetal testis.
Human fetal testes (9.1–11.3 GW) xenografted in castrated Nude (host) mice. Host mice received vehicle (Control) or 10μM BPA in the drinking water. (A) Host mouse six weeks after xenografting demonstrating xenograft tissue below the skin (green circles). (B) Xenografted explants (green circles) in the back muscle of the host mouse at the end of the experiment, in a control and a BPA-treated mouse. Scale bar: 5 mm. (C) Histological section of a xenograft after haematoxylin-eosin-saffron staining, T: testis; M: mouse muscle; C: connective tissue. Scale bar: 100 μm. (D) Histological section of a xenograft showing a blood vessel (BV) in the interstitial tissue. LC: Leydig cell. Scale bar: 10 μm.
Histological sections of both control and BPA-treated xenografted testes exhibited a well-maintained architecture with Sertoli cells surrounding the germ cells in the seminiferous cords and the Leydig cells in the interstitial compartment (Fig 1C and 1D). Furthermore, in histological sections blood vessels were present inside the xenografted tissues. There was no evidence of necrosis, demonstrating that the size of xenografted pieces of testis was suitable for supporting good vascularization of tissues and that the xenografting procedure preserved normal histological appearance of the testis (Fig 1C and 1D).
Treatment of host mice
All the mice were injected subcutaneously every 72h with 20 IU hCG from the day of surgery until euthanasia. After one recovery week, host mice received drinking water containing 0.1% ethanol (control mice) or BPA diluted in 0.1% ethanol to obtain 10 μM BPA as the final concentration (BPA-treated mice) for the five subsequent weeks. During the treatment period, mice were weighed every week. BPA treatment did not affect the body weight of the mice compared to controls. As one adult mouse weighs ~30 g and drinks ~7 mL of water per day the evaluated daily intake of BPA by treated mice is ~500 μg/kg of body weight/day. For comparison, the US Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) have determined the NOAEL for BPA to be equal to 5,000 and 8,960 μg/kg of body weight/day in rodents respectively.
Sampling in xenograft model
At the end of the grafting period, host mice were anesthetized by isoflurane inhalation and blood was extracted by cardiac puncture for testosterone and BPA measurements. Mice were killed by cervical dislocation and seminal vesicles were removed and weighed. Xenografts were retrieved and fixed in Bouin’s fluid overnight.
Internal BPA concentration in the host mice
In order to characterize this model, free and total (free + glucuronide and sulfate conjugates) BPA concentration levels were assayed in plasma of both exposed and control mice. Each sample (20 to 50 μL) was fortified with 10 ng of 13C-BPA (internal standard for quantification according to the isotope dilution method), vortexed for 30 sec and followed by equilibration for 3h. For a total BPA determination, i.e.: free + phase II metabolites (glucuronide + sulfate), an enzymatic hydrolysis was carried out using a purified Helix pomatia preparation (50°C for 12h). Ethyl acetate (500 μL) was added, the sample was shaken for around 30 sec and centrifuged at 5000 rpm for 15 min at 4°C. The organic phase was collected into a 4 mL glass tube. This liquid/liquid extraction was repeated twice. The resulting organic phase was evaporated to dryness under a gentle nitrogen stream (45°C). The dry residue was reconstituted in 100 μL acetonitrile and transferred to an injection vial, and finally evaporated to dryness. Then, 20 μL of MSTFA [N-Methyl-N-(trimethylsilyl) trifluoroacetamide] were added to the dry residue and the vial was heated for 30 min at 45°C. Detection was achieved using gas chromatography coupled to tandem mass spectrometry (GC-MS/MS) operating in selected reaction monitoring (SRM) acquisition mode as previously described [51,52]. The limit of detection of BPA in the plasma was 0.13 nM (0.03μg/L) and the limit of quantitation was 0.44 nM (0.1μg/L). All the samples were above the limit of quantitation.
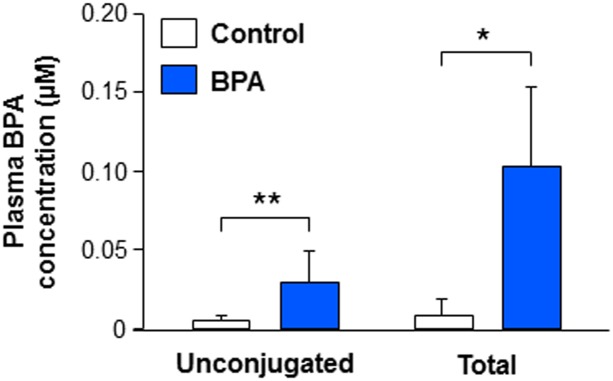
Plasma of BPA-treated mice contained 0.10 ± 0.04 μM total BPA, one third of which was unconjugated (0.038 ± 0.010 μM) (Fig 2). Control mice exhibited detectable concentrations of total and unconjugated BPA (0.02 ± 0.01 μM and 0.007 ± 0.001 μM respectively). In the literature, generally, internal level of BPA is not assayed in both control and treated animals. However, a recent study performed by the National Program of Toxicology carefully evaluated total and unconjugated levels of BPA in the plasma of control rats and reported values in the same order of magnitude as the present results [53]. Importantly, in the present study, both unconjugated and total plasma BPA concentrations were significantly higher in the treated group compared with controls.
Fig 2. Effect of BPA exposure on plasma BPA concentration in xenografted mice.
Plasma levels of BPA were quantified by gas chromatography coupled to tandem mass spectrometry (GC-MS/MS) from castrated Nude male mice xenografted with first trimester human fetal testis (9.1–11.3 GW, mean 10.2 ± 0.2 GW; n = 6–7) and exposed to vehicle (Control) or BPA (10 μM in the drinking water) for five weeks. For each fetus, all the pieces from one testis were grafted in a control mouse and all the pieces from the contralateral testis were grafted in a BPA-treated mouse. Statistical analysis was performed using the Mann-Whitney test. *p<0.05, **p<0.01.
Histological analyses
After dehydration the tissue pieces were embedded in paraffin wax and sectioned at 5 μm thickness. Serial testes sections were deparaffinized, rehydrated and haematoxylin-eosin-saffron staining or immunohistochemistry were performed as previously described [54,55].
To evaluate the percentage of apoptotic and proliferative germ cells, immunohistochemistry was performed using anti-cleaved caspase-3 (CC3; 1:100, Cell Signaling, 9661S) and anti-Ki67 (1:50, BD Pharmingen, cat 556609) antibodies respectively. Anti-AMH antibody (1:200, Santa Cruz, sc6886) [48] was used to stain the Sertoli cells in order to aid identification of the AMH-negative GC within the seminiferous cords. Immunohistochemistry was conducted using anti-AP-2γ (1:100, Santa Cruz, sc12762) or anti-MAGE-A4 (1:1000, GeneTex, GTX116357) antibodies to determine germ cell differentiation status. The primary antibodies were detected using species-specific secondary antibodies conjugated with a peroxidase activity polymer (ImmPRESS reagent kit; Vector Laboratories, Burlingame, CA). Peroxidase activity was visualized in brown using DAB (3,3’-diaminobenzidine) as substrate or visualized in green using Permanent HRP Green Kit (Zytomed Systems).
To quantify germ cell density, germ cells were identified based on morphological appearance after haematoxylin-eosin-saffron staining and counted in several randomly chosen areas each one measuring 0.464 mm2.
All counts were carried out blind using the Histolab analysis software (Microvision Instruments, Evry, France). A minimum of 500 germ cells were counted per testis.
Testosterone radioimmunoassay
Serum testosterone concentration of Nude host mice was measured as previously described [56]. The limit of detection was 80 pg/mL. The intra-assay coefficient of variation (CoV) is 2% and the inter-assay CoV is 5%.
RNA extraction, reverse transcription and real-time Polymerase Chain Reaction
A piece of each grafted testis was frozen in RLT buffer (Qiagen, Courtaboeuf, France) and stored at– 20°C. Total RNA from frozen samples was extracted using the RNeasy Mini-Kit (Qiagen, Courtaboeuf, France) and reverse transcribed using the High Capacity cDNA Reverse, followed by real-time PCR as previously described (48). The StepOne real-time PCR system (ThermoFisher Scientific) and Quantifast Sybr green (Qiagen) were used for RT–qPCR. The comparative ΔΔcycle threshold method was used to determine the relative quantities of specific key steroidogenic mRNA (STAR, CYP11A1, CYP17A1 and CYP19) using β-ACTIN, RPL0 and Cyclophilin A (CYPA) as reference genes. Each sample was run in duplicate. The sequences of oligonucleotides used with SYBR-green detection were designed with Primer express Software (Table 1).
Table 1. Sequences of the primers.
| Genes | Forward sequence | Reverse sequence |
|---|---|---|
| STAR | CGTGGTACTCAGCATCGA | TGGGCACAGTTGGGAACA |
| CYP11A1 | TGGGTCGCCTATCACCAGTAT | CCACCGGTCTTTCTTCCA |
| CYP17A1 | CCACCTTTGCCCTGTTCAAG | GCCAGCATATCACACAATGTACT |
| CYP19 | TCACTGGCCTTTTTTCTCTTGGT | GGGTCCAATTCCCATGCA |
| β-ACTIN | TGACCCAGATCATGTTTGAGA | TACGGCGAGAGGCGTACAGG |
| RPLP0 | TCCCACTTGCTGAAAAGGTCA | CAAAGGCAGATGGATCAGCC |
| CYPA | CGCGTCTCCTTTGAGCTGTT | ATTTTCTGCTGTCTTTGGGACC |
Statistical analysis
All data are presented as means ± SEM. The statistical significance of the difference between control and BPA-treated data were evaluated using the non-parametric test: Wilcoxon for paired comparisons and Mann-Whitney for unpaired ones. Statistical significance was set as p<0.05.
Second trimester human fetal testis xenograft studies
Collection of second trimester human fetal testis
Human testes (GW 14–18) were collected from second-trimester fetuses (n = 4), obtained after the medical termination of pregnancy following written informed consent. Gestation was determined by ultrasound scan and subsequent direct measurement of foot length. Ethical approval for obtaining the tissue was given by the South East Scotland Research ethics committee (reference number LREC08/S1101/1).
Host mice for xenografting
Male CD1 nude mice (aged 4–6 weeks; n = 64; Charles River UK) were housed in individually ventilated cages with access to soy-free diet and water ad libitum. In preparation for xenografting, mice were anesthetised by inhalation of isofluorane and castrated through a scrotal incision. Castration was performed at least 2 weeks prior to xenografting. Following castration, mice received analgesia (Carprofen; Pfizer) in the drinking water for 3 days post-surgery.
Xenografting procedure
Human fetal testis xenografts were performed as previously described [41]. Briefly, human fetal testes were cut into approximately 1 mm3 pieces and grafted subcutaneously on either side of the midline of nude mice. Testis tissue pieces from each human fetus testis were grafted into 1–3 host mice. Specific approval for the animal studies, including ethical approval was given by the UK Home office and all procedures were conducted in accordance with the Animal (Scientific Procedures) Act 1986. Mice received corn oil containing either vehicle (absolute ethanol) or BPA (Sigma-Aldrich) by daily gavage at a dose of 0.5 or 50μg/kg of body weight for a period of 5 weeks. Mice were euthanized by inhalation of CO2 and cervical dislocation. Blood was extracted by cardiac puncture for testosterone measurements and seminal vesicles were removed and weighed.
Testosterone assay
Serum testosterone concentration of Nude host mice was measured as described above for the first trimester. To perform relevant comparisons between values observed in first and the second trimester xenografted mice hosts, all the plasma testosterone extraction and assay were performed by the same investigator.
Statistical analysis
The method is the same as that used above for the studies of the first trimester testis.
Results
Effects of BPA exposure on first trimester human fetal testis
Effects of BPA on gonocyte apoptosis and proliferation in first trimester human fetal testis using FeTA model
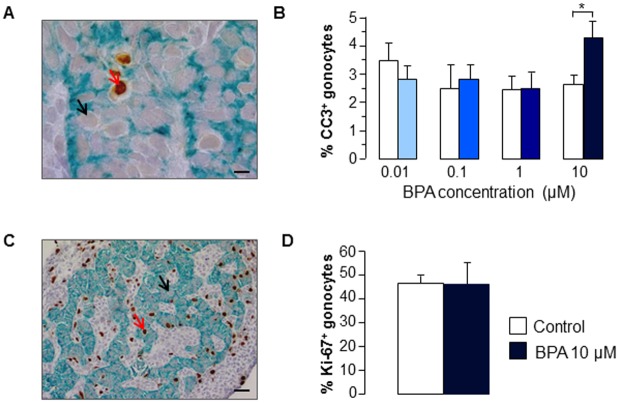
Human fetal testes from 6 to 12 GW, were cultured for 72 hours in the presence or absence of BPA at various concentrations ranging from 0.01μM to 10 μM to evaluate the effect of BPA on germ cells apoptosis (Fig 3A and 3B). Exposure to 10 μM BPA significantly increased germ cells apoptosis ex vivo by 76 ± 37% (n = 8; p<0.05). However, lower concentrations of BPA did not affect germ cells apoptosis.
Fig 3. Effect of BPA exposure on germ cell apoptosis and proliferation in first trimester human fetal testes cultured using the FeTA system.
Human fetal testes (6–12 GW, mean 8.7 ± 0.6 GW) were cultured using the ex vivo human Fetal Testis Assay system (hFeTA). After 24 hours in control medium, explants were cultured with 100 ng/mL of LH for the 3 subsequent days in the presence of ethanol vehicle (control explants) or BPA at concentrations ranging from 0.01 to 10 μM. Control and BPA-treated explants were paired samples from the same testis. (A) Histological sections after labeling with anti-cleaved caspase-3 antibody (brown) and anti-AMH antibody (green). Positive (red arrows) and negative (black arrows) germ cells can be identified. Scale bar: 10 μm. (B) Quantification of cleaved caspase-3 positive cells (mean ± SEM; n = 4–8). (C) Histological sections after labeling with anti-Ki-67 antibody (brown) and anti-AMH antibody (green). Positive (red arrows) and negative (black arrows) germ cells can be identified. Scale bar: 50 μm. (D) Quantification of Ki67 positive gonocytes (mean ± SEM; n = 4–8).
Moreover, exposure to 10 μM BPA did not affect proliferation of human fetal germ cells based on quantification of immunostaining for Ki-67 (Fig 3C and 3D).
Effect of BPA on germ cell apoptosis, proliferation and density in first trimester human fetal testis xenografts
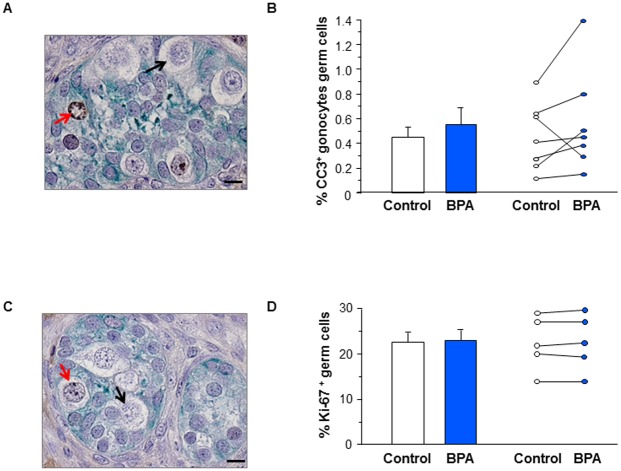
The percentage of apoptotic germ cells (CC3+) was increased by 35±22% (n = 7) in the BPA-treated group compared with the controls, although this did not reach statistically significance (Fig 4A and 4B). BPA exposure did not affect the percentage of gonocyte proliferating (Ki67+) gonocytes (Fig 4C and 4D).
Fig 4. Effect of BPA exposure on germ cell apoptosis and proliferation in first trimester human fetal testis xenografts.
Human fetal testes (9.1–11.3 GW) were xenografted into castrate Nude (host) mice. Host mice received vehicle (Control) or 10μM BPA in the drinking water for five weeks. (A) Histological sections of the testis after labeling with anti-CC3 antibody (brown) and anti-AMH antibody (green). (B) Quantification of CC3+ cells displayed as mean ± SEM (n = 7) on the left panel and as individual values with a line drawn between the control and the corresponding BPA-treated testis from the same fetus on the right panel. (C) Histological sections after labeling with anti-Ki-67 antibody (brown) and anti-AMH antibody (green). Positive (red arrows) and negative (black arrows) germ cells can be identified. (D) Quantification of Ki67+ gonocytes displayed as mean ± SEM (n = 5) on the left panel and as individual values with a line drawn between the control and the corresponding BPA-treated testis from the same fetus on the right panel. Scale bars: 15 μm. Data analyzed using Wilcoxon paired test. For both apoptosis and proliferation, the differences between vehicle and BPA-exposed groups were not statistically significant.
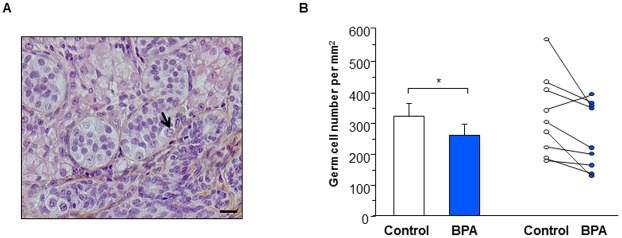
We hypothesized that a modest but sustained negative effect of BPA on germ cell apoptosis during a longer period would impact on the germ cell density. Indeed, we observed that 5 weeks of treatment with BPA significantly reduced the density of germ cells by 19±7% (n = 9) as compared with control (Fig 5).
Fig 5. Effect of BPA exposure on germ cell density in first trimester human fetal testis xenografts.
Human fetal testes (9.1–11.3 GW) were xenografted into castrate Nude (host) mice. Host mice received vehicle (Control) or 10μM BPA in the drinking water for five weeks. (A) Histological sections after haematoxylin-eosin-saffron staining. Germ cells (black arrow) can be easily identified. Scale bar: 20 μm. (B) Quantification of germ cell density displayed as mean ± SEM (n = 9) on the left panel and as individual values with a line drawn between the control and the corresponding BPA-treated testis from the same fetus on the right panel. Data analyzed using the Wilcoxon paired-test. *p<0.05 compared with control condition.
Effect of BPA on germ cell differentiation in first trimester human fetal testis xenografts
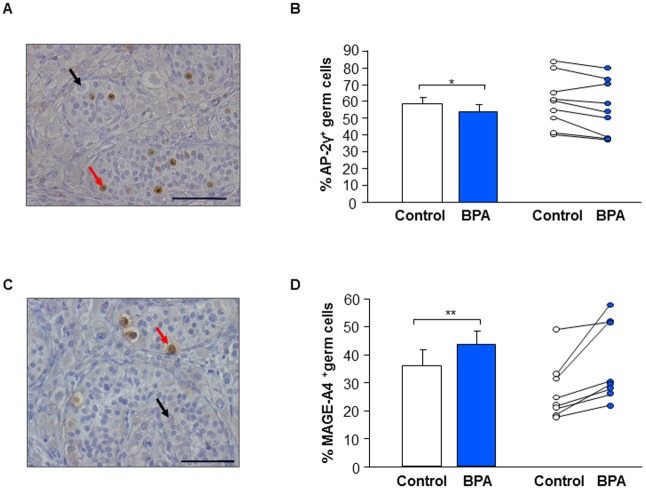
Using immunohistochemistry, we quantified the percentage of germ cells expressing a gonocyte marker (AP-2γ), and a pre-spermatogonial (MAGE-A4) marker (Fig 6A and 6C). After BPA treatment, percentage of germ cells expressing AP-2γ was significantly decreased by 6.9 ± 2.7% (n = 9) whereas percentage of germ cells expressing MAGE-A4 was significantly increased by 21.2 ± 6.2% (n = 8) compared with control (Fig 6B and 6D).
Fig 6. Effect of BPA exposure on germ cell differentiation in first trimester human fetal testis xenografts.
Human fetal testes (9.1–11.3 GW) were xenografted into castrate Nude (host) mice. Host mice received vehicle (Control) or 10μM BPA in the drinking water for five weeks. (A) Histological sections of testes after immunostaining for AP-2γ (gonocytes). Positive (red arrows) and negative (black arrows) germ cells can be identified. Scale bar: 60 μm. (B) Quantification of AP-2γ-positive cells displayed as mean ± SEM (n = 9) on the left panel and as individual values with a line drawn between the control and the corresponding BPA-treated testis from the same fetus on the right panel. (C) Histological sections of testes after immunostaining for MAGE-A4 (prespermatogonia). Positive (red arrows) and negative (black arrows) germ cells can be identified. Scale bar: 60 μm. (D) Quantification of MAGE-A4-positive cells displayed as mean ± SEM (n = 8) on the left part and as individual values with a line drawn between the control and the corresponding BPA-treated testis from the same fetus on the right part. Data analyzed using the Wilcoxon paired-test. *p<0.05, **p<0.01.
Effect of BPA on hCG-stimulated androgenic production in first trimester human fetal testis xenografts
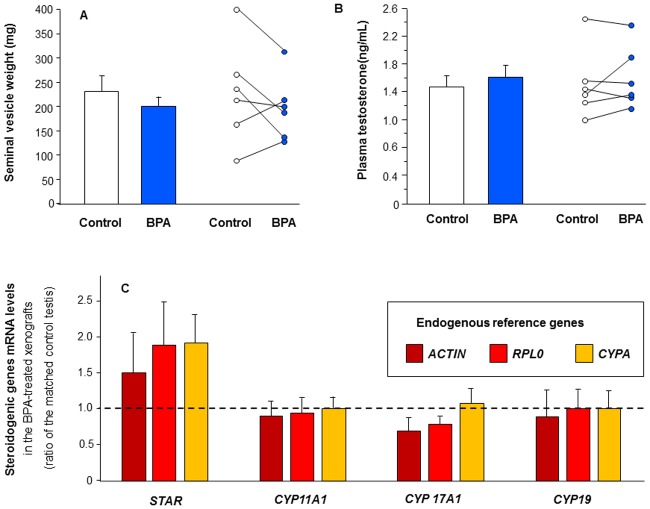
We analyzed endocrine function of control xenografts at the end of the experiment. Seminal vesicle (SV) weight (an indirect measure of androgen production) of host control mice was 230 ± 43 mg (Fig 7A) indicating secretion of testosterone during the six weeks of xenografting. This is similar to SV weight (~280 mg) in non-castrated non-xenografted Nude mice whilst castrated non-xenografted Nude mice have SV weights of ~5–10 mg. No significant differences were found in SV weight (Fig 7A) or plasma testosterone level (Fig 7B) between BPA-treated and control mice. It should be noted that SV weight and plasma testosterone concentration were significantly positively correlated both in controls (r = 0.816, n = 6) and in BPA-treated mice (r = 0.845, n = 6).
Fig 7. Effect of BPA exposure on plasma testosterone level and seminal vesicle weight in the host mice xenografted with first trimester human testes and on steroidogenic genes expression in the xenografts.
Human fetal testes (9.1–11.3 GW) were xenografted into castrate Nude (host) mice. Host mice received vehicle (Control) or 10 μM BPA in the drinking water for five weeks. (A) seminal vesicle weight displayed as mean ± SEM (n = 6) on the left panel and as individual values with a line drawn between the control and the corresponding BPA-exposed testis from the same fetus on the right panel. B) plasma testosterone concentration in host mice displayed as mean ± SEM (n = 6) on the left panel and as individual values with a line drawn between the control and the corresponding BPA-exposed testis from the same fetus on the right panel. C) Expression of key genes in the steroidogenic pathway (STAR, CYP11A1, CYP17A1 and CYP19) using quantitative RT-PCR standardized to either β-ACTIN (ACTIN) or RPLP0 or CYPA as endogenous control. Results are presented as a percentage of the control value (mean ± SEM; n = 4). Data analyzed by Wilcoxon test. No significant difference between BPA-treated and control mice were identified.
To confirm the lack of effect of BPA on steroidogenesis, we measured the mRNA levels of various key regulators in the steroidogenic pathway. BPA treatment did not affect the expression of STAR, CYP11A1, CYP17A1, and CYP19 regardless of the reference gene (Fig 7C).
Effects of BPA exposure on second trimester human fetal testis
Effect of BPA on hCG-stimulated androgenic production in second trimester human fetal testis xenografts
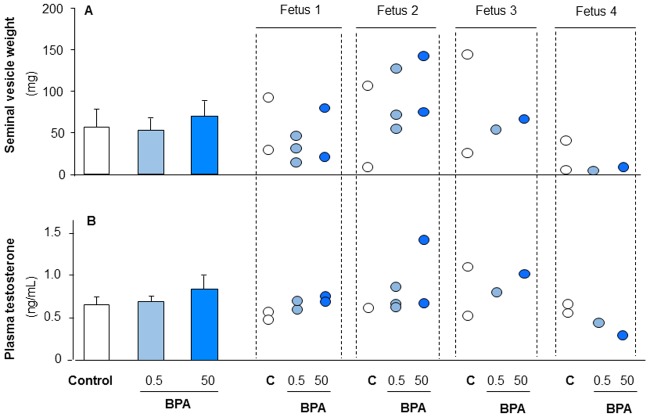
Seminal vesicle weight and serum testosterone were measured in host mice to determine testosterone production over the grafting period. No difference was observed for seminal vesicle weight (Fig 8A) or serum testosterone (Fig 8B) after 5 weeks exposure to 0.5 or 50 μg/kg/d BPA compared with vehicle-exposed control.
Fig 8. Effect of BPA exposure on testosterone plasma level and seminal vesicle weight in the host mice carrying second trimester human fetal testis xenografts.
Human fetal testes (14-18GW) xenografted into castrated Nude (host) mice. Each human fetal testis was grafted into 1–3 host mice which received the same treatment. Host mice received vehicle (Control), BPA (0.5μg/kg or 50μg/kg/d) daily by oral gavage for five weeks. A) seminal vesicle weight as overall mean ± SEM (n = 4) is displayed on the left with individual values on the right. B) plasma testosterone concentration in host mice as overall mean ± SEM (n = 4) is displayed on the left with individual values on the right. Data analysed by Mann-Whitney test. No significant differences were observed for serum testosterone or seminal vesicle weight between BPA-treated mice compared to control.
Discussion
The present study is the first experimental demonstration that exposure to environmentally relevant BPA concentrations induces quantitative and qualitative effects in the development of germ cells in the human fetal testis.
First, using the FeTA system, we demonstrated here that BPA has the potentiality to increase germ cell apoptosis in first trimester human fetal testis ex vivo compared with control conditions. However, this was only apparent at higher concentrations (10μM), whilst exposure to lower BPA concentrations (1, 0.1 and 0.01μM) had no effect. Using the FeTA system, we were able to demonstrate a similar effect of BPA exposure on gonocyte apoptosis in the mouse testis (S1 Fig). In this case, a concentration of 0.1 μM BPA was sufficient to significantly increase apoptosis. This demonstrates, for the first time, BPA-induced apoptosis in the fetal germ cells of rodents and human similar to that which has previously in adult rodents. Oral administration of BPA in adult rodent (from 2 μg to 960 mg/ kg/day) induces germ cell apoptosis [57–59] and increases oxidative stress in testis of adult rats at concentrations of 20 and 50 mg/kg/day [16,60]. In the same way a single injection of 50mg/kg BPA induced germ cell apoptosis in the pubertal rat testis via an activation of p38 MAPK, a well-known marker of cellular stress [61]. Furthermore, DNA damage has been demonstrated in cultured CHO-K1 and Chicken DT40 cells exposed to BPA at concentrations ranging from 30 to 600 μM [62,63] and in meiotic germ cells of C. elegans exposed to 1mM BPA and of mice exposed orally with 10mg/kg/day [64,65]. These cytotoxic effects of BPA may be responsible for the increase in apoptosis and further investigation is required to determine whether such effects on oxidative stress and DNA damages also occurs in male human fetal germ cells. Whilst these results show consistent effects, the concentration of BPA to which the cells/tissues/animals were exposed is often higher than those reported in the serum of humans. To focus on environmental BPA concentrations, we utilized a xenograft model which allows longer term exposure using low doses.
One important question when assessing one ED risk for human health is to appreciate whether the chosen protocol reproduces BPA concentrations relevant to environmental exposure in human. No data are available regarding the BPA level in the plasma of human fetuses during intrauterine life. In the adult, although there remains some uncertainty and dispute, about the level of effective exposure in humans [66,67], the overall consensus is that internal exposure to unconjugated BPA, which is the only biologically active form of BPA [68], is in the range of 0.002–0.043μM (i.e. 0.5–10μg/L) in blood and serum samples from healthy men and non-pregnant women [69]. Internal BPA concentrations in pregnant women may be slightly higher since different studies reported a mean value of 0.017 μM (3.88μg/L) [69], which this was confirmed by a recent study [70]. Concentrations of unconjugated BPA in umbilical cord blood of newborn are reported to range from 0.0006 to 0.021μM (i.e. 0.14 to 4.76μg/L) with a mean value equal to 0.005 μM (1.12 μg/L) [71]. Lastly, it has been proposed that BPA might accumulate particularly in younger fetuses because of lower metabolic clearance or conjugation at this developmental stage [72]. We took this into consideration when selecting our dosing schedule. In the first series of experiments, it was an administration of BPA via the drinking water and thus, the level of exposure had to be quantified in this model. We observed that the plasma level of BPA, and hence the concentration to which the human testicular xenografts are exposed, was 0.1 and 0.038μM (i.e. 22,8 and 8.7μg/L) for total and unconjugated BPA respectively. In the second series of experiments using host mice carrying second trimester human fetal testis xenografts, we administrated doses of 0.5 and 50μg/kg/day of BPA to the treated host. By comparison, it is interesting to recall that 4 μg/kg/day, is the tolerable dose intake (TDI) estimated by the EFSA. This TDI was derived from a Point of Departure which is the equivalent to a Non Observable Adverse Level Effect (NOAEL) equal to 8,960μg/kg/day i.e. higher than all the doses we used in the current series of experiments (0.5, 50, 500μg/kg/d). Taken together, these data suggest that the three doses of BPA that we chose are relevant to environmental exposure in human.
With the xenografting model, germ cell density in the grafts from the first trimester was statistically reduced after a 5-weeks exposure to BPA. This suggests that low doses of BPA provoke a weak increase in germ cell apoptosis that is cumulative over the time, ultimately resulting in a decrease in the germ cell number. Whether this degree of germ cell loss is subsequently compensated and the potential clinical significance is uncertain and would require further investigation.
Importantly, we observed that BPA changed not only the germ cell number but also germ cell differentiation status in first trimester testis xenografts. Exposure to BPA significantly decreased the proportion of germ cells expressing AP-2γ a gonocyte marker and pluripotency factor, whilst the proportion of germ cells expressing the pre-spermatogonial marker MAGE-A4 was increased. It is known that the proportion of germ cells expressing AP-2γ decreases during intrauterine life and finally disappears around 36 GW whilst the percentage of germ cells expressing the pre-spermatogonial marker MAGE-A4 increases from the end of the first trimester onwards [37–40,42]. Thus, our results suggest that BPA exposure could accelerate progression of human germ cell differentiation from gonocyte to spermatogonia, although the clinical importance of such a finding is unknown.
This study is the first to investigate the effect of BPA exposure on male germ cells differentiation in human fetal testis; however, there are limited data on BPA effects on male fetal germ cells differentiation in rodents. Using mouse ES cells, Aoki and Takada have shown that BPA at 50 μM induces an up-regulation of Stra8, a meiotic entry gene, an increase in ovarian markers expression such as Wnt4 and Foxl2, and a decrease in testicular markers expression such as Sox9 and Fgf9 [73]. Another study has described that treatment of pregnant mice with BPA at 40, 80 and 160μg BPA/kg body weight/day may induce epigenetic and gene expression modifications in fetal germ cells in both sexes [74]. This may be related to the fact that BPA alters the meiotic process in fetal oocytes in humans and non-human primates resulting in the production of chromosomally abnormal eggs [75,76].
We also investigated the effect of BPA exposure on androgenic production in the first and the second trimester human fetal testis. Androgen action during fetal life is important for the process of masculinization of the male fetus. It has been shown in rats that a decrease in testosterone synthesis or action during a critical period (15.5 and 18.5 day post conception), named “the masculinizing programming window” (MPW), causes masculinization defects that occur from 18.5 dpc onwards, whilst a decrease in androgen production after this period does not affect the ontogenesis of male reproductive organs but reduces their final size [77,78]. In humans, the putative MPW extends from 8 to 14 GW [77,79] and masculinization begins on GW 10 with subsequent growth throughout intrauterine life.
Importantly, we have demonstrated that five weeks exposure to low BPA concentrations does not alter the hCG-stimulated testosterone secretion by xenografted human fetal testis. This was observed for both first trimester and second trimester xenografts although there were differences in the exposure route, the BPA doses and the grafting procedure between the two series of experiments. Taken together, these results suggest that the effect of BPA on germ cells described in the present study is unlikely to result from altered testosterone production despite the importance of testosterone for gonocyte development [80,81]. The absence of a BPA effect on hCG-stimulated testosterone production in the xenografting model is also in agreement with previous results obtained using the FeTA system for which, we and others have shown that concentrations of BPA lower than 10μM do not alter LH- or hCG-stimulated testosterone production by human fetal testes [15,32]. However, using the same FeTA approach we have also shown that 0.01μM and higher BPA concentrations reduce basal testosterone production of the human fetal testes [31,32]. Given that hCG stimulation more accurately reflects the physiological situation, we would consider the results obtained in the presence of LH or hCG to be more representative of the situation in human pregnancy than those obtained under basal conditions. However, hCG plasma concentrations change during development and can vary considerably from one fetus to another [79] thus making precise replication of these in vivo conditions challenging. Another challenge is that the endocrine milieu contains a mixture of LH and hCG with a ratio changing from the end of the first trimester onwards.
Limited epidemiological studies have resulted in conflicting results in relation to associations between BPA exposure in utero and male reproductive abnormalities. A Chinese study involving the sons of workers who were occupationally exposed to BPA during pregnancy were shown to have a shorter anogenital distance (an index of fetal testicular testosterone production) compared to a control group [82]. However, no increase in BPA concentration in umbilical cord blood was observed in newborns with cryptorchidism [71], and no association was found between concentrations of BPA and testosterone in umbilical cord blood in a population-based cohort [83].
Conclusion
This is the first study performed by the same group comparing organ culture and xenograft approaches for determining the effects of environmental chemical exposures in the human fetal testis. Importantly, both ex vivo (FeTA) and in vivo (xenografts) resulted in similar effects in terms of germ cell loss as a result of BPA exposure, whilst hCG-stimulated testosterone production was unaffected in both systems. They appear as two complementary approaches since FeTA can be used for high-throughput screening, dose-response and mechanism of action studies, whereas xenografting can be used for long-term in vivo exposure studies.
Although the fetal testis is well described as a potential target of EDs [15], we developed here, using the xenograft model, the first experimental in vivo study of the effects of BPA on the development and function of the human fetal testis using BPA exposures that have been reported to reflect environmental exposure. With these conditions, BPA did not affect hCG-stimulated androgenic function of the xenografted fetal testis but we did identify effects on fetal germ cell number and differentiation that could potentially impact on fertility at adulthood.
Supporting information
Testes were removed from NMRI mice at 12.5 day-post conception and cultured as previously described using the FeTA system [29,31,48,49]. After 24 hours in control medium, explants were cultured under basal conditions for the subsequent 24 hours in the presence of ethanol (vehicle control) or 0.1 μM BPA. The effect of BPA on germ cell apoptosis was estimated by the comparison between the percentage of cleaved caspase-3 positive gonocytes in the testis cultured in the presence of BPA and that measured in the other testis from the same fetus cultured without BPA (control) as previously described [29,48]. Quantification of cleaved caspase-3 positive cells are presented as mean ± SEM (n = 9) in the left panel and as individual values with a line drawn between the control and the BPA-exposed testis from the same fetus in the right panel. Data was analysed using the Wilcoxon paired test. The increase in apoptosis in response to 0.1μM BPA was statistically significant (p = 0.027).
(TIF)
Acknowledgments
We are grateful to Aurélie Gouret and Arielle Leliard for skillful secretarial assistance, Véronique Neuville for animal care and the staff of the Department of Obstetrics and Gynecology of the Antoine Béclère Hospital (Clamart, France) for collecting human fetuses.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
All the authors have nothing to declare. The authors received no specific funding for this work.
References
- 1.Auger J, Kunstmann JM, Czyglik F, Jouannet P (1995) Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med 332: 281–285. doi: 10.1056/NEJM199502023320501 [DOI] [PubMed] [Google Scholar]
- 2.Leridon H, Slama R (2008) The impact of a decline in fecundity and of pregnancy postponement on final number of children and demand for assisted reproduction technology. Hum Reprod 23: 1312–1319. doi: 10.1093/humrep/den106 [DOI] [PubMed] [Google Scholar]
- 3.Main KM, Skakkebaek NE, Virtanen HE, Toppari J (2010) Genital anomalies in boys and the environment. Best Pract Res Clin Endocrinol Metab 24: 279–289. doi: 10.1016/j.beem.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 4.Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, Toppari J, Andersson AM, et al. (2016) Male Reproductive Disorders and Fertility Trends: Influences of Environment and Genetic Susceptibility. Physiol Rev 96: 55–97. doi: 10.1152/physrev.00017.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olesen IA, Sonne SB, Hoei-Hansen CE, Rajpert-De Meyts E, Skakkebaek NE (2007) Environment, testicular dysgenesis and carcinoma in situ testis. Best Pract Res Clin Endocrinol Metab 21: 462–478. doi: 10.1016/j.beem.2007.04.002 [DOI] [PubMed] [Google Scholar]
- 6.Sharpe RM, Skakkebaek NE (2008) Testicular dysgenesis syndrome: mechanistic insights and potential new downstream effects. Fertil Steril 89: e33–38. doi: 10.1016/j.fertnstert.2007.12.026 [DOI] [PubMed] [Google Scholar]
- 7.Skakkebaek NE, Rajpert-De Meyts E, Main KM (2001) Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 16: 972–978. [DOI] [PubMed] [Google Scholar]
- 8.van den Driesche S, Kilcoyne KR, Wagner I, Rebourcet D, Boyle A, et al. (2017) Experimentally induced testicular dysgenesis syndrome originates in the masculinization programming window. JCI Insight 2: e91204 doi: 10.1172/jci.insight.91204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharpe RM, Skakkebaek NE (1993) Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet 341: 1392–1395. [DOI] [PubMed] [Google Scholar]
- 10.Rouiller-Fabre V, Guerquin MJ, N'Tumba-Byn T, Muczynski V, Moison D, et al. (2015) Nuclear receptors and endocrine disruptors in fetal and neonatal testes: a gapped landscape. Front Endocrinol (Lausanne) 6: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skakkebaek NE (2016) A Brief Review of the Link between Environment and Male Reproductive Health: Lessons from Studies of Testicular Germ Cell Cancer. Horm Res Paediatr 86: 240–246. doi: 10.1159/000443400 [DOI] [PubMed] [Google Scholar]
- 12.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr., et al. (2012) Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 33: 378–455. doi: 10.1210/er.2011-1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rouiller-Fabre V, Habert R, Livera G (2014) Effects of endocrine disruptors on the human fetal testis. Ann Endocrinol (Paris) 75: 54–57. [DOI] [PubMed] [Google Scholar]
- 14.Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, et al. (2014) Bisphenol a and reproductive health: update of experimental and human evidence, 2007–2013. Environ Health Perspect 122: 775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eladak S, Grisin T, Moison D, Guerquin MJ, N'Tumba-Byn T, et al. (2015) A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil Steril 103: 11–21. doi: 10.1016/j.fertnstert.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 16.Kabuto H, Hasuike S, Minagawa N, Shishibori T (2003) Effects of bisphenol A on the metabolisms of active oxygen species in mouse tissues. Environ Res 93: 31–35. [DOI] [PubMed] [Google Scholar]
- 17.Salian S, Doshi T, Vanage G (2009) Perinatal exposure of rats to Bisphenol A affects the fertility of male offspring. Life Sci 85: 742–752. doi: 10.1016/j.lfs.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 18.Tinwell H, Haseman J, Lefevre PA, Wallis N, Ashby J (2002) Normal sexual development of two strains of rat exposed in utero to low doses of bisphenol A. Toxicol Sci 68: 339–348. [DOI] [PubMed] [Google Scholar]
- 19.Vilela J, Hartmann A, Silva EF, Cardoso T, Corcini CD, et al. (2014) Sperm impairments in adult vesper mice (Calomys laucha) caused by in utero exposure to bisphenol A. Andrologia 46: 971–978. doi: 10.1111/and.12182 [DOI] [PubMed] [Google Scholar]
- 20.Watanabe S, Wang RS, Miyagawa M, Kobayashi K, Suda M, et al. (2003) Imbalance of testosterone level in male offspring of rats perinatally exposed to bisphenol A. Ind Health 41: 338–341. [DOI] [PubMed] [Google Scholar]
- 21.Xi W, Wan HT, Zhao YG, Wong MH, Giesy JP, et al. (2011) Effects of perinatal exposure to bisphenol A and di(2-ethylhexyl)-phthalate on gonadal development of male mice. Environ Sci Pollut Res Int 19: 2515–2527. doi: 10.1007/s11356-012-0827-y [DOI] [PubMed] [Google Scholar]
- 22.Tanaka M, Nakaya S, Katayama M, Leffers H, Nozawa S, et al. (2006) Effect of prenatal exposure to bisphenol A on the serum testosterone concentration of rats at birth. Hum Exp Toxicol 25: 369–373. doi: 10.1191/0960327106ht638oa [DOI] [PubMed] [Google Scholar]
- 23.Thuillier R, Manku G, Wang Y, Culty M (2009) Changes in MAPK pathway in neonatal and adult testis following fetal estrogen exposure and effects on rat testicular cells. Microsc Res Tech 72: 773–786. doi: 10.1002/jemt.20756 [DOI] [PubMed] [Google Scholar]
- 24.Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM (2007) Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure. Reprod Toxicol 23: 383–390. doi: 10.1016/j.reprotox.2006.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howdeshell KL, Furr J, Lambright CR, Wilson VS, Ryan BC, et al. (2008) Gestational and lactational exposure to ethinyl estradiol, but not bisphenol A, decreases androgen-dependent reproductive organ weights and epididymal sperm abundance in the male long evans hooded rat. Toxicol Sci 102: 371–382. doi: 10.1093/toxsci/kfm306 [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi K, Miyagawa M, Wang RS, Sekiguchi S, Suda M, et al. (2002) Effects of in utero and lactational exposure to bisphenol A on somatic growth and anogenital distance in F1 rat offspring. Ind Health 40: 375–381. [DOI] [PubMed] [Google Scholar]
- 27.Tyl RW, Myers CB, Marr MC, Thomas BF, Keimowitz AR, et al. (2002) Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol Sci 68: 121–146. [DOI] [PubMed] [Google Scholar]
- 28.Habert R, Devif I, Gangnerau MN, Lecerf L (1991) Ontogenesis of the in vitro response of rat testis to gonadotropin-releasing hormone. Mol Cell Endocrinol 82: 199–206. [DOI] [PubMed] [Google Scholar]
- 29.Livera G, Delbes G, Pairault C, Rouiller-Fabre V, Habert R (2006) Organotypic culture, a powerful model for studying rat and mouse fetal testis development. Cell Tissue Res 324: 507–521. doi: 10.1007/s00441-006-0167-7 [DOI] [PubMed] [Google Scholar]
- 30.Lambrot R, Coffigny H, Pairault C, Donnadieu AC, Frydman R, et al. (2006) Use of organ culture to study the human fetal testis development: effect of retinoic acid. J Clin Endocrinol Metab 91: 2696–2703. doi: 10.1210/jc.2005-2113 [DOI] [PubMed] [Google Scholar]
- 31.N'Tumba-Byn T, Moison D, Lacroix M, Lecureuil C, Lesage L, et al. (2012) Differential effects of bisphenol A and diethylstilbestrol on human, rat and mouse fetal leydig cell function. PLoS One 7: e51579 doi: 10.1371/journal.pone.0051579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben Maamar M, Lesne L, Desdoits-Lethimonier C, Coiffec I, Lassurguere J, et al. (2015) An investigation of the endocrine-disruptive effects of bisphenol a in human and rat fetal testes. PLoS One 10: e0117226 doi: 10.1371/journal.pone.0117226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gondos B, Bhiraleus P, Hobel CJ (1971) Ultrastructural observations on germ cells in human fetal ovaries. Am J Obstet Gynecol 110: 644–652. [DOI] [PubMed] [Google Scholar]
- 34.Fujimoto T, Miyayama Y, Fuyuta M (1977) The origin, migration and fine morphology of human primordial germ cells. Anat Rec 188: 315–330. doi: 10.1002/ar.1091880305 [DOI] [PubMed] [Google Scholar]
- 35.Olaso R, Habert R (2000) Genetic and cellular analysis of male germ cell development. J Androl 21: 497–511. [PubMed] [Google Scholar]
- 36.Rouiller-Fabre V, Muczynski V, Lambrot R, Lecureuil C, Coffigny H, et al. (2009) Ontogenesis of testicular function in humans. Folia Histochem Cytobiol 47: S19–24. doi: 10.2478/v10042-009-0065-4 [DOI] [PubMed] [Google Scholar]
- 37.Gaskell TL, Esnal A, Robinson LL, Anderson RA, Saunders PT (2004) Immunohistochemical profiling of germ cells within the human fetal testis: identification of three subpopulations. Biol Reprod 71: 2012–2021. doi: 10.1095/biolreprod.104.028381 [DOI] [PubMed] [Google Scholar]
- 38.Pauls K, Jager R, Weber S, Wardelmann E, Koch A, et al. (2005) Transcription factor AP-2gamma, a novel marker of gonocytes and seminomatous germ cell tumors. Int J Cancer 115: 470–477. doi: 10.1002/ijc.20913 [DOI] [PubMed] [Google Scholar]
- 39.Hoei-Hansen CE, Nielsen JE, Almstrup K, Sonne SB, Graem N, et al. (2004) Transcription factor AP-2gamma is a developmentally regulated marker of testicular carcinoma in situ and germ cell tumors. Clin Cancer Res 10: 8521–8530. doi: 10.1158/1078-0432.CCR-04-1285 [DOI] [PubMed] [Google Scholar]
- 40.Pauls K, Schorle H, Jeske W, Brehm R, Steger K, et al. (2006) Spatial expression of germ cell markers during maturation of human fetal male gonads: an immunohistochemical study. Hum Reprod 21: 397–404. doi: 10.1093/humrep/dei325 [DOI] [PubMed] [Google Scholar]
- 41.Mitchell RT, Saunders PT, Childs AJ, Cassidy-Kojima C, Anderson RA, et al. (2010) Xenografting of human fetal testis tissue: a new approach to study fetal testis development and germ cell differentiation. Hum Reprod 25: 2405–2414. doi: 10.1093/humrep/deq183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell RT, Cowan G, Morris KD, Anderson RA, Fraser HM, et al. (2008) Germ cell differentiation in the marmoset (Callithrix jacchus) during fetal and neonatal life closely parallels that in the human. Hum Reprod 23: 2755–2765. doi: 10.1093/humrep/den295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukuda T, Hedinger C, Groscurth P (1975) Ultrastructure of developing germ cells in the fetal human testis. Cell Tissue Res 161: 55–70. [DOI] [PubMed] [Google Scholar]
- 44.Wartenberg H (1976) Comparative cytomorphologic aspects of the male germ cells, especially of the "Gonia". Andrologia 8: 117–130. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell RT, Childs AJ, Anderson RA, van den Driesche S, Saunders PT, et al. (2012) Do phthalates affect steroidogenesis by the human fetal testis? Exposure of human fetal testis xenografts to di-n-butyl phthalate. J Clin Endocrinol Metab 97: E341–348. doi: 10.1210/jc.2011-2411 [DOI] [PubMed] [Google Scholar]
- 46.van den Driesche S, McKinnell C, Calarrao A, Kennedy L, Hutchison GR, et al. (2015) Comparative effects of di(n-butyl) phthalate exposure on fetal germ cell development in the rat and in human fetal testis xenografts. Environ Health Perspect 123: 223–230. doi: 10.1289/ehp.1408248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evtouchenko L, Studer L, Spenger C, Dreher E, Seiler RW (1996) A mathematical model for the estimation of human embryonic and fetal age. Cell Transplant 5: 453–464. [DOI] [PubMed] [Google Scholar]
- 48.Lambrot R, Livera G, Coffigny H, Pairault C, Frydman R, et al. (2006) A new method for toxicity assays on human and mouse fetal testis. Biochimie 88: 1831–1835. doi: 10.1016/j.biochi.2006.09.022 [DOI] [PubMed] [Google Scholar]
- 49.Habert R, Muczynski V, Grisin T, Moison D, Messiaen S, et al. (2014) Concerns about the widespread use of rodent models for human risk assessments of endocrine disruptors. Reproduction 147: R119–129. doi: 10.1530/REP-13-0497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poulain M, Frydman N, Tourpin S, Muczynski V, Souquet B, et al. (2014) Involvement of doublesex and mab-3-related transcription factors in human female germ cell development demonstrated by xenograft and interference RNA strategies. Mol Hum Reprod 20: 960–971. doi: 10.1093/molehr/gau058 [DOI] [PubMed] [Google Scholar]
- 51.Deceuninck Y, Bichon E, Durand S, Bemrah N, Zendong Z, et al. (2014) Development and validation of a specific and sensitive gas chromatography tandem mass spectrometry method for the determination of bisphenol A residues in a large set of food items. J Chromatogr A 1362: 241–249. doi: 10.1016/j.chroma.2014.07.105 [DOI] [PubMed] [Google Scholar]
- 52.Deceuninck Y, Bichon E, Marchand P, Boquien CY, Legrand A, et al. (2015) Determination of bisphenol A and related substitutes/analogues in human breast milk using gas chromatography-tandem mass spectrometry. Anal Bioanal Chem 407: 2485–2497. doi: 10.1007/s00216-015-8469-9 [DOI] [PubMed] [Google Scholar]
- 53.Churchwell MI, Camacho L, Vanlandingham MM, Twaddle NC, Sepehr E, et al. (2014) Comparison of life-stage-dependent internal dosimetry for bisphenol A, ethinyl estradiol, a reference estrogen, and endogenous estradiol to test an estrogenic mode of action in Sprague Dawley rats. Toxicol Sci 139: 4–20. doi: 10.1093/toxsci/kfu021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lecerf L, Rouiller-Fabre V, Levacher C, Gautier C, Saez JM, et al. (1993) Stimulatory effect of follicle-stimulating hormone on basal and luteinizing hormone-stimulated testosterone secretions by the fetal rat testis in vitro. Endocrinology 133: 2313–2318. doi: 10.1210/endo.133.5.8404683 [DOI] [PubMed] [Google Scholar]
- 55.Gautier C, Levacher C, Avallet O, Vigier M, Rouiller-Fabre V, et al. (1994) Immunohistochemical localization of transforming growth factor-beta 1 in the fetal and neonatal rat testis. Mol Cell Endocrinol 99: 55–61. [DOI] [PubMed] [Google Scholar]
- 56.Habert R, Picon R (1984) Testosterone, dihydrotestosterone and estradiol-17 beta levels in maternal and fetal plasma and in fetal testes in the rat. J Steroid Biochem 21: 193–198. [DOI] [PubMed] [Google Scholar]
- 57.Jin P, Wang X, Chang F, Bai Y, Li Y, et al. (2013) Low dose bisphenol A impairs spermatogenesis by suppressing reproductive hormone production and promoting germ cell apoptosis in adult rats. J Biomed Res 27: 135–144. doi: 10.7555/JBR.27.20120076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li YJ, Song TB, Cai YY, Zhou JS, Song X, et al. (2009) Bisphenol A exposure induces apoptosis and upregulation of Fas/FasL and caspase-3 expression in the testes of mice. Toxicol Sci 108: 427–436. doi: 10.1093/toxsci/kfp024 [DOI] [PubMed] [Google Scholar]
- 59.Wang Q, Zhao XF, Ji YL, Wang H, Liu P, et al. (2010) Mitochondrial signaling pathway is also involved in bisphenol A induced germ cell apoptosis in testes. Toxicol Lett 199: 129–135. doi: 10.1016/j.toxlet.2010.08.014 [DOI] [PubMed] [Google Scholar]
- 60.Othman AI, Edrees GM, El-Missiry MA, Ali DA, Aboel-Nour M, et al. (2016) Melatonin controlled apoptosis and protected the testes and sperm quality against bisphenol A-induced oxidative toxicity. Toxicol Ind Health 32: 1537–1549. doi: 10.1177/0748233714561286 [DOI] [PubMed] [Google Scholar]
- 61.Urriola-Munoz P, Lagos-Cabre R, Moreno RD (2014) A mechanism of male germ cell apoptosis induced by bisphenol-A and nonylphenol involving ADAM17 and p38 MAPK activation. PLoS One 9: e113793 doi: 10.1371/journal.pone.0113793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tayama S, Nakagawa Y, Tayama K (2008) Genotoxic effects of environmental estrogen-like compounds in CHO-K1 cells. Mutat Res 649: 114–125. doi: 10.1016/j.mrgentox.2007.08.006 [DOI] [PubMed] [Google Scholar]
- 63.Lee S, Liu X, Takeda S, Choi K (2013) Genotoxic potentials and related mechanisms of bisphenol A and other bisphenol compounds: a comparison study employing chicken DT40 cells. Chemosphere 93: 434–440. doi: 10.1016/j.chemosphere.2013.05.029 [DOI] [PubMed] [Google Scholar]
- 64.Allard P, Colaiacovo MP (2010) Bisphenol A impairs the double-strand break repair machinery in the germline and causes chromosome abnormalities. Proc Natl Acad Sci U S A 107: 20405–20410. doi: 10.1073/pnas.1010386107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naik P, Vijayalaxmi KK (2009) Cytogenetic evaluation for genotoxicity of bisphenol-A in bone marrow cells of Swiss albino mice. Mutat Res 676: 106–112. doi: 10.1016/j.mrgentox.2009.04.010 [DOI] [PubMed] [Google Scholar]
- 66.Vandenberg LN, Hunt PA, Myers JP, Vom Saal FS (2013) Human exposures to bisphenol A: mismatches between data and assumptions. Rev Environ Health 28: 37–58. doi: 10.1515/reveh-2012-0034 [DOI] [PubMed] [Google Scholar]
- 67.Teeguarden JG, Twaddle NC, Churchwell MI, Doerge DR (2016) Urine and serum biomonitoring of exposure to environmental estrogens I: Bisphenol A in pregnant women. Food Chem Toxicol 92: 129–142. doi: 10.1016/j.fct.2016.03.023 [DOI] [PubMed] [Google Scholar]
- 68.Lacroix MZ, Puel S, Collet SH, Corbel T, Picard-Hagen N, et al. (2011) Simultaneous quantification of bisphenol A and its glucuronide metabolite (BPA-G) in plasma and urine: applicability to toxicokinetic investigations. Talanta 85: 2053–2059. doi: 10.1016/j.talanta.2011.07.040 [DOI] [PubMed] [Google Scholar]
- 69.Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, et al. (2010) Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 118: 1055–1070. doi: 10.1289/ehp.0901716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gerona RR, Pan J, Zota AR, Schwartz JM, Friesen M, et al. (2016) Direct measurement of Bisphenol A (BPA), BPA glucuronide and BPA sulfate in a diverse and low-income population of pregnant women reveals high exposure, with potential implications for previous exposure estimates: a cross-sectional study. Environ Health 15: 50 doi: 10.1186/s12940-016-0131-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fenichel P, Dechaux H, Harthe C, Gal J, Ferrari P, et al. (2012) Unconjugated bisphenol A cord blood levels in boys with descended or undescended testes. Hum Reprod 27: 983–990. doi: 10.1093/humrep/der451 [DOI] [PubMed] [Google Scholar]
- 72.Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y (2002) Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod 17: 2839–2841. [DOI] [PubMed] [Google Scholar]
- 73.Aoki T, Takada T (2012) Bisphenol A modulates germ cell differentiation and retinoic acid signaling in mouse ES cells. Reprod Toxicol 34: 463–470. doi: 10.1016/j.reprotox.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 74.Zhang XF, Zhang LJ, Feng YN, Chen B, Feng YM, et al. (2012) Bisphenol A exposure modifies DNA methylation of imprint genes in mouse fetal germ cells. Mol Biol Rep 39: 8621–8628. doi: 10.1007/s11033-012-1716-7 [DOI] [PubMed] [Google Scholar]
- 75.Brieno-Enriquez MA, Reig-Viader R, Cabero L, Toran N, Martinez F, et al. (2012) Gene expression is altered after bisphenol A exposure in human fetal oocytes in vitro. Mol Hum Reprod 18: 171–183. doi: 10.1093/molehr/gar074 [DOI] [PubMed] [Google Scholar]
- 76.Hunt PA, Lawson C, Gieske M, Murdoch B, Smith H, et al. (2012) Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc Natl Acad Sci U S A 109: 17525–17530. doi: 10.1073/pnas.1207854109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, et al. (2008) Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest 118: 1479–1490. doi: 10.1172/JCI34241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Macleod DJ, Sharpe RM, Welsh M, Fisken M, Scott HM, et al. (2010) Androgen action in the masculinization programming window and development of male reproductive organs. Int J Androl 33: 279–287. doi: 10.1111/j.1365-2605.2009.01005.x [DOI] [PubMed] [Google Scholar]
- 79.Fowler PA, Childs AJ, Courant F, MacKenzie A, Rhind SM, et al. (2014) In utero exposure to cigarette smoke dysregulates human fetal ovarian developmental signalling. Hum Reprod 29: 1471–1489. doi: 10.1093/humrep/deu117 [DOI] [PubMed] [Google Scholar]
- 80.Merlet J, Moreau E, Habert R, Racine C (2007) Development of fetal testicular cells in androgen receptor deficient mice. Cell Cycle 6: 2258–2262. doi: 10.4161/cc.6.18.4654 [DOI] [PubMed] [Google Scholar]
- 81.Merlet J, Racine C, Moreau E, Moreno SG, Habert R (2007) Male fetal germ cells are targets for androgens that physiologically inhibit their proliferation. Proc Natl Acad Sci U S A 104: 3615–3620. doi: 10.1073/pnas.0611421104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miao M, Yuan W, He Y, Zhou Z, Wang J, et al. (2011) In utero exposure to bisphenol-A and anogenital distance of male offspring. Birth Defects Res A Clin Mol Teratol 91: 867–872. doi: 10.1002/bdra.22845 [DOI] [PubMed] [Google Scholar]
- 83.Chevalier N, Brucker-Davis F, Lahlou N, Coquillard P, Pugeat M, et al. (2015) A negative correlation between insulin-like peptide 3 and bisphenol A in human cord blood suggests an effect of endocrine disruptors on testicular descent during fetal development. Hum Reprod 30: 447–453. doi: 10.1093/humrep/deu340 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Testes were removed from NMRI mice at 12.5 day-post conception and cultured as previously described using the FeTA system [29,31,48,49]. After 24 hours in control medium, explants were cultured under basal conditions for the subsequent 24 hours in the presence of ethanol (vehicle control) or 0.1 μM BPA. The effect of BPA on germ cell apoptosis was estimated by the comparison between the percentage of cleaved caspase-3 positive gonocytes in the testis cultured in the presence of BPA and that measured in the other testis from the same fetus cultured without BPA (control) as previously described [29,48]. Quantification of cleaved caspase-3 positive cells are presented as mean ± SEM (n = 9) in the left panel and as individual values with a line drawn between the control and the BPA-exposed testis from the same fetus in the right panel. Data was analysed using the Wilcoxon paired test. The increase in apoptosis in response to 0.1μM BPA was statistically significant (p = 0.027).
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.