Abstract
Background
Vesicular stomatitis (VS) is an important viral disease of livestock. The main feature of VS is irregular blisters that occur on the lips, tongue, oral mucosa, hoof crown and nipple. Humans can also be infected with vesicular stomatitis and develop meningitis. This study analyses 2014 American VS outbreaks in order to accurately predict vesicular stomatitis outbreak trends.
Methods
American VS outbreaks data were collected from OIE. The data for VS keywords were obtained by inputting 24 disease-related keywords into Google Trends. After calculating the Pearson and Spearman correlation coefficients, it was found that there was a relationship between outbreaks and keywords derived from Google Trends. Finally, the predicted model was constructed based on qualitative classification and quantitative regression.
Results
For the regression model, the Pearson correlation coefficients between the predicted outbreaks and actual outbreaks are 0.953 and 0.948, respectively. For the qualitative classification model, we constructed five classification predictive models and chose the best classification predictive model as the result. The results showed, SN (sensitivity), SP (specificity) and ACC (prediction accuracy) values of the best classification predictive model are 78.52%,72.5% and 77.14%, respectively.
Conclusion
This study applied Google search data to construct a qualitative classification model and a quantitative regression model. The results show that the method is effective and that these two models obtain more accurate forecast.
Introduction
Vesicular stomatitis is a highly contagious zoonotic infectious disease caused by the vesicular stomatitis virus (VSV). VSV is an RNA virus in the family Rhabdoviridae and genus Vesiculovirus, which includes the vesicular stomatitis virus New Jersey (VSV-NJ), Indiana (VSV-IN), and Alagoas serotypes (VSV-AV) and the Cocal virus [1]. New Jersey and Indiana are the two major serotypes of vesicular stomatitis. VSV-NJ belongs to the New Jersey serotype [2]. Vesicular stomatitis mainly affects cattle, sheep, camels and other ruminants. Approximately 10–15% of adult animals show clinical signs. Vesicular stomatitis is characterized by vesicles, papules, erosion and ulcer. These lesions mainly occur in the mouth or on the feet, teats and prepuce[3]. The disease varies by species. For example, blisters in horses usually appear on the upper surface of the tongue, lips and nostrils around the mouth and gums. Lesions in cattle occur mainly on the tongue, lips, gums, hard palate, and sometimes on the muzzle, and ulcers in pigs occur in the snout. Although these diseases do not cause death, they result in pain and anorexia in the animals. These diseases can give rise to secondary bacterial mastitis, which causes a loss of meat and milk production and seriously affects the development of animal husbandry [4]. Some vesicular stomatitis viruses also infect humans, although these infections are rare. Vesicular stomatitis has been classified as a grade A infectious disease by the World Organization for Animal Health (OIE) and as a class II infectious disease by the Animal Epidemic Prevention Law of the People's Republic of China [5].
Vesicular stomatitis mainly occurs in the Western hemisphere and is most common in the United States, Panama, Mexico, Peru and Venezuela. The United States has a higher prevalence rate of vesicular stomatitis than other countries. As early as 1926, the United States reported vesicular stomatitis in horses, followed by pigs, cattle and sheep [6]. Data from the United States Department of Agriculture (USDA) show that vesicular stomatitis outbreaks have continued in America since 2004, with vesicular stomatitis outbreaks in 2005 and 2014. In 2014, a vesicular stomatitis outbreak occurred in four American states (Arizona, Colorado, Nebraska, and Texas) [7].
Due to the danger of vesicular stomatitis, the need for an early warning system has received much attention. An early warning system has always been advocated as an important research field by some countries. Furthermore, an effective early warning system can significantly reduce the losses of the livestock industry [8]. However, traditional monitoring systems contain some defects, including inappropriate monitoring methods, data resource intensive, slow speed and difficulties with the collection of virus specimens [9]. Hence, the supplement and reform of traditional warning systems have become particularly significant. The appearance of new digital surveillance sources has brought new developments to animal epidemic monitoring, such as Google Trends.
Google Trends is a web-based tool for real-time surveillance of disease outbreaks that offers real-time information about the disease [10]. Currently, millions of people worldwide search online for health-related information every day, which makes web search queries a valuable source of information for the collection of health trends. In comparison with the network monitoring system, Google Trends shows great promise. The surveillance system offers timely, robust, and sensitive information and is widely used in disease surveillance research [11]. In recent years,“Google Trends” is realizing wider applications in epidemiological research. For example, Google Trends’ timely and accurate surveillance is commonly used in seasonal and pandemic influenza, which requires early detection of the outbreak [12–14]. The surveillance of other diseases, including Dengue, gastroenteritis, chickenpox and Tuberculosis, is also analysed by the near real-time search query data of Google Trends, which compensates for the deficiencies of traditional, healthcare-based, government-implemented surveillance [15–17].
The most common predictive early warning models include quantitative and qualitative models [18]. Multiple regressions are a quantitative model used to analyse the association between one independent variable and two or more dependent variables. They have been widely applied in epidemiology[19, 20]. For example, the seasonal autoregressive model was constructed using the SPSS software to analyse Hand-Foot-Mouth disease outbreaks [21]. Linear regressions and step regressions are quite commonly seen in the research of respiratory and cardiovascular diseases. They are also used to establish forecasting models for childhood colds and senile cerebrovascular diseases [22].
The classification forecasting model as a qualitative model has been extensively used in many fields, including medicine, epidemiology and molecular biology [23]. For example, Naïve Bayes with its unique strength was widely used in the forecasting system for heart disease. The detection system of cardiac arrhythmias within ECG signals was constructed using a Bayesian artificial neural network (ANN) classifier [24, 25].
In this study, the VS forecasting model was constructed using qualitative and quantitative approaches with data from OIE and Google Trends to predict the epidemic trend of vesicular stomatitis outbreaks. The keywords were selected from definitions and clinical symptoms of vesicular stomatitis. The trend data was available on Google Trends which include the numbers and geographical locations of searches [26].
We found that the keyword “vesicular stomatitis” in daily or weekly Google Trends had a higher relevance to outbreaks. After calculating the Pearson and Spearman correlation coefficients, 15 keywords that were positively correlated with outbreaks were chosen to construct a quantitative model. Thirteen keywords were used to build a classification forecasting model. Of those 13 keywords, 7 were negatively correlated with outbreaks and the remaining 6 keywords were randomly selected from 15 positively correlated keywords. The purpose of regression models is to use known Google Trends data to predict unknown outbreaks. The quantitative regression modelling method can illustrate the system’s development and direction with specific numerical values. Nine negative keywords cannot be used in the multiple linear regressions and multiple stepwise regressions since they are not compliant with the previous intentions of the regression models. However, all disease keywords represent real-time disease information and should not be ignored. When the relevance of the keywords is not good, the classification model can be used to analyse the disease outbreaks. Compared with regression models, the classification model is more tolerant to keywords. It accommodates almost all of the keyword-related information and is widely used in drug design and virtual screening [27]. Additionally, classification models have also achieved great success in disease monitoring [28]. Weka is a classification workbench for data mining that includes almost all mainstream classification algorithms [29]. AdaBoost is a supervised learning algorithm in Weka that has been applied as a very successful technique to solve the two-class classification problem [30]. Our approach uses AdaBoost to train a set of classifiers for outbreaks.
First, the vesicular stomatitis related keywords are individually filtered. After removing keywords that have a negative effect on the model, 13 keywords were selected to build a classification forecasting model. We constructed the classification model based on 5 classification thresholds and found that the classification model based on a threshold of 4 was optimal, with SN, SP and ACC values on the training set of 78.52%, 72.5% and 77.14%, respectively. The classification threshold was defined by vesicular stomatitis outbreaks. For example, if an outbreak data threshold of 4 is set as the boundary, then less than 4 cases are acceptable low frequency for outbreaks with 4 cases of tolerance. Outbreaks data for more than 4 cases are high frequency outbreaks, and urgent measures should be taken.
This study uses 2014 American vesicular stomatitis outbreaks as an example. First, the VS outbreak data were collected from the OIE. Then, Google search data were gathered by inputting disease-related keywords into Google Trends. Pearson and Spearman correlation analyses were performed between the disease outbreak and Google search data. Qualitative classification and quantitative regression models were constructed to predict vesicular stomatitis outbreaks and reduce the risk of disease.
Data preparation
Vesicular stomatitis outbreaks
The American vesicular stomatitis outbreaks from May 18th to November 25th, 2014 were coll-ected from the World Animal Health Information System (WAHIS) on OIE. The total number of VS outbreaks during that period was 433 [31]. (The data can be obtained from the link: http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review/viewsummary?fupser=&dothis=&reportid=15320). The regression model was built with weekly data that was collected by setting s-unday as the first day of the week and daily data were collected to build a classification mod-el (see supplementary materials S1 and S2 Tables). All data collection is complied with the terms of service for OIE.
Google Trends data
The keyword based Google Trends data were also divided into two types (weekly data and dai-ly data) and were collated from the Google Trends Platform. (The data can be obtained from the link: https://trends.google.com/trends/). The daily data were collated by keeping the dates in sync with the disease outbreaks date in OIE (May to December, 2014). The timeframe was li-mited to the whole of 2014 to obtain the weekly Google Trends data (see supplementary mat-erials S1 and S2 Tables). All data collection is complied with the terms of service for Google Trend-s.
Keyword selection and correlation calculations
Twenty-four vesicular stomatitis related keywords were selected from the definitions and clinical symptoms of vesicular stomatitis [32]. From a comparison between keyword “vesicular stomatitis” Google Trends data and VS outbreaks, we deduced that there is a correlation between Google Trends data and outbreaks. Then, Pearson and Spearman correlation coefficients were calculated between each keyword's Google Trends data and the vesicular stomatitis outbreaks using IBM SPSS Statistics 20. The regression model was built with fifteen keywords (vesicular stomatitis, mouth ulcer, sore mouth, vesicular stomatitis virus, VSV, ulcer in mouth, inappetence, pyrexia, lameness, papules, ulcers, vesicular lesions, blister, blister lip, and lip blister) that were positively correlated with vesicular stomatitis outbreaks. The classification forecasting model was built using 13 keywords. Eight (mouth ulcer, sore mouth, ulcer in mouth, inappetence, pyrexia, lameness, blister lip, and lip blister) of the keywords were negatively correlated with outbreaks and the remaining 5 variables (vesicle, tongue blister, molar, excessive salivation, and lethargy) were randomly selected from the positively correlated keywords (see supplementary materials S1 Table).
Methods
Linear regression model
(1) Multiple linear regression and multiple stepwise regression methods
C was the number of outbreaks, and X1—X15 represented the 15 keywords that are positively correlated with outbreaks. A multiple linear regression model was constructed between the vesicular stomatitis outbreaks and the 15 disease-related keywords on the R 3.32 platform [33]. These 15 keywords are the independent variables used to predict the dependent variable (the number of vesicular stomatitis outbreaks). To optimize the multivariate the linear regression equation, a stepwise regression model was established to select the independent variables that had more influence on the dependent variables.
(2) Model prediction
The predicted outbreaks of the multiple linear regression and stepwise multiple regression methods were calculated after the model was assessed on R 3.32 [34]. Then, the correlation between the predicted value and VS outbreaks was calculated to compare the trend of the two sets of data and infer the accuracy of the model. The specific steps are as follows:
Input: S = {(Ci, Xi_1, Xi_2, …, Xi_15), i = 1, 2, …, 28}
Process:
Step1 // Multiple linear regression analysis on training set S
Ms <- lm (C~X1+X2+X3+X4+X6+X7+X8+X9+X10+X11+X12+X13+X14+X15,S)
Step2 // The outbreaks prediction based on multiple linear regression
ps <-predict (Ms, S)
Step3 // Stepwise regression
Ss <- step (Ms)
Step4 // The outbreaks prediction based on stepwise regression
Ps <-predict (Ss,S)
Output: Ps
Classification forecasting model
(1) Data classification and variable screening
At first, a VS outbreak number of 1 was set as a threshold to classify the 13 keywords included in the 192 daily data sets from Google Trends in Excel (S3 Table). The Google search data corresponding to less than 1 outbreak were classified as A, and the data corresponding to greater than or equal to 1 outbreaks were classified as B. Second, the data set was randomly divided into a training set (175) and a test set (17). The training set was used to construct the training model, and the test set was used to evaluate the performance of the final model after each training run. Additionally, to avoid over-fitting, the test data set is used only once. The Google Trends data were classified for thresholds 2, 3, 4, and 5 using the same method used for threshold 1 (S4 and S5 Tables). The 13 keywords were filtered individually from the first keyword “vesicle”, and keywords that had a negative effect on the model were removed. The AdaBoost classifier in Weka 3.6.12 was chosen to construct the model [35, 36]. After deleting the variables that decreased the model’s sensitivity, specificity and accuracy, 13 keywords were significantly correlated with vesicular stomatitis outbreaks.
(2) Model construction and testing
The classification machine AdaBoost was combined with the trees, Bayes, functions, mi, misc, lazy, rules, meta and nested dichotomies functions to construct the threshold 1, 2, 3, 4, and 5 training set classification predicted models [37]. The modelling method with the best sensitivity (SN), specificity (SP) and accuracy (ACC) was selected [38]. Then, the test sets classified as thresholds 1, 2, 3, 4, and 5 were substituted into the constructed model, and the SN, SP and ACC values were calculated.
(3) AdaBoost
AdaBoost is the main classifier in our study and the classification model was constructed by combining AdaBoost with different weak classifiers. The processes of classic the AdaBoost algorithm (binary classification) are described below:
Set sample set:
| (1) |
Initialization
For each (xi, yi) ∈ S Dt(xi, yi) = 1/m; AdaBoost repeatedly calls a given weak or basic learning classifier over a series of time intervals t = 1, 2,…, T, where Dt(xi, yi) is the weight of sample (xi, yi) on the tth cycle [39, 40].
We calling the WeakLearn program algorithm with parameter Dt and obtain the basic classification rule ht:X → Y. Namely, the classification rules of the tth round are generated by the weak algorithm (Usually, a collection of rules).
We choose the correct αt to describe the importance of ht based on the measurement of the prediction error. αt ∈ R, where αt is the evaluation of classification rule ht on round t. A larger αt, indicates a more important ht.
- We generate the weight of each sample by running the algorithm to the (t+1)th cycle. Incorrectly classified samples will have a greater weight on the tth cycle. The specific weight update rule can be expressed as
where Zt is the normalized constant. This results in(2) (3)
Output: The final classifier
Each round classification rule ht acts on X such that
| (4) |
Results
Correlation calculation and analysis
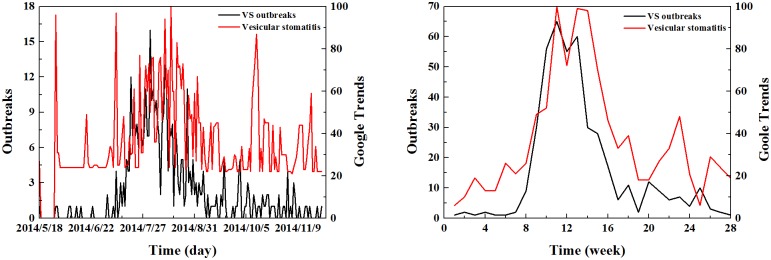
After obtaining the 2014 American vesicular stomatitis outbreak data and the Google search data of the “vesicular stomatitis” keywords, the relationship of the two kinds of data was presented in the trend curve (Fig 1).
Fig 1. The trend for outbreaks and Google Trends data from the “vesicular stomatitis” keywords.
(A) Daily Google Trends data. (B) Weekly Google Trends data.
There is a correlation between VS outbreaks and Google Trends data from comparing these two sets of data. The outbreaks (black curve) and “vesicular stomatitis” keywords (red curve) are overlapped from June 22nd to August 31st, 2014 (using the daily Google Trends data). The trend of outbreaks (black curve) and “vesicular stomatitis” keywords (red curve) appear to be highly correlated especially from the fourth week to the twentieth week (using the weekly Google Trends data). Therefore, we can deduce that the Google Trends data for vesicular stomatitis keywords are correlated with vesicular stomatitis outbreaks. After collecting the 2014 American vesicular stomatitis outbreak data and the Google search data, Pearson and Spearman correlation analyses were performed between the 24 keywords, the weekly Google Trends data and the actual outbreak data. The results are shown in Table 1.
Table 1. The correlation coefficient value between the outbreaks and Google Trends data.
| Parameter | Keywords | Correlation coefficient value | |
|---|---|---|---|
| Pearson | Spearman | ||
| X1 | vesicular stomatitis | 0.853** | 0.774** |
| X2 | mouth ulcer | 0.429* | 0.215 |
| X3 | sore mouth | 0.396* | 0.208 |
| X4 | Vesicular Stomatitis Virus | 0.387* | 0.505** |
| X5 | VSV | 0.441* | 0.566** |
| X6 | ulcer in mouth | 0.34 | 0.267 |
| X7 | inappetence | 0.181 | 0.159 |
| X8 | pyrexia | 0.151 | 0.013 |
| X9 | Lameness | 0.145 | 0.11 |
| X10 | papules | 0.349 | 0.113 |
| X11 | ulcers | 0.187 | 0.264 |
| X12 | Vesicular lesions | 0.228 | 0.149 |
| X13 | blister | 0.337 | 0.075 |
| X14 | blister lip | 0.244 | 0.045 |
| X15 | lip blister | 0.255 | 0.077 |
| X16 | vesicle | -0.430* | -0.180 |
| X17 | gum blister | -0.061 | -0.105 |
| X18 | tongue blister | -0.027 | 0.027 |
| X19 | molar | -0.489* | -0.298 |
| X20 | pruritus | -0.058 | 0.318 |
| X21 | anorexia | -0.442* | -0.306 |
| X22 | Sore nose | -0.365 | -0.266 |
| X23 | excessive salivation | -0.133 | -0.144 |
| X24 | lethargy | -0.234 | -0.089 |
** Significant correlations at 0.01 level (bilateral)
* Significant correlation at 0.05 level (bilateral)
The correlation coefficient value from variables X1 –X15 are positive, indicating a positive interrelated relationship between vesicular stomatitis keywords and vesicular stomatitis outbreaks. The remaining vesicular stomatitis keywords (X16—X24) have a negative correlation with vesicular stomatitis outbreaks.
Linear regression model
Regression parameter estimation
According to the R output, the multiple linear regression equation between the actual number of outbreaks Y and the vesicular stomatitis keywords X is:
The stepwise regression equation is:
Significance test of the regression equation
To test the significance of individual and overall variables, the T test, F test, and R2 (coefficient of determination) were calculated in R. The multivariate linear regression T-test showed that only the independent vesicular stomatitis variable X1 was significant (***, Table 2). All of the variables were significant after the multiple stepwise regression analysis (Table 3).
Table 2. Significance test of multiple linear regression equations.
| parameter | Std. Error | t value | value Pr(>|t|) |
|---|---|---|---|
| C | 59.76067 | -1.899 | 0.081845 . |
| X1 | 0.11353 | 5.543 | 0.000127 *** |
| X2 | 0.6045 | 0.137 | 0.89305 |
| X3 | 0.39954 | -0.988 | 0.342599 |
| X4 | 0.37123 | -0.605 | 0.55656 |
| X5 | 0.40409 | 0.40409 | 0.307755 |
| X6 | 0.32297 | 0.339 | 0.740694 |
| X7 | 0.21058 | 0.728 | 0.480373 |
| X8 | 0.10581 | -0.268 | 0.793231 |
| X9 | 1.10831 | -0.671 | 0.515061 |
| X10 | 0.53168 | 1.322 | 0.210789 |
| X11 | 0.16166 | 1.647 | 0.125561 |
| X12 | 0.35742 | 1.556 | 0.145608 |
| X13 | 0.14746 | 1.688 | 0.117302 |
| X14 | 0.15665 | 0.889 | 0.391436 |
| X15 | 0.26838 | -1.433 | 0.177433 |
Signif. codes: ‘***’ 0.001 ‘.’ 0.1
Table 3. Significance test of stepwise multiple regression equations.
| parameter | Std. Error | t value | value Pr(>|t|) |
|---|---|---|---|
| C | 33.84799 | -2.87 | 0.0098 ** |
| X1 | 0.08638 | 7.796 | 2.45e-07 *** |
| X3 | 0.20588 | -1.744 | 0.0972 . |
| X10 | 0.43447 | 2.032 | 0.0564 . |
| X11 | 0.13101 | 2.034 | 0.0561 . |
| X12 | 0.23147 | 2.213 | 0.0393 * |
| X13 | 0.1026 | 2.005 | 0.0594 . |
| X14 | 0.11143 | 1.598 | 0.1266 |
| X15 | 0.20456 | -2.548 | 0.0197 * |
Signif. codes: ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 In the F and R2 tests, the P value of the stepwise multiple regression was 4.772e-07, which was less than the P value of 0.0005248 obtained in the multiple linear regression analysis. The stepwise multiple regression’s R2 was 0.8746 and the adjusted R2 was 0.8218, which were close to 1 (Table 4). An R2 is closer to 1 indicates that the majority of the dependent variable’s uncertainty can be explained by the regression equation, indicating a better goodness of fit. Based on the above results, the stepwise multiple regression results were superior.
Table 4. F test and R2 of regression model.
| P value | R2 | Adjusted R2 | |
|---|---|---|---|
| Multiple linear regression | 0.000525 | 0.8853 | 0.7617 |
| Stepwise multiple regression | 4.77E-07 | 0.8746 | 0.8218 |
Model prediction
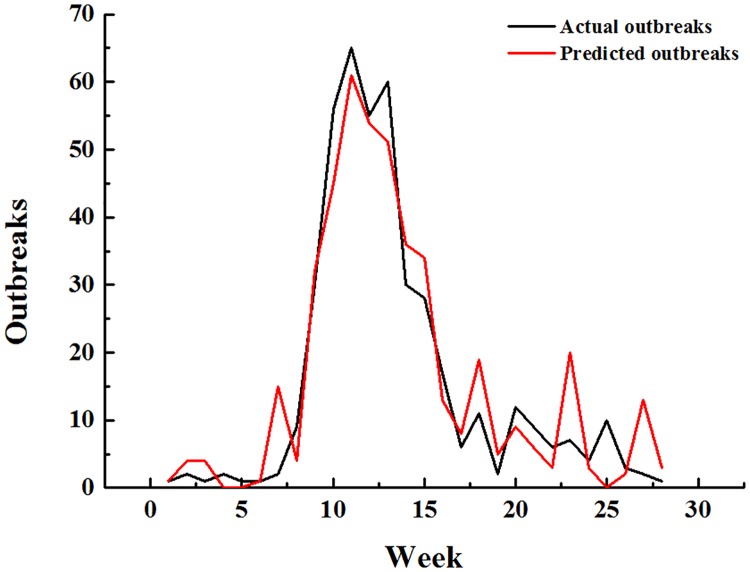
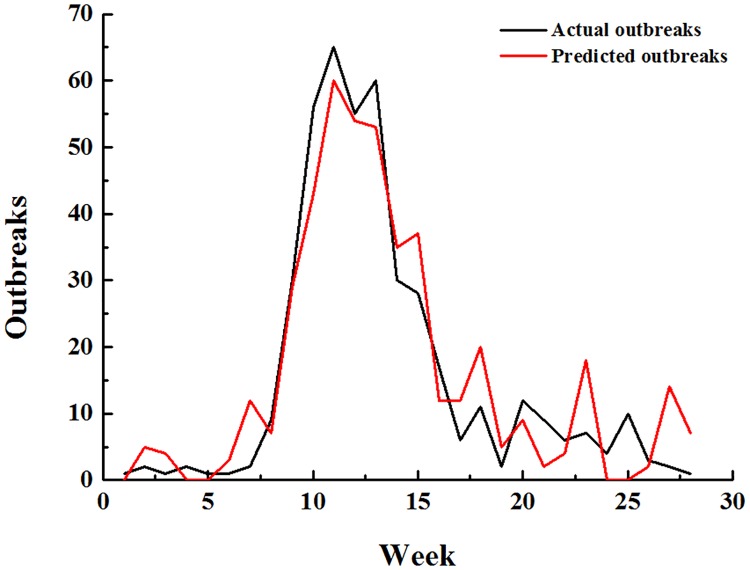
We used the predict () function in R to predict the multiple linear regression and multiple stepwise regression data. The results are shown in Figs 2 and 3. The red curve (actual outbreaks) and the black curve (predicted outbreaks) have similar trends under the multiple linear regression and the multiple stepwise regression. The fit for the multiple linear regression is better than the multiple stepwise regression since the trend differences only appeared in the 23rd week in the multiple linear regression, while appeared in the 20th week in the multiple stepwise regression. The Pearson correlation coefficient calculations were conducted between the actual outbreaks and the predicted outbreaks. The results of the multiple linear regression and the multiple stepwise regression are 0.953 and 0.948, respectively.
Fig 2. Comparison between the actual American vesicular stomatitis outbreaks and the predicted outbreaks using the multiple linear regression model.
Fig 3. Comparison between the actual American vesicular stomatitis outbreaks and the predicted outbreaks using the stepwise multiple regression model.
Classification model
The classifier AdaBoost was combined with different weak classifiers to construct the classification model. After inputting the training set of thresholds 1, 2, 3, 4, and 5, the model was constructed and tested with the independent test set. The classification model’s SN, SP and ACC from the training set exceeded 60% are shown in Table 5. In the 11 classification models, the prediction accuracy (ACC) of thresholds 3, 4 and 5 reached 70% and AdaBoost combined with DecisionStump of threshold 4 reached the highest value at 77.14%. The SP of the independent test set thresholds from thresholds 1 and 2 are under 50% and the model lacks stability. After the classification calculation, the model constructed by AdaBoost combined with DecisionStump from threshold 4 was found to be the best classification model, with SN, SP and ACC values of 78.52%, 72.5%, and 77.14%, respectively.
Table 5. AdaBoost combined with weak classifiers’ model.
| Classification threshold | Classifier | Training set | Independent test set | ||||
|---|---|---|---|---|---|---|---|
| SN(%) | SP(%) | ACC(%) | SN(%) | SP(%) | ACC(%) | ||
| 1 | NbTree | 67.14 | 60 | 62.86 | 57.14 | 50 | 52.94 |
| 2 | DecisionStump | 71.17 | 60.94 | 67.43 | 81.82 | 33.33 | 64.71 |
| BayesNet | 72.07 | 64.06 | 69.14 | 81.82 | 33.33 | 64.71 | |
| MultiBoostAB | 72.97 | 64.06 | 69.71 | 90.91 | 33.33 | 70.59 | |
| 3 | ComplementNaiveBayes | 65.63 | 80.85 | 69.71 | 41.67 | 80.00 | 52.94 |
| NaiveBayesMultinomialUpdateable | 76.56 | 61.7 | 72.57 | 66.67 | 80.00 | 70.59 | |
| 4 | DecisionStump | 78.52 | 72.5 | 77.14 | 69.23 | 75.00 | 70.59 |
| ComplementNaiveBayes | 61.48 | 95 | 69.14 | 46.15 | 75.00 | 52.94 | |
| 5 | NaiveBayesMultinomial | 72.73 | 71.88 | 72.57 | 53.85 | 75.00 | 58.82 |
| NaiveBayesMultinomialUpdateable | 71.33 | 68.75 | 70.86 | 53.85 | 75.00 | 58.82 | |
| VIF | 72.73 | 81.25 | 74.29 | 69.23 | 75.00 | 70.59 | |
Single variable predictions
The training and test sets were separately constructed using 4 as the classification threshold, and the integrated learning algorithm AdaBoost combined with DecisionStump was applied to examine the effect of a single variable on the model. The results are shown in Table 6. The ACC of the disease-related keywords exceeded 70%, indicating that the classifier AdaBoost combined with DecisionStump was a useful method for model classification. In addition, the ACC of each keyword is balanced, which means that there is no strong influencing factor. Therefore, the 13 keywords should be combined to construct a more accurate classification model.
Table 6. Single variable model constructed by AdaBoost+ DecisionStump.
| Parameter | Training set | ||
|---|---|---|---|
| SN(%) | SP(%) | ACC(%) | |
| X2 | 98.52% | 7.50% | 77.71% |
| X3 | 95.56% | 0.00% | 73.71% |
| X6 | 95.56% | 0.00% | 73.71% |
| X7 | 97.78% | 2.50% | 76.00% |
| X8 | 100.00% | 0.00% | 77.14% |
| X9 | 100.00% | 0.00% | 77.14% |
| X14 | 100.00% | 0.00% | 77.14% |
| X15 | 99.26% | 0.00% | 76.57% |
| X16 | 100.00% | 0.00% | 77.14% |
| X18 | 99.26% | 0.00% | 76.57% |
| X19 | 99.26% | 0.00% | 76.57% |
| X23 | 99.26% | 0.00% | 76.57% |
| X24 | 99.26% | 0.00% | 76.57% |
Discussion
A model is used to predict the future development of events of interest. The model is usually divided into two subsets, including (a qualitative prediction method and a quantitative prediction method) [41]. The qualitative analysis indicates a link between each disease keyword and VS outbreaks. In our study, the multiple stepwise regression results are almost the same as the disease outbreaks, with a correlation coefficient between the two variables of 0.948. Many keywords are associated with vesicular stomatitis, while not all of the Google search data are positively correlated with vesicular stomatitis outbreaks. Some disease keywords, such as “vesicles”, even presented a negative correlation. The inclusion of these keywords in the regression modelling process will affect the regression trend and be automatically deleted by the stepwise multiple regression process. However, all disease keywords represent real-time disease information and should not be ignored. When the relevance of the keywords is not ideal, the classification model with its tolerance can be used to analyse the disease outbreaks. At first, 21 vesicular stomatitis related keywords were used to construct the classification model by AdaBoost combined with combined with different weak classifiers in order to conceal the defects of other keywords. This was done after removing the keywords “vesicular stomatitis”, “vesicular stomatitis virus” and “VSV” that were strongly correlated with vesicular stomatitis outbreaks. However, the classification results using 21 keywords are not good. S8 Table (see supplementary materials S8 Table) shows that no accuracy (ACC) exceeds 60% for the classification models constructed by thresholds 1 and 3, and the ACC for thresholds 2, 4, and 5 are generally below 70%. We believe some keywords among the 21 vesicular stomatitis relative keywords have a negative impact on classification. Therefore, 21 vesicular stomatitis relative keywords were individually filtered. After removing the negative keywords, 13 keywords were selected to build a classification forecasting model. Seven of the keywords were negatively correlated with outbreaks and the remaining 6 keywords were randomly selected from the positively correlated keywords. The best classification model was AdaBoost combined with DecisionStump from classification threshold 4, with SN, SP and ACC values of 78.52%, 72.5%, and 77.14%, respectively. The classification threshold was defined by vesicular stomatitis outbreaks.
Classification models from thresholds 3, 4 and 5 are better than thresholds 1 and 2 since the uneven classification exists in the Google Trends keywords data. The unbalanced data set may affect the model’s accuracy. Due to the lack of an outbreak data set, the model is unable to provide any further classifications and thus warrants further exploration. The majority of American vesicular stomatitis outbreaks are 1 and 0. When we set a VS outbreak number as the threshold to classify the Google Trends data, the data of classes A and B obtained by the classification are not accurate. For example, the number in classes A from thresholds 1 is 78, while in threshold 5 it is 156 (S3 Table). The classified data is more balanced with thresholds 3 and 4.
In addition, the weak classifier combined with AdaBoost also played a very important role in the classification model’s construction. As a supervised learning algorithm, AdaBoost is used to correct the misclassified samples by increasing the weight so that the next iteration will focus on these samples and the classifier with higher accuracy, and the weight is also relatively higher after each iteration [42]. In this paper, the best classification model was obtained by using decision stumps as a weak classifier. Decision stumps are the simplest form of binary decision trees are often used as components ("weak learners" or "base learners") [43]. Decision stumps with one internal node make immediately connect the classification decision to the terminal nodes. The AdaBoost with the decision stump classifier acts as a feature selection mechanism to quickly and accurately make classification decisions. Moreover, AdaBoost with the weak classifiers NaiveBayesMultinomial, NaiveBayesMultinomialUpdateable and VIF also achieved good results. However, the classification model constructed by the common classifier BayesNet was not good and the ACC was 62.86%. Although BayesNet is widely used in various classification models due to its an ability to obtain the probability density functions (PDFs) of individual pattern classes from learning samples, it is not suitable for the samples in this study [44].
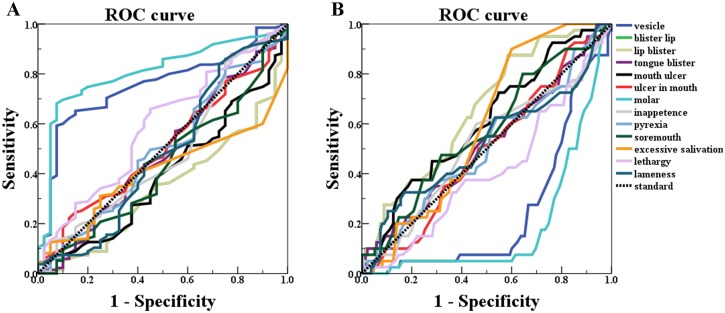
The 13 keywords receiver operating characteristic (ROC) curve was created using SPSS to determine the most influential keywords in the model classification [45]. Fig 4(A) shows the ROC curve of the threshold 4 classification model. It can be easily concluded that keyword “Blister lip” (blue curve) is better than or at least as good as the others and “excessive salivation” (orange curve) (in Fig 4(B)) is the most influential keyword of Google Trends data classified by outbreaks greater than 4.
Fig 4. ROC curve of 13 keywords for vesicular stomatitis.
(A) State variable is 0 (number of outbreaks<4) (B) State variable is 1 (number of outbreaks≥4).
Traditional disease models are constructed using a single quantitative or qualitative method. For example, Chen Jinhong et al useda BP neural network to predict cardiovascular and cerebrovascular disease [46], Sang Youn Kim et al, used clinical decision support systems to predict advanced prostate cancer by comparing support vector machines and artificial neural networks [47]. In the United States, Berger used the Bayesian classification algorithm developed by the GIDEON system, which is a smart identification system for infectious diseases [48]. Feldman et al proposed using a decision tree technique on psychiatric diagnoses [49]. However, the two methods are rarely used to construct a disease prediction model. Therefore, the construction of one model is not complete due to the loss of accuracy and sensitivity.
In this study, the model was constructed using qualitative and quantitative approaches. Relevant keywords were used to construct a stepwise regression model and to explain the relationship between the disease keywords search data and the disease outbreaks. This model predicted the development and direction of vesicular stomatitis outbreaks. The classification model was constructed using negatively correlated keywords or keywords that were not significantly related to the disease to predict how many cases of VS were classed as high frequency outbreaks. This approach will allow the relevant departments to quickly take preventive measures. Although this article has made the most of the VS keywords, the search engine has some flaws. For example, the search engine cannot search for some keywords in Google Trends, resulting in the loss of some important disease information. In addition, 24 vesicular stomatitis related keywords were selected from the definition and clinical symptoms of vesicular stomatitis and should theoretically not be negatively correlated with VS outbreaks. The negative keywords are not compliant with previous regression models and cannot be used in multiple linear regressions or multiple stepwise regressions. There is still a need to combine more accurate information searches for disease information collection.
Conclusion
In this work, we used two types of methods to construct the disease prediction model. The discovery of Google Trends is an important research area in mathematical modelling. As a timely, robust, and sensitive surveillance system, Google Trends is widely used in disease surveillance research. This study applied Google search data to construct a qualitative classification model and a quantitative regression model. The results show that the method is effective and that these two models obtain more accurate forecasting values.
Supporting information
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability
The American vesicular stomatitis outbreaks from May 18th to November 25th, 2014 were collected from the World Animal Health Information System (WAHIS) on OIE. The total number of VS outbreaks during that period was 433 [31]. (The data can be obtained from the link: http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review/viewsummary?fupser=&dothis=&reportid=15320). The regression model was built with weekly data that was collected by setting Sunday as the first day of the week and daily data were collected to build a classification model (see Supporting Information files S1 and S2). All data collection is complied with the Terms of Service for OIE. The keyword-based Google Trends data were also divided into two types (weekly data and daily data) and were collated from the Google Trends Platform. (The data can be obtained from the link: https://trends.google.com/trends/). World Organisation for Animal Health (OIE) and World Animal Health Information Database (WAHIS) Interface 2013 are available from: http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review/viewsummary?fupser=&dothis=&reportid=15320.
Funding Statement
QC and BN are funded by The National Key Research and Development Program of China (grant no. 2016YFD0501101) and High Performance Computing Center Program of Shanghai University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zhang M, Ge J, Li X, Chen W, Wang X, Wen Z, et al. Protective efficacy of a recombinant Newcastle disease virus expressing glycoprotein of vesicular stomatitis virus in mice. Virology Journal. 2016;13(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrera JD, Letchworth GJ. Persistence of vesicular stomatitis virus New Jersey RNA in convalescent hamsters. Virology. 1996;219(2):453–64. doi: 10.1006/viro.1996.0271 [DOI] [PubMed] [Google Scholar]
- 3.USA CfFS, Health P. Vesicular stomatitis. Vesicular Stomatitis. 2008;7(4):205–7. [Google Scholar]
- 4.Walton TE, Webb PA, Kramer WL. Epizootic vesicular stomatitis in Colorado, 1982: epidemiologic and entomologic studies. American Journal of Tropical Medicine & Hygiene. 1987;36(1):166–76. [DOI] [PubMed] [Google Scholar]
- 5.Chu XL, Cheng J, Zhou XZ, Gao F. Research Progress on Vesicular Stomatitis. Jilin Journal of Animal Hu Sbandry & Veterinary Medicine. 2004. [Google Scholar]
- 6.Mccluskey BJ, Hurd HS, Mumford EL. Review of the 1997 outbreak of vesicular stomatitis in the western United States. Journal of the American Veterinary Medical Association. 1999;215(9):1259–62. [PubMed] [Google Scholar]
- 7.USDA. Vesicular stomatitis 2016. www.aphis.usda.gov/vs/nahss/equine/vsv/docs/usaha_2006_VSV_presentation.pdf.
- 8.Mateus JC, Carrasquilla G. Predictors of local malaria outbreaks: an approach to the development of an early warning system in Colombia. Memórias Do Instituto Oswaldo Cruz. 2011;106(Suppl 1):107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng CKY, Lau EHY, Ip DKM, Yeung ASY, Ho LM, Cowling BJ. A profile of the online dissemination of national influenza surveillance data. Bmc Public Health. 2009;9 Artn 339 doi: 10.1186/1471-2458-9-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carneiro HA, Mylonakis E. Google trends: a web-based tool for real-time surveillance of disease outbreaks. Clinical Infectious Diseases. 2009;49(10):1557 doi: 10.1086/630200 [DOI] [PubMed] [Google Scholar]
- 11.Dugas AF, Jalalpour M, Gel Y, Levin S, Torcaso F, Igusa T, et al. Influenza Forecasting with Google Flu Trends. Online Journal of Public Health Informatics. 2013;8(2):e56176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortiz JR, Zhou H, Shay DK, Neuzil KM, Fowlkes AL, Goss CH. Monitoring Influenza Activity in the United States: A Comparison of Traditional Surveillance Systems with Google Flu Trends. Plos One. 2011;6(4):e18687 doi: 10.1371/journal.pone.0018687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valdivia A, Lopez-Alcalde J, Vicente M, Pichiule M, Ruiz M, Ordobas M. Monitoring influenza activity in Europe with Google Flu Trends: comparison with the findings of sentinel physician networks—results for 2009–10. Eurosurveillance: bulletin europeen sur les maladies transmissibles = European communicable disease bulletin. 2010;15(29):2–7. [DOI] [PubMed] [Google Scholar]
- 14.Kang M, Zhong H, He J, Rutherford S, Yang F. Using Google Trends for influenza surveillance in South China. Plos One. 2013;8(1):e55205 doi: 10.1371/journal.pone.0055205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gluskin RT, Johansson MA, Santillana M, Brownstein JS. Evaluation of Internet-based dengue query data: Google Dengue Trends. Plos Neglected Tropical Diseases. 2014;8(2):e2713 doi: 10.1371/journal.pntd.0002713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelat C, Turbelin C, Barhen A, Flahault A, Valleron AJ. More diseases tracked by using google trends. Emerging Infectious Diseases. 2009;15(8):1327–8. doi: 10.3201/eid1508.090299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X, Ye J, Feng Y. Tuberculosis surveillance by analyzing Google trends. IEEE transactions on bio-medical engineering. 2011;58(8):2247–54. [DOI] [PubMed] [Google Scholar]
- 18.Peace UIO. Using Quantitative and Qualitative Models to Forecast Instability. United States Institute of Peace. 2008.
- 19.Hu M, Li Z, Wang J, Lin J, Liao Y, Lai S, et al. Determinants of the Incidence of Hand, Foot and Mouth Disease in China Using Geographically Weighted Regression Models. Plos One. 2012;7(6):e38978 doi: 10.1371/journal.pone.0038978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin H, Pan XP, Yuan LI. THE INTRODUCTION AND APPLICATION OF REGRESSION MODELS FOR ORDINAL CATEGORICAL RESPONSE IN EPIDEMIOLOGY. Modern Preventive Medicine. 2006.
- 21.Wan H S, Huang CH, Tong H, Liu XF. The prediction of the weekly incidence of hand-foot-mouth disease (HFMD) by three regression models. Modern Preventive Medicine. 2016;43(16):2889–92. [Google Scholar]
- 22.Hangzhou. STUDY ON NEW FORECAST MODELS FOR THE NUMBER OF DISEASE INCIDENCE CASE. Scientia Meteorologica Sinica. 2006;26(4):462–6. [Google Scholar]
- 23.Lisboa P, Chabaud S, Bachelot T. Comparison of artificial neural network with logistic regression as classification models for variable selection for prediction of breast cancer patient outcomes. Advances in Artificial Neural Systems. 2010;2010(3):2. [Google Scholar]
- 24.Gao D, Madden M, Chambers D, Lyons G, editors. Bayesian ANN classifier for ECG arrhythmia diagnostic system: a comparison study. IEEE International Joint Conference on Neural Networks, 2005 IJCNN '05 Proceedings; 2005.
- 25.Medhekar DS, Bote MP, Deshmukh SD. Heart disease prediction system using naive Bayes. Int J Enhanced Res Sci Technol Eng. 2013;2(3). [Google Scholar]
- 26.Seifter A, Schwarzwalder A, Geis K, Aucott J. The utility of "Google Trends" for epidemiological research: Lyme disease as an example. Geospat Health. 2010;4(2):135–7. doi: 10.4081/gh.2010.195 [DOI] [PubMed] [Google Scholar]
- 27.Burbidge R, Trotter M, Buxton B, Holden S. Drug design by machine learning: support vector machines for pharmaceutical data analysis. Computers & Chemistry. 2001;26(1):5. [DOI] [PubMed] [Google Scholar]
- 28.Boursalie O, Samavi R, Doyle TE. M4CVD: Mobile Machine Learning Model for Monitoring Cardiovascular Disease. Procedia Computer Science. 2015;63:384–91. [Google Scholar]
- 29.Frank E, Hall M, Holmes G, Kirkby R, Pfahringer B, Witten IH, et al. Weka-A Machine Learning Workbench for Data Mining Data Mining & Knowledge Discovery Handbook. 2009:1269–77. [Google Scholar]
- 30.Zhu J, Zou H, Rosset S, Hastie T. Multi-class AdaBoost. Statistics & Its Interface. 2006;2(3):349–60. [Google Scholar]
- 31.World Organisation for Animal Health (OIE). World Animal Health Information Database (WAHIS) Interface 2013. http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review/viewsummary?fupser=&dothis=&reportid=15320.
- 32.Tan XL L H. Correlation Analysis between Web Search and H7N9 Bird Flu Trends. Hubei Journal of Traditional Chinese Medicine | Hubei J Tradit Chin Med. 2015;37((10)):15–8. [Google Scholar]
- 33.Cankurt S, Subasi A, editors. Comparison of linear regression and neural network models forecasting tourist arrivals to Turkey. Issd; 2012.
- 34.Wang JJ Z Y, Peng YS, Li KL, Jang TJ. ON PREDICTION OF DENGUE EPIDEMICS BASED ON BAIDU INDEX. Computer Applications and Software. 2016;33((7)):41–6. [Google Scholar]
- 35.Niu B, Cai YD, Lu WC, Li GZ, Chou KC. Predicting protein structural class with AdaBoost Learner. Protein & Peptide Letters. 2006;13(5):489–92. [DOI] [PubMed] [Google Scholar]
- 36.Niu B, Jin YH, Feng KY, Lu WC, Cai YD, Li GZ. Using AdaBoost for the prediction of subcellular location of prokaryotic and eukaryotic proteins. Molecular Diversity. 2008;12(1):41 doi: 10.1007/s11030-008-9073-0 [DOI] [PubMed] [Google Scholar]
- 37.Niu B, Zhao M, Su Q, Zhang M, Lv W, Chen Q, et al. 2D-SAR and 3D-QSAR analyses for acetylcholinesterase inhibitors. Molecular Diversity. 2017:1–14. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Liu H, Yang J, Chou KC. Prediction of linear B-cell epitopes using amino acid pair antigenicity scale. Amino Acids. 2007;33(3):423–8. doi: 10.1007/s00726-006-0485-9 [DOI] [PubMed] [Google Scholar]
- 39.Duffy N, Helmbold D, editors. A geometric approach to leveraging weak learners European Conference on Computational Learning Theory; 1999. [Google Scholar]
- 40.Schapire RE. The boosting approach to machine learning: An overview. Nonlinear estimation and classification: Springer; 2003: 149–71. [Google Scholar]
- 41.Kuhn M, Johnson K. Applied predictive modeling: Springer; 2013. [Google Scholar]
- 42.Venkataraju KU, Paiva ARC, Jurrus E, Tasdizen T. Automatic Markup of Neural Cell Membranes Using Boosted Decision Stumps. Proceedings. 2009:1039–42.
- 43.Reyzin L, Schapire RE, editors. How boosting the margin can also boost classifier complexity. Proceedings of the 23rd international conference on Machine learning; 2006: ACM.
- 44.Lee S, Shimoji S, editors. BAYESNET: Bayesian classification network based on biased random competition using Gaussian kernels. Neural Networks, 1993, IEEE International Conference on; 1993: IEEE.
- 45.Kai FG. Sensitivity of fusion performance to classifier model variations. Proc Spie. 2003;5099:39–46. [Google Scholar]
- 46.Chen Jin-hong W H-y, He Yao, et al. Construction and evaluation of predictive model for ischemic cardiovascular diseases of senior men based on BP neural network. J Third Mil Med Univ. 2011;33(8):797–9. [Google Scholar]
- 47.Kim SY, Moon SK, Jung DC, Hwang SI, Sung CK, Cho JY, et al. Pre-Operative Prediction of Advanced Prostatic Cancer Using Clinical Decision Support Systems: Accuracy Comparison between Support Vector Machine and Artificial Neural Network. Korean J Radiol. 2011;12(5):588–94. doi: 10.3348/kjr.2011.12.5.588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berger S.A.. GIDEON: a comprehensive Web-based resource for geographic medicine. Int J health Geoger. 2005;4(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.S. Feldman K DF, Honigfeld G. The raliability of a decision tree technique applied to psychiatric diagnosis. biometrics. 1972;28(3):831–40. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
The American vesicular stomatitis outbreaks from May 18th to November 25th, 2014 were collected from the World Animal Health Information System (WAHIS) on OIE. The total number of VS outbreaks during that period was 433 [31]. (The data can be obtained from the link: http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review/viewsummary?fupser=&dothis=&reportid=15320). The regression model was built with weekly data that was collected by setting Sunday as the first day of the week and daily data were collected to build a classification model (see Supporting Information files S1 and S2). All data collection is complied with the Terms of Service for OIE. The keyword-based Google Trends data were also divided into two types (weekly data and daily data) and were collated from the Google Trends Platform. (The data can be obtained from the link: https://trends.google.com/trends/). World Organisation for Animal Health (OIE) and World Animal Health Information Database (WAHIS) Interface 2013 are available from: http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review/viewsummary?fupser=&dothis=&reportid=15320.