Abstract
ATP Binding Cassette family efflux proteins ABCB1 and ABCG2 have previously been demonstrated to interact with Tyrosine Kinase Inhibitors (TKIs); however, evidence for the interaction of other potentially relevant drug transporters with TKIs is lacking. Through Taqman transporter array technology we assessed the impact of nilotinib on mRNA expression of ABC transporters, with ABCC6 identified as a transporter of interest. Additionally, increased expression of ABCC6 mRNA was observed during in vitro development of nilotinib resistance in BCR-ABL1-expressing cell lines. K562 cells exposed to gradually increasing concentrations of nilotinib (to 2 μM) expressed up to 57-fold higher levels of ABCC6 mRNA when compared with control cells (p = 0.002). Analogous results were observed in nilotinib resistant K562-Dox cells (up to 33-fold higher levels of ABCC6, p = 0.002). IC50 experiments were conducted on patient mononuclear cells in the absence and presence of three ABCC6 inhibitors: indomethacin, probenecid and pantoprazole. Results demonstrated that all three inhibitors significantly reduced nilotinib IC50 (p<0.001) indicating ABCC6 is likely involved in nilotinib transport. Cell line data confirmed these findings. Similar results were obtained for dasatinib, but not imatinib. Combined, these studies suggest that nilotinib and dasatinib are likely substrates of ABCC6 and to our knowledge, this is the first report of ABCC6 involvement in TKI transport. In addition, ABCC6 overexpression may also contribute to nilotinib and dasatinib resistance in vitro. With nilotinib and dasatinib now front line therapy options in the treatment of CML, concomitant administration of ABCC6 inhibitors may present an attractive option to enhance TKI efficacy.
Introduction
Chronic Myeloid Leukaemia (CML) is caused by the Breakpoint cluster region-abelson (Bcr-Abl) oncoprotein, which is effectively inhibited by Tyrosine Kinase Inhibitors (TKIs) such as imatinib[1] and the second generation inhibitors nilotinib[2] and dasatinib[3]. Resistance to TKIs can occur due to numerous factors including Bcr-Abl overexpression and most commonly because of mutations in the Bcr-Abl kinase domain[4]. However, mounting evidence emphasises the importance of cellular drug efflux transporters, in particular members of the ATP-Binding Cassette (ABC) family, and attendant intracellular drug levels in TKI response. Indeed, recent data has highlighted P-glycoprotein (ABCB1) overexpression as a contributor to TKI resistance[5] and as a potential biomarker for predicting patient response to imatinib[6].
Members of the ABC family of transporters are responsible for the extrusion of small molecules, chemotherapeutics and other xenobiotics, including TKIs, from cells[7]. In particular, ABCB1 and breast cancer resistance protein (BCRP/ABCG2) have previously been implicated in multidrug resistance and their interaction with TKIs has been thoroughly investigated and reviewed[8]. More recently the role of ABCC1, ABCC3, ABCC4[9] and ABCA3[10] in TKI transport and resistance has also been examined. However, to date, no investigations into the TKI:ABCC6 relationship have been conducted.
ABCC6 (MRP6) belongs to the Multidrug Resistance Protein (MRP) superfamily and is capable of extruding a wide variety of substrates from cells[11]. ABCC6 bears striking sequence homology to ABCC1 and is thought to have arisen from a gene duplication[12–14]. While a biological substrate has not yet been elucidated, ABCC6 is known to transport lipophilic molecules of negative charge[15, 16] and is primarily expressed at points of drug extrusion such as the liver and the kidney[12, 17, 18]. The involvement of ABCC6 in an autosomal recessive connective tissue disorder, pseudoxanthoma elasticum, is well established despite the fact that ABCC6 is not expressed in the tissue within which this disease occurs[19, 20].
The involvement of ABCC6 in the transport of, and resistance to, a number of anticancer agents including etoposide, doxorubicin and daunorubicin has been demonstrated[15, 21]. However, ABCC6 involvement in the transport of other small molecule inhibitors such as TKIs has not been investigated. Our previous study[22] demonstrated a reduction in nilotinib IC50 (based on Bcr-Abl kinase inhibition) in ABCB1 overexpressing K562-Dox cells in the presence of proton pump inhibitors (PPI) known to inhibit ABCB1[23, 24]. The reduction observed suggests that PPIs block ABCB1-mediated efflux of nilotinib, increasing the intracellular concentration and subsequent kinase inhibition thus reducing the IC50. Intriguingly, the IC50 was also reduced in K562 cells, albeit to a lesser extent, which do not overexpress ABCB1[22]. These results suggest that an alternate transporter expressed in both K562-Dox and K562 cells may also be responsible for the efflux of nilotinib leading to the lowered IC50. PPIs have been previously demonstrated to inhibit ABCC6[15, 23, 25] and given the overlapping substrate specificities of ABC transporters, further investigations into the TKI:ABCC6 relationship were warranted. Here we provide the first evidence that ABCC6 may be involved in TKI transport and that overexpression of ABCC6 likely causes resistance to nilotinib in vitro.
Materials and methods
Ethics statement
This research involved human clinical samples and these correlative studies were approved by the Royal Adelaide Hospital Research Ethics Committee (RAH protocol number: 070718). All samples were collected with informed consent in accordance with the Institutional Ethics approved protocols and with reference to the Declaration of Helsinki.
Cell lines
BCR-ABL1-expressing cell lines KU812 and K562 were obtained from American Type Culture Collection (ATCC; Manassas, VA). K562 cells were transfected as described previously and the resultant K562-ABCG2 cells cultured in 500 μg/mL G418 (Invitrogen, Carlsbad, CA) and assessed for appropriate ABCG2 expression by quantitative PCR and flow cytometry[26]. K562-Dox cells (ABCB1-overexpressing) were generated after the continuous culture of K562 parental cells in the ABCB1 substrate, doxorubicin. K562-Dox cells now stably express ABCB1 in the absence of doxorubicin (kindly provided by Prof. Leonie Ashman, University of Newcastle, Callaghan, NSW). HepG2 cells were kindly provided by Prof. Andrew Zannettino (South Australian Health and Medical Research Institute/University of Adelaide, Adelaide, SA). All suspension cells were cultured as described previously[27]; HepG2 cells were cultured in Dulbecco’s Modified Eagle Media (DMEM; Sigma). Prior to reculture (1:20 dilution), cells were trypsinised (0.25%; Sigma), separated by pipetting and resuspended in fresh media devoid of trypsin. Nilotinib-[5], dasatinib-[28] and imatinib-resistant[28] cell lines were generated as described previously. Briefly, cell lines were maintained in liquid culture and were gradually exposed to escalating concentrations of TKI. TKI concentrations were increased once cells demonstrated tolerance to the current concentration (>80% survival in culture for >10 days).
Patient cells
Peripheral blood was obtained from de novo chronic phase CML patients before commencement of TKI therapy and mononuclear cells (MNCs) were isolated using Lymphoprep (Axis Shield, Oslo, Norway) density gradient centrifugation.
TKIs and efflux transporter inhibitors
Imatinib mesylate (Glivec®) and nilotinib (Tasigna®) were provided by Novartis Pharmaceuticals (Basel, Switzerland), dasatinib (Sprycel®) was provided by Bristol-Myers Squibb (Victoria, Australia). Stock solutions of imatinib were prepared at 10 mM in distilled water, sterile filtered and stored at -80°C. Stock solutions of nilotinib and dasatinib were prepared at 10 mM in dimethylsulfoxide (DMSO; Sigma, St Louis, MO) and stored at 4°C. Verapamil (Royal Adelaide Hospital (RAH) Pharmacy) was used at 50 μM from a 2.5 mg/mL stock; pantoprazole (RAH Pharmacy) was used at 200 μM from a 10 mM stock; indomethacin (Sigma) was used at 100 μM from a 10 mg/mL stock; probenecid (Sigma) was used at 1 mM from a 175 mM stock; PSC-833 is a Cyclosporin A derivative kindly provided by Novartis Pharmaceuticals and was used at 10 μM from 8.23 mM stock. The concentrations of inhibitors were chosen based on specificity of ABC transporter inhibition and previous in vitro experimentation (S1 Table).
p-CRKL determined IC50 and western blotting
BCR-ABL1-expressing cells (2×105 cells) or 2×106 patient mononuclear cells (MNCs) were incubated for 2 h at 37°C/5%CO2 with concentrations of nilotinib (NIL) ranging 0–100 μM, imatinib (IM) ranging 0–100 μM and dasatinib (DAS) ranging 0–5000 nM. Following incubation, cells were lysed in Laemmli’s buffer[29] before resolution by 12% SDS-PAGE and electrophoretic transfer to PVDF membrane (GE Healthcare, Buckinghamshire, UK) at 65 mA overnight. Western blotting for phosphorylated CT10 regulator of kinase like (p-CRKL) was performed as previously described[30]. IC50 values (IC50NIL, IC50DAS, IC50IM) were determined as the dose of drug required to reduce p-CRKL levels by 50% and are presented as mean ± SEM. Western blotting for ABCC6 was performed as described in Supplemental methods.
Taqman® array—Human drug transporters
The Taqman® transporter arrays (Cat# 4414118; Thermo Fisher Scientific, Waltham, MA, USA) utilise gene-specific primer and probe sets in order to compare gene expression between treated and untreated samples. The arrays were carried out according to the manufacturer’s instructions.
Real time quantitative polymerase chain reaction (RQ-PCR)
1×107 intermediately resistant cells (produced during TKI resistance generation[5]) were stored in TRIzol stabilization solution (Invitrogen Life Technologies) at -80°C. RNA was extracted using the phenol/chloroform method[31] and cDNA synthesized using random hexamers (GeneWorks, Hindmarsh, SA, Australia) and Superscript II reverse transcriptase (Invitrogen Life Technologies). Primers were designed using Primer Express software v2.0 (Applied Biosystems, Foster City, CA, USA), and sequences were as described in Supplemental methods. Amplification was performed using RT2 real-time SYBR Green/ROX PCR Master Mix (SuperArray Bioscience, Frederick, MD, USA) on a RotorGene real time PCR machine (Corbett Research, San Francisco, CA, USA). Results were analysed using Rotor-Gene 6000 Series software (Corbett Research) and the relative expression levels of the transporters were calculated by the comparative Ct method using the 2ΔΔCt formula to achieve results for relative quantification. The ABCC6 control cell line HepG2 was used as a calibrator and all samples were normalized to the house keeping gene BCR.
Statistics
Statistical tests were performed using the GraphPad Prism 6 statistical software (GraphPad Prism Inc, La Jolla, CA, USA). Normality tests were performed on each data set using the D’Agostino & Pearson omnibus normality test. The Mann-Whitney Rank Sum or the Student’s t-test were used to determine differences between experimental groups depending on whether the data sets failed or passed the normality test, respectively. Differences were considered to be statistically significant when the probability value (p-value) was <0.05.
Results
ABCB1 inhibition does not significantly affect nilotinib-mediated kinase inhibition in patient mononuclear cells
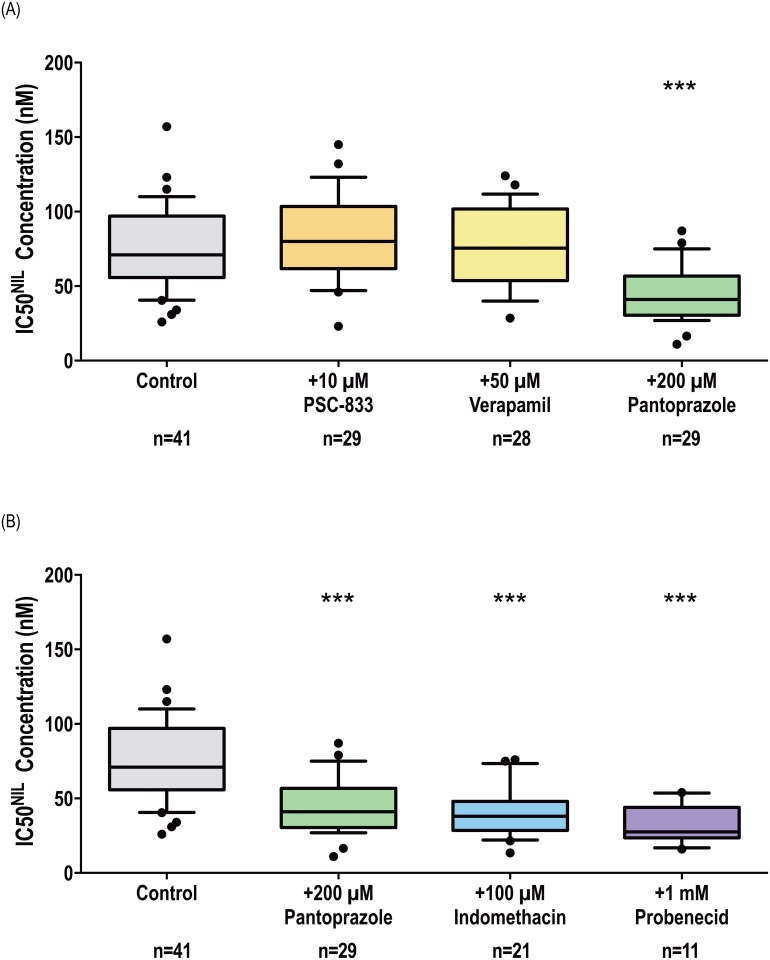
The prognostic value of monitoring early alterations in ABCB1 mRNA expression levels in CML patient cells in order to predict patient response to imatinib has recently been described[6]. ABCB1 overexpression has also been implicated in nilotinib, imatinib and dasatinib resistance development in vitro[5]. For this reason, the effect of three ABCB1 inhibitors (pantoprazole, verapamil, PSC-833, S1 Table) on IC50NIL was assessed in patient MNCs prior to the start of TKI therapy. The IC50 assay is a surrogate for sensitivity to Bcr-Abl kinase inhibition and it was expected that if ABCB1 is involved in the efflux of nilotinib from patient MNCs, inhibition of this transporter should increase concentrations of intracellular nilotinib resulting in a reduction in IC50NIL. Unexpectedly, a significant decrease in IC50NIL was observed only in those cells incubated in the presence of 200 μM pantoprazole (Fig 1A, S1A Fig): 41 nM versus 71 nM in control cells cultured in the absence of inhibitors (p<0.001). The addition of 10 μM PSC-833 and 50 μM verapamil had no effect on IC50NIL: 76 nM and 80 nM respectively (p>0.05;).
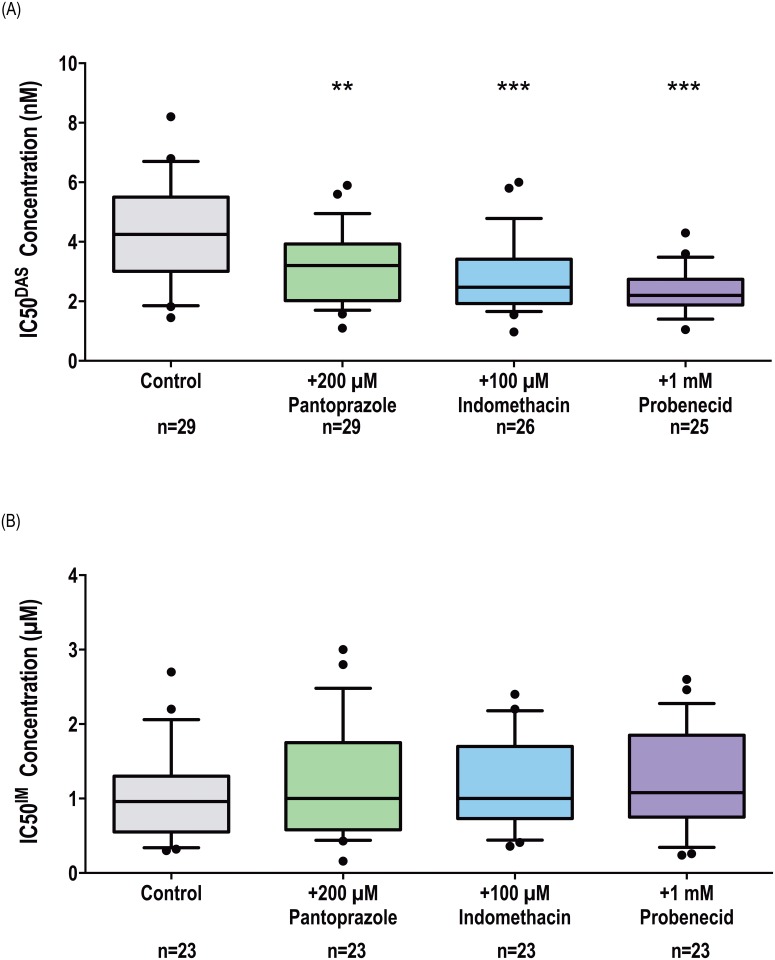
Fig 1. Chemical inhibition of ABCC6 but not ABCB1 increases the efficacy of nilotinib in patient MNCs.
p-CRKL determined IC50NIL was determined via incubating patient MNCs with increasing concentrations of nilotinib in the absence and presence of (A) three ABCB1 inhibitors: PSC-833, verapamil and pantoprazole and (B) three ABCC6 inhibitors: pantoprazole, indomethacin, probenecid. CRKL western blotting was performed to determine the concentration of nilotinib required for 50% Bcr-Abl kinase inhibition (IC50NIL). Representative western blots are shown in S1 Fig. The box plots depict the median, the upper 25th and the lower 75th percentiles while the whiskers encompass the 10th and 90th percentiles. Statistical analyses were performed using Student’s t-test with statistically significant p-values denoted by asterisks (*** p<0.001). NIL = nilotinib.
Nilotinib may be effluxed by other previously unidentified ABC transporters
We have also previously observed a significant decrease in IC50NIL in BCR-ABL1+ cell lines in the presence of pantoprazole and to a lesser extent esomeprazole (dual ABCB1/ABCG2 inhibitors)[22]. Interestingly, comparable results were observed in two different cells lines regardless of ABCB1 expression: K562-Dox cells (ABCB1-overexpressing) and the parental K562 cells (negligible ABCB1/ABCG2). Thus, for the current study we investigated the effect of different concentrations of pantoprazole in a further two cell lines: KU812 (negligible ABCB1/ABCG2) and K562-ABCG2 cells (ABCG2-overexpressing)[27]. Results demonstrated a significant reduction in IC50NIL in the presence of pantoprazole in all cell lines regardless of the ABCB1/ABCG2 expression status that was proportionate to the dose of pantoprazole (Table 1). The greatest reduction occurred in K562 cells (71% reduction in the presence of 500 μM pantoprazole, p<0.001), which express negligible levels of ABCB1 and ABCG2. These data, combined with observations in patient MNCs, suggest there may be other clinically relevant ABC efflux transporter/s that are involved in nilotinib transport and are inhibitable by pantoprazole.
Table 1. The effect of increasing concentrations of pantoprazole on IC50NIL in BCR-ABL1+ cell lines.
| Cell Line | Transporter Expression | IC50NIL (nM) | Decrease (%) | p-value | |
|---|---|---|---|---|---|
| ABCB1 | ABCG2 | ||||
| K562 | |||||
| Control (n = 6) | ✘ | ✘ | 388 | ||
| +50 μM PP (n = 3) | ✘ | ✘ | 254 | 34 | p = 0.012 |
| +200 μM PP (n = 5) | ✘ | ✘ | 217 | 44 | p = 0.002 |
| +500 μM PP (n = 4) | ✘ | ✘ | 114 | 71 | p = 0.0002 |
| K562-Dox | |||||
| Control (n = 5) | ✔ | ✘ | 463 | ||
| +50 μM PP (n = 3) | ✔ | ✘ | 202 | 56 | p = 0.021 |
| +200 μM PP (n = 4) | ✔ | ✘ | 201 | 57 | p = 0.010 |
| +500 μM PP (n = 3) | ✔ | ✘ | 145 | 69 | p = 0.010 |
| K562-ABCG2 | |||||
| Control (n = 6) | ✘ | ✔ | 261 | ||
| +50 μM PP (n = 5) | ✘ | ✔ | 122 | 53 | p = 0.007 |
| +100 μM PP (n = 5) | ✘ | ✔ | 157 | 40 | p = 0.041 |
| +200 μM PP (n = 5) | ✘ | ✔ | 120 | 54 | p = 0.011 |
| KU812 | |||||
| Control (n = 5) | ✘ | ✘ | 305 | ||
| +50 μM PP (n = 5) | ✘ | ✘ | 149 | 51 | p = 0.010 |
| +100 μM PP (n = 5) | ✘ | ✘ | 146 | 52 | p = 0.011 |
| +250 μM PP (n = 5) | ✘ | ✘ | 117 | 62 | p = 0.004 |
Statistical analyses were performed using Student’s t-test; NIL = nilotinib, PP = pantoprazole.
Exposure to nilotinib increases expression of ABCC6 in vitro
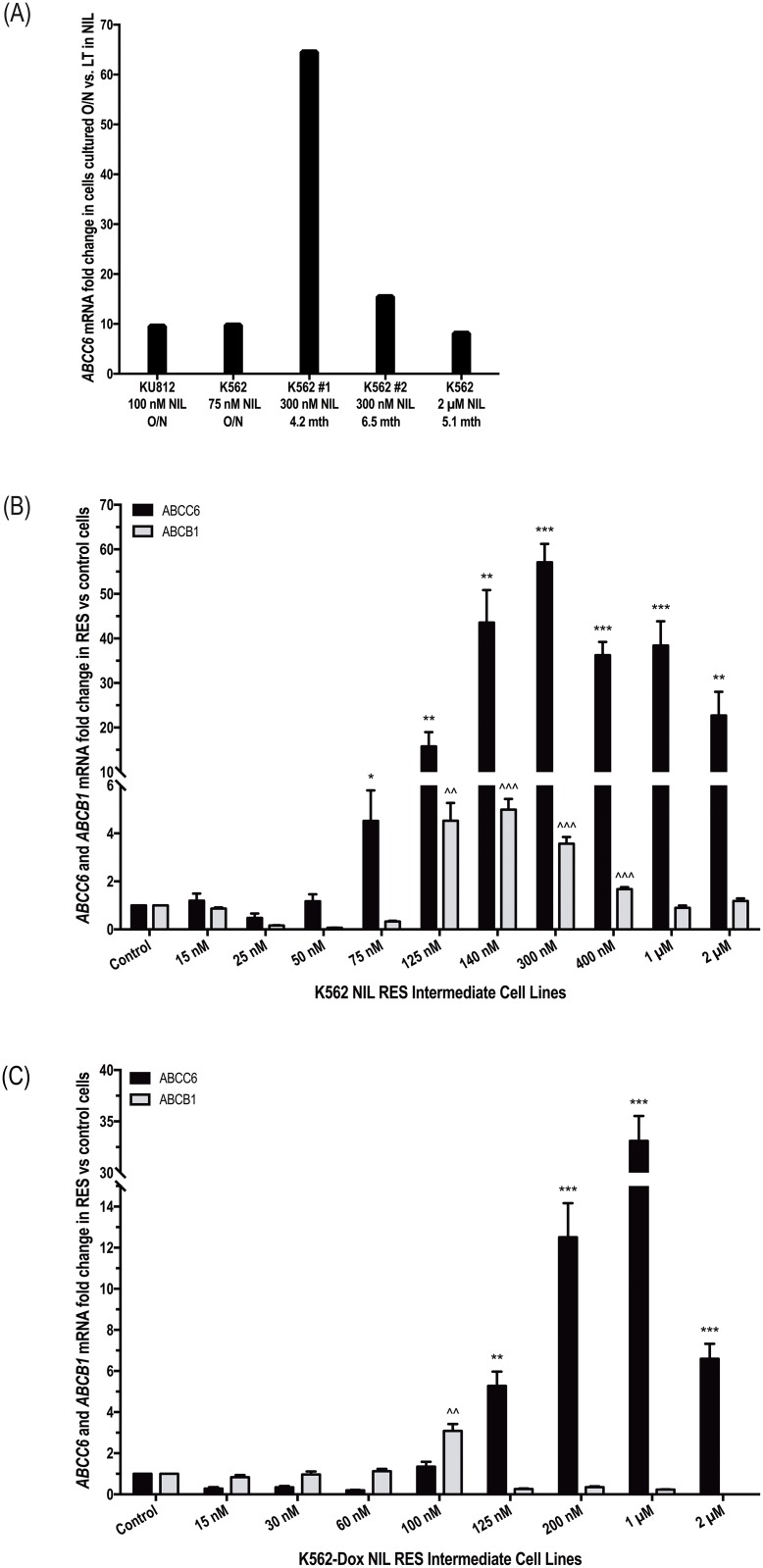
Previous studies have demonstrated that incubation with various cytotoxic agents results in increased mRNA expression of transporters known to be relevant for those drugs[32, 33]. Thus, in order to identify other candidate transporters potentially involved in the efflux of nilotinib, commercially available Taqman® transporter array plates were used to assess mRNA expression levels in BCR-ABL1+ K562 and KU812 cells incubated overnight in the absence and presence of 75 nM and 100 nM nilotinib respectively. Additionally, K562 cells that had been cultured long term in nilotinib[5] were also assessed for alterations in transporter expression compared with control cells (Fig 2A). Results demonstrated a consistent increase in ABCC6 mRNA in response to nilotinib exposure, highlighting ABCC6 as a likely candidate for nilotinib transport. In K562 and KU812 cells exposed transiently to nilotinib, ABCC6 mRNA levels were increased 9.7- and 9.5-fold respectively compared with cells incubated in the absence of nilotinib; in K562 cells exposed long term to 300 nM and 2 μM nilotinib, ABCC6 mRNA levels increased up to 64-fold compared with control cells (Fig 2A). These results were validated through assessment of ABCC6 mRNA levels over the course of nilotinib resistance generation in K562 and K562-Dox intermediately resistant cells[5]. ABCC6 mRNA levels increased significantly at the onset of nilotinib resistance in both cell lines (Fig 2B and 2C). In K562 cells, levels peaked at 57-fold greater in the 300 nM NIL cells compared with control cells (p = 0.002) while in K562-Dox cells the 1 μM NIL cells demonstrated 33-fold greater ABCC6 mRNA levels compared with control cells (p = 0.002).
Fig 2. ABCB1 and ABCC6 mRNA levels increase in concert during development of nilotinib resistance in BCR-ABL1+ cell lines.
(A) Expression levels of ABCC6 mRNA were assessed by Taqman® transporter array in K562 and KU812 cells exposed transiently (overnight, O/N) and long term to nilotinib Expression levels of ABCC6 and ABCB1 mRNA were assessed in (B) K562 and (C) K562-Dox cells gradually made resistant to nilotinib by exposure to increasing concentrations over time. (A) ABCC6 levels were normalized to selected control genes (as determined by Thermo Fisher Scientific DataAssist Software v1.0) and fold change in cells cultured in the presence of nilotinib calculated relative to cells cultured in the absence of nilotinib. (B-C) ABCC6 and ABCB1 levels were normalized to the housekeeping gene BCR and fold change in resistance intermediates calculated relative to control cells (control cell fold change was set at 1). The mRNA expression represents the mean of six independent experiments performed in triplicate. Statistical analyses were performed using Student’s t-test. Statistically significant p-values are denoted by asterisks (ABCC6) and carets (ABCB1) * p<0.05; ** p<0.01; *** p<0.001). Error bars represent SEM. NIL = nilotinib; RES = resistant.
ABCC6 protein is expressed in BCR-ABL1+ cell lines and ABCC6 inhibition increases efficacy of nilotinib in vitro
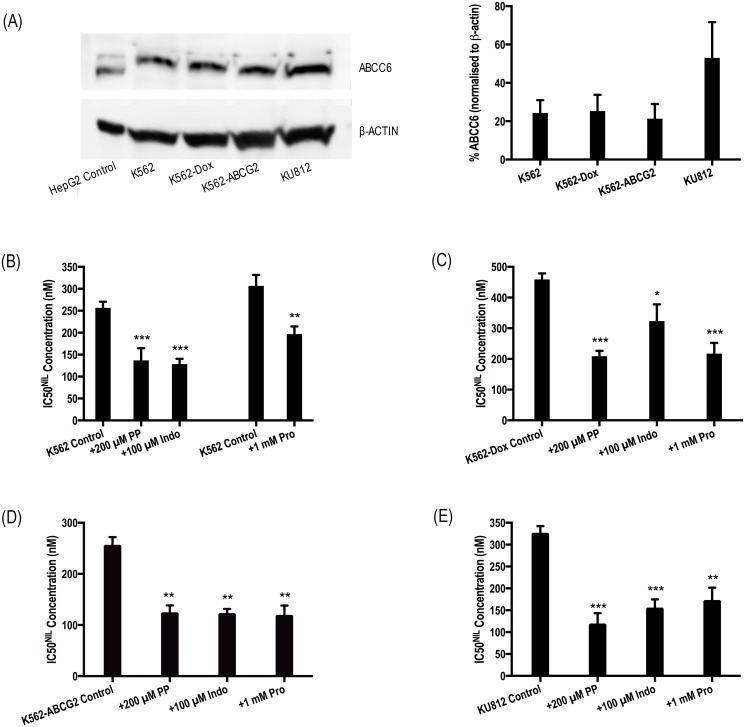
Before ABCC6 could be validated as a likely transporter of nilotinib, confirmation was required of a) ABCC6 expression in BCR-ABL1+ cell lines and b) pantoprazole-mediated inhibition of ABCC6. Indeed, all four cell lines expressed detectable levels of ABCC6 (Fig 3A) and a comprehensive literature search for inhibitor cross reactivity revealed pantoprazole likely inhibits ABCC6 (S1 Table). Thus, the ABCC6:TKI interaction warranted further investigation in cell lines and patient MNCs. Confoundingly, promiscuity in transporter inhibition for drugs such as pantoprazole make it difficult to isolate the transporter upon which a given inhibitor is acting. For this reason, a panel of ABCC6 inhibitors, with overlapping inhibition profiles, was used to ascertain the involvement of ABCC6 in the transport of nilotinib. This approach that has been successfully employed previously to eliminate transporters unlikely to interact with imatinib[34].
Fig 3. Chemical inhibition of ABCC6 increases the efficacy of nilotinib in BCR-ABL1+ cell lines.
(A) Expression levels of ABCC6 protein were assessed in K562, K562-Dox, K562-ABCG2 and KU812 BCR-ABL1+ cell lines. The western blot analyses are representative and the corresponding densitometry analysis represents the mean of three experiments. HepG2 cells were used as a positive control and protein levels were normalised to β-ACTIN. HepG2 cells express two splice variants of ABCC6; BCR-ABL1+ cell lines express the N-glycosylated isoform only. (B) K562 (C) K562-Dox (D) K562-ABCG2 and (E) KU812 cells were incubated with increasing concentrations of nilotinib in the absence and presence of three ABCC6 inhibitors (200 μM pantoprazole, 100 μM indomethacin, 1 mM probenecid). CRKL western blotting was performed to determine the concentration of TKI required for 50% Bcr-Abl kinase inhibition (IC50NIL). Two K562 control bars are shown as experiments in the two groups were performed months apart. Error bars represent SEM. Representative western blots are shown in S2 Fig. NIL = nilotinib; PP = pantoprazole; Indo = Indomethacin; Pro = probenecid.
IC50NIL experiments were repeated in the presence of pantoprazole as well as two additional ABCC6 inhibitors: indomethacin and probenecid. The addition of all inhibitors significantly reduced IC50NIL in all cell lines studied (Fig 3B–3D, S2 Fig). In KU812 cells: IC50NIL was reduced from 325 nM to 117 nM (p<0.001), to 154 nM (p<0.01) and to 171 nM (p<0.05) in the presence of pantoprazole, indomethacin and probenecid respectively. Similar results were observed in K562, K562-Dox and K562-ABCG2 cells. The relevance of ABCC6-mediated transport of nilotinib was then investigated in the patient setting (Fig 1B, S1B Fig). Importantly, when patient MNCs were cultured in the presence of indomethacin or probenecid, a reduction in IC50NIL was observed, confirming observations made in the presence of pantoprazole as well as those made in cell lines. ABCC6 inhibition reduced IC50NIL from 71 nM in cells incubated in the absence of inhibitors to 38 nM and 28 nM in cells cultured with indomethacin and probenecid respectively (p<0.001). Taken together, these results indicate the likely role of ABCC6 in the transport of nilotinib.
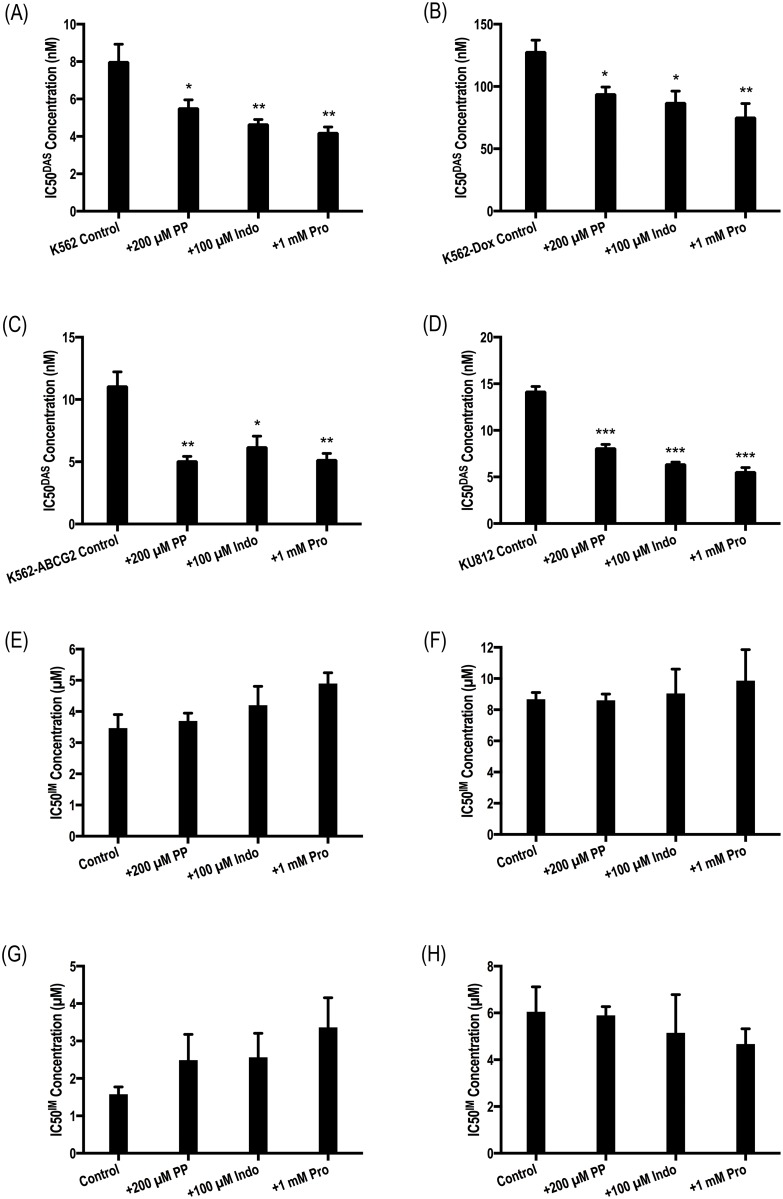
ABCC6 inhibition increases the efficacy of dasatinib, but not imatinib, in both BCR-ABL1+ cell lines and patient MNCs
Analogous experiments testing the effect of ABCC6 inhibitors on the efficacy of dasatinib and imatinib were performed in BCR-ABL1+ cell lines. Data demonstrated that the addition of pantoprazole, indomethacin or probenecid resulted in a significant reduction in IC50DAS in all cell lines tested (p<0.05; Fig 4A–4D). No effect of ABCC6 inhibitors on IC50IM was observed (Fig 4E and 4F) denoting imatinib as an unlikely substrate for ABCC6. The role of ABCC6 in the efflux of dasatinib and imatinib from CML patient MNCs was also investigated (Fig 5A, S5A Fig). Again, IC50 experiments were performed in the absence and presence of pantoprazole, indomethacin and probenecid. Results confirmed those observed in cell lines and demonstrated a significant decrease in IC50DAS in the presence of all three inhibitors: IC50DAS was reduced from 4.3 nM in the absence of inhibitor to 3.2 nM (p = 0.014), 2.5 nM (p<0.001) and 2.2 nM (p<0.001) in the presence of pantoprazole, indomethacin and probenecid respectively. In contrast, equivalent experiments investigating the effect of ABCC6 inhibition on imatinib efficacy in CML patient MNCs demonstrated no change in IC50IM in the absence or presence of ABCC6 inhibition (Fig 5B, S5B Fig): IC50IM in the absence of inhibitor = 0.96 μM versus 1 μM, 1 μM and 1.1 μM (p>0.05) in the presence of pantoprazole, indomethacin and probenecid respectively. Thus, while ABCC6 inhibition significantly increases the efficacy of dasatinib presumably through reduced dasatinib efflux, it is unlikely ABCC6 plays a role in the transport of imatinib in patient MNCs.
Fig 4. Chemical inhibition of ABCC6 increases the efficacy of dasatinib but not imatinib in BCR-ABL1+ cell lines.
(A,E) K562 (B,F) K562-Dox (C,G) K562-ABCG2 and (D,H) KU812 cells were incubated with the indicated concentrations of (A-D) dasatinib and (E-H) imatinib in the absence and presence of three ABCC6 inhibitors (200 μM pantoprazole, 100 μM indomethacin, 1 mM probenecid). CRKL western blotting was performed to determine the concentration of TKI required for 50% Bcr-Abl kinase inhibition (IC50). Error bars represent SEM. Representative western blots are shown in S3 and S4 Figs. IM = imatinib; DAS = dasatinib; PP = pantoprazole; Indo = Indomethacin; Pro = probenecid.
Fig 5. Chemical inhibition of ABCC6 increases the efficacy of dasatinib but not imatinib in patient MNCs.
p-CRKL determined IC50 was determined via incubating patient MNCs with increasing concentrations of (A) dasatinib and (B) imatinib in the absence and presence of three ABCC6 inhibitors: pantoprazole, indomethacin, probenecid. CRKL western blotting was performed to determine the concentration of TKI required for 50% Bcr-Abl kinase inhibition (IC50). Representative western blots are shown in S3 and S4 Figs. The box plots depict the median, the upper 25th and the lower 75th percentiles while the whiskers encompass the 10th and 90th percentiles. Statistical analyses were performed using Student’s t-test with statistically p-values denoted by asterisks (** p<0.01; *** p<0.001). IM = imatinib. DAS = dasatinib.
ABCC6 is the most important transporter in development of nilotinib resistance and contributes to dasatinib resistance in vitro
We have previously examined ABCB1 mRNA expression in cells made resistant to nilotinib[5] and now we compare this with ABCC6 mRNA expression. Data suggest that ABCB1 and ABCC6 work in concert during the development of nilotinib resistance (Fig 2). Interestingly, ABCC6 overexpression appears to contribute to nilotinib resistance to a greater extent than ABCB1. In K562 cells, ABCC6 expression levels increased in concordance with exposure to increasing nilotinib concentrations (up to 57-fold higher than expression levels in control cells, p<0.001) and remained high for the duration of nilotinib dose escalation (Fig 2B). In contrast, while ABCB1 levels increased initially, 5-fold greater levels were observed in cells resistant to 140 nM nilotinib compared with control cells (p<0.001), continued exposure to escalating nilotinib concentrations resulted in reduction of ABCB1 to levels comparable with those observed in control cells (Fig 2B). Similar results were observed during the generation of nilotinib resistance in K562-Dox cells (Fig 2C). Comparable results were obtained for dasatinib resistant cells but not imatinib resistant cells supporting IC50 data and a dasatinib:ABCC6 interaction (S6 Fig).
Discussion
ABCC6 is primarily expressed in the liver and kidney[12, 17, 18] and confers resistance to a number of anti-cancer agents[15]. While it presents a likely and novel candidate to function as a TKI transporter, the ABCC6:TKI relationship has not previously been studied. Here we demonstrate for the first time, that ABCC6 plays a role in the export of nilotinib and dasatinib from CML patient MNCs and that ABCC6 inhibition increases the efficacy of these TKIs. Importantly, ABCC6 inhibition had no effect on imatinib efficacy indicating an unlikely role for this transporter in imatinib efflux and confirming the specificity of the ABCC6:nilotinib/dasatinib interaction. ABCC6 is known to transport substrates anionic in nature[35]. Imatinib predominantly exists in vivo as a cation owing to the large degree of protonation at physiological pH[36]. In contrast, nilotinib and dasatinib exist more often as anions and nilotinib is transported by a number of Organic Anion Transporters (OATs)[37]. These observations make nilotinib and dasatinib likely ABCC6 substrates, a notion supported by the data presented here.
ABCB1 has previously been implicated as an important transporter of TKIs in BCR-ABL1+ cell lines[8] and increases in expression during development of resistance to nilotinib have been observed in vitro[5, 38, 39]. In this study we investigated the impact of ABCB1 inhibitors on nilotinib-mediated Bcr-Abl kinase inhibition (IC50NIL). If nilotinib is indeed transported by ABCB1 one would expect the IC50NIL to decrease in the presence of ABCB1 inhibition. However, results were unexpected with only pantoprazole demonstrating a significant effect on IC50NIL in patient MNCs. There are two possible explanations for this; firstly, nilotinib is not transported by ABCB1, however, this seems unlikely given the large volume of in vitro data supporting ABCB1 transport. Alternatively, ABCB1 may not be a relevant transporter in patient MNCs. This hypothesis is more plausible given the low expression levels of ABCB1 protein our laboratory has observed in routine screening of de novo CML patients (unpublished data). However, high expression of ABCB1 has been demonstrated in primitive CD34+CD38– and CD34+CD38+ CML cells[40] as well as cells at the blood-brain barrier[41]. Thus, ABCB1-mediated TKI efflux may predominantly occur in primitive subsets of cells and in cells at blood-tissue barriers (CNS, brain, testes) enhancing exclusion of TKIs and providing a reservoir of residual leukaemic cells. In contrast, ABCC6-mediated transport of nilotinib may be more relevant in the MNC populations present in patients prior to receiving TKI therapy.
In addition to providing evidence that ABCC6 is the predominant transporter of nilotinib in patient MNCs, this study also suggests that ABCC6 is the most important transporter involved in development of nilotinib resistance. Previous studies have demonstrated that, upon exposure to certain xenobiotics, cells increase mRNA expression of transporters known to interact with the substrate under investigation[32, 33, 42–44]. ABCC6 mRNA expression was investigated in two BCR-ABL1+ cell lines during development of nilotinib resistance in vitro with expression increasing concomitantly upon exposure to escalating nilotinib concentrations and remaining high. In contrast, increased ABCB1 expression appears to provide the initial platform required for development of additional mechanisms of resistance to TKIs in vitro, including the overexpression of ABCC6 presented here. This hypothesis is supported by previous research in both cell lines[5] and primary patient cells[6]. A similar pattern of transporter expression was observed in BCR-ABL1+ cell lines made resistant to dasatinib where increased ABCB1 was followed by persistent overexpression of ABCC6. Increases in ABCC6 expression did not occur during development of imatinib resistance in the cell line models here.
The results presented here offer an explanation for the clinical observation that the concomitant use of a PPI such as pantoprazole results in a better response to nilotinib in CML patients[45]. Those patients receiving the standard 300 mg twice daily (BID) nilotinib therapy who also received a PPI for >50% of their time undergoing nilotinib therapy demonstrated higher rates of Major Molecular Response (MMR, <0.1% BCR-ABL1IS) at 12 months compared with those patients who received nilotinib alone. The difference in MMR rates was even greater in patients receiving a 400 mg BID dose. While superior rates of MMR were observed in both newly diagnosed patients as well as imatinib-resistant or–intolerant patients receiving PPI co-treatment, the differences failed to reach statistical significance. This was likely due to the small sample sizes in each of the patient cohorts. Importantly, our data support those findings and provide justification for a standardized trial comparing the long-term responses to nilotinib with and without co-administration of an ABCC6 inhibitor such as pantoprazole.
Taken together these data support ABCC6-mediated transport of nilotinib and dasatinib in cell lines as well as in patient MNCs. In vitro evidence also suggests that ABCC6 overexpression may play a role in development of resistance to both nilotinib and dasatinib although the contribution to resistance in the clinical setting remains to be elucidated. Concomitant administration of inhibitors of ABC transporters have previously been demonstrated to increase the efficacy of various cytotoxic agents in vitro[46, 47]. Importantly, simultaneous administration of pantoprazole in patients receiving nilotinib therapy has been investigated with no adverse effect on TKI efficacy described[22, 45]; indeed increased rates of MMR were observed. In conclusion, our data suggest that ABCC6 functions as a novel efflux transporter of nilotinib and dasatinib, but not imatinib in BCR-ABL+ cell lines as well as patient MNCs. The difference in ABCC6 affinity we observed between first and second generation inhibitors could potentially be due to the cationic and anionic forms in which imatinib and nilotinib/dasatinib exist at physiological pH. Finally, inhibition of ABCC6 significantly decreases IC50NIL and IC50DAS in patient MNCs, most likely due to an increase in intracellular TKI concentrations. The data presented here provide a strong rationale for a trial investigating the concomitant use of pantoprazole with nilotinib and dasatinib in the treatment of de novo CP-CML patients to enhance the retention of these TKIs in the target MNC population.
Supporting information
p-CRKL determined IC50NIL was determined via incubating patient MNCs with the indicated concentrations of nilotinib in the absence and presence of a) three ABCB1 inhibitors: PSC-833, verapamil and pantoprazole and b) three ABCC6 inhibitors: pantoprazole, indomethacin, probenecid. CRKL western blotting was performed to determine the concentration of nilotinib required for 50% Bcr-Abl kinase inhibition (IC50NIL). The western blots depict one patient and are representative of typical results. NIL = nilotinib; Ver = verapamil; PP = pantoprazole; Indo = Indomethacin; Pro = probenecid.
(TIF)
p-CRKL determined IC50NIL was determined via incubating a) K562 b) K562-Dox c) K562-ABCG2 and d) KU812 cells with the indicated concentrations of nilotinib in the absence and presence of three ABCC6 inhibitors (200 μM pantoprazole, 100 μM indomethacin, 1 mM probenecid). CRKL western blotting was performed to determine the concentration of TKI required for 50% Bcr-Abl kinase inhibition (IC50NIL). The western blots are representative of typical results. The arrows depict approximate nilotinib concentration required for achievement of equal levels of CRKL and p-CRKL proteins. NIL = nilotinib. PP = pantoprazole; Indo = Indomethacin; Pro = probenecid.
(TIF)
p-CRKL determined IC50DAS was determined via incubating a) K562 b) K562-Dox c) K562-ABCG2 and d) KU812 cells with the indicated concentrations of dasatinib in the absence and presence of three ABCC6 inhibitors (200 μM pantoprazole, 100 μM indomethacin, 1 mM probenecid). CRKL western blotting was performed to determine the concentration of TKI required for 50% Bcr-Abl kinase inhibition (IC50DAS). The western blots are representative of typical results. The arrows depict approximate dasatinib concentration required for achievement of equal levels of CRKL and p-CRKL proteins. DAS = dasatinib; PP = pantoprazole; Indo = Indomethacin; Pro = probenecid.
(TIF)
p-CRKL determined IC50IM was determined via incubating a) K562 b) K562-Dox c) K562-ABCG2 and d) KU812 cells with the indicated concentrations of imatinib in the absence and presence of three ABCC6 inhibitors (200 μM pantoprazole, 100 μM indomethacin, 1 mM probenecid). CRKL western blotting was performed to determine the concentration of TKI required for 50% Bcr-Abl kinase inhibition (IC50IM). The western blot analyses are representative of typical results. The arrows depict approximate imatinib concentration required for achievement of equal levels of CRKL and p-CRKL proteins. IM = imatinib; PP = pantoprazole; Indo = Indomethacin; Pro = probenecid.
(TIF)
p-CRKL determined IC50IM and IC50DAS were determined via incubating patient MNCs with the indicated concentrations of a) dasatinib and b) imatinib in the absence and presence of three ABCC6 inhibitors: pantoprazole, indomethacin, probenecid. CRKL western blotting was performed to determine the concentration of imatinib or dasatinib required for 50% Bcr-Abl kinase inhibition (IC50). The western blots depict one patient and are representative of typical results. DAS = dasatinib; IM = imatinib; PP = pantoprazole; Indo = Indomethacin; Pro = probenecid.
(TIF)
Expression levels of ABCC6 and ABCB1 mRNA were assessed in a) K562 b-c) K562-Dox cells and c) KU812 gradually made resistant to a-b) dasatinib or c-d) imatinib by exposure to increasing concentrations of TKI over time. ABCC6 and ABCB1 levels were normalized to the housekeeping gene GUSB and fold change in resistance intermediates calculated relative to control cells (control cell fold change was set at 1). The mRNA expression represents a single experiment performed in triplicate. DAS = dasatinib; IM = imatinib; RES = resistant.
(TIF)
Acknowledgments
This research was supported by the Leukaemia Foundation of Australia from whom Laura Eadie received a PhD scholarship. Thank you to Verity Saunders and Sue Heatley for their critical appraisal of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
LNE performed this research during her doctoral studies which were funded by the Leukaemia Foundation of Australia (LFA). However, the LFA had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2(5):561–6. Epub 1996/05/01. . [DOI] [PubMed] [Google Scholar]
- 2.Weisberg E, Manley PW, Breitenstein W, Bruggen J, Cowan-Jacob SW, Ray A, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7(2):129–41. Epub 2005/02/16. doi: 10.1016/j.ccr.2005.01.007 . [DOI] [PubMed] [Google Scholar]
- 3.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47(27):6658–61. Epub 2004/12/24. doi: 10.1021/jm049486a . [DOI] [PubMed] [Google Scholar]
- 4.Branford S, Rudzki Z, Walsh S, Parkinson I, Grigg A, Szer J, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2003;102(1):276–83. Epub 2003/03/08. doi: 10.1182/blood-2002-09-2896 . [DOI] [PubMed] [Google Scholar]
- 5.Eadie LN, Hughes TP, White DL. ABCB1 Overexpression Is a Key Initiator of Resistance to Tyrosine Kinase Inhibitors in CML Cell Lines. PLoS One. 2016;11(8):e0161470 doi: 10.1371/journal.pone.0161470 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eadie LN, Dang P, Saunders VA, Yeung DT, Osborn MP, Grigg AP, et al. The clinical significance of ABCB1 overexpression in predicting outcome of CML patients undergoing first-line imatinib treatment. Leukemia. 2017;31(1):75–82. doi: 10.1038/leu.2016.179 . [DOI] [PubMed] [Google Scholar]
- 7.Szakacs G, Varadi A, Ozvegy-Laczka C, Sarkadi B. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME-Tox). Drug Discov Today. 2008;13(9–10):379–93. Epub 2008/05/13. doi: 10.1016/j.drudis.2007.12.010 . [DOI] [PubMed] [Google Scholar]
- 8.Eadie LN, Hughes TP, White DL. Interaction of the efflux transporters ABCB1 and ABCG2 with imatinib, nilotinib, and dasatinib. Clin Pharmacol Ther. 2014;95(3):294–306. doi: 10.1038/clpt.2013.208 . [DOI] [PubMed] [Google Scholar]
- 9.Giannoudis A, Davies A, Harris RJ, Lucas CM, Pirmohamed M, Clark RE. The clinical significance of ABCC3 as an imatinib transporter in chronic myeloid leukaemia. Leukemia. 2014;28(6):1360–3. doi: 10.1038/leu.2014.38 . [DOI] [PubMed] [Google Scholar]
- 10.Hupfeld T, Chapuy B, Schrader V, Beutler M, Veltkamp C, Koch R, et al. Tyrosinekinase inhibition facilitates cooperation of transcription factor SALL4 and ABC transporter A3 towards intrinsic CML cell drug resistance. Br J Haematol. 2013;161(2):204–13. doi: 10.1111/bjh.12246 . [DOI] [PubMed] [Google Scholar]
- 11.Sodani K, Patel A, Kathawala RJ, Chen ZS. Multidrug resistance associated proteins in multidrug resistance. Chinese journal of cancer. 2012;31(2):58–72. Epub 2011/11/22. doi: 10.5732/cjc.011.10329 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kool M, van der Linden M, de Haas M, Baas F, Borst P. Expression of human MRP6, a homologue of the multidrug resistance protein gene MRP1, in tissues and cancer cells. Cancer Res. 1999;59(1):175–82. Epub 1999/01/19. . [PubMed] [Google Scholar]
- 13.Slot AJ, Molinski SV, Cole SP. Mammalian multidrug-resistance proteins (MRPs). Essays in biochemistry. 2011;50(1):179–207. doi: 10.1042/bse0500179 . [DOI] [PubMed] [Google Scholar]
- 14.Vanakker OM, Hosen MJ, Paepe AD. The ABCC6 transporter: what lessons can be learnt from other ATP-binding cassette transporters? Frontiers in genetics. 2013;4:203 doi: 10.3389/fgene.2013.00203 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belinsky MG, Chen ZS, Shchaveleva I, Zeng H, Kruh GD. Characterization of the drug resistance and transport properties of multidrug resistance protein 6 (MRP6, ABCC6). Cancer Res. 2002;62(21):6172–7. Epub 2002/11/05. . [PubMed] [Google Scholar]
- 16.Chen CJ, Chin JE, Ueda K, Clark DP, Pastan I, Gottesman MM, et al. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986;47(3):381–9. Epub 1986/11/07. . [DOI] [PubMed] [Google Scholar]
- 17.Scheffer GL, Hu X, Pijnenborg AC, Wijnholds J, Bergen AA, Scheper RJ. MRP6 (ABCC6) detection in normal human tissues and tumors. Lab Invest. 2002;82(4):515–8. Epub 2002/04/16. . [DOI] [PubMed] [Google Scholar]
- 18.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab Pharmacokinet. 2005;20(6):452–77. Epub 2006/01/18. . [DOI] [PubMed] [Google Scholar]
- 19.Boraldi F, Quaglino D, Croce MA, Garcia Fernandez MI, Tiozzo R, Gheduzzi D, et al. Multidrug resistance protein-6 (MRP6) in human dermal fibroblasts. Comparison between cells from normal subjects and from Pseudoxanthoma elasticum patients. Matrix Biol. 2003;22(6):491–500. Epub 2003/12/12. . [DOI] [PubMed] [Google Scholar]
- 20.Ilias A, Urban Z, Seidl TL, Le Saux O, Sinko E, Boyd CD, et al. Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6). J Biol Chem. 2002;277(19):16860–7. Epub 2002/03/07. doi: 10.1074/jbc.M110918200 . [DOI] [PubMed] [Google Scholar]
- 21.Chen ZS, Tiwari AK. Multidrug resistance proteins (MRPs/ABCCs) in cancer chemotherapy and genetic diseases. FEBS J. 2011;278(18):3226–45. Epub 2011/07/12. doi: 10.1111/j.1742-4658.2011.08235.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White DL, Eadie LN, Saunders VA, Hiwase DK, Hughes TP. Proton pump inhibitors significantly increase the intracellular concentration of nilotinib, but not imatinib in target CML cells. Leukemia. 2013;27(5):1201–4. doi: 10.1038/leu.2012.295 . [DOI] [PubMed] [Google Scholar]
- 23.Luciani F, Spada M, De Milito A, Molinari A, Rivoltini L, Montinaro A, et al. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst. 2004;96(22):1702–13. Epub 2004/11/18. doi: 10.1093/jnci/djh305 . [DOI] [PubMed] [Google Scholar]
- 24.Pauli-Magnus C, Rekersbrink S, Klotz U, Fromm MF. Interaction of omeprazole, lansoprazole and pantoprazole with P-glycoprotein. Naunyn Schmiedebergs Arch Pharmacol. 2001;364(6):551–7. Epub 2002/01/05. . [DOI] [PubMed] [Google Scholar]
- 25.De Milito A, Luciani F, Fais S. How to Overcome Cisplatin Resistance Through Proton Pump Inhibitors In: Bonetti A, Leone R, Muggia F, Howell S, editors. Platinum and Other Heavy Metal Compounds in Cancer Chemotherapy. Cancer Drug Discovery and Development: Humana Press; 2009. p. 109–14. [Google Scholar]
- 26.Hiwase DK, Saunders V, Hewett D, Frede A, Zrim S, Dang P, et al. Dasatinib cellular uptake and efflux in chronic myeloid leukemia cells: therapeutic implications. Clin Cancer Res. 2008;14(12):3881–8. Epub 2008/06/19. 14/12/3881 doi: 10.1158/1078-0432.CCR-07-5095 . [DOI] [PubMed] [Google Scholar]
- 27.Eadie LN, Saunders VA, Hughes TP, White DL. Degree of kinase inhibition achieved in vitro by imatinib and nilotinib is decreased by high levels of ABCB1 but not ABCG2. Leuk Lymphoma. 2013;54(3):569–78. doi: 10.3109/10428194.2012.715345 . [DOI] [PubMed] [Google Scholar]
- 28.Tang C, Schafranek L, Watkins DB, Parker WT, Moore S, Prime JA, et al. Tyrosine kinase inhibitor resistance in chronic myeloid leukemia cell lines: investigating resistance pathways. Leuk Lymphoma. 2011;52(11):2139–47. Epub 2011/07/02. doi: 10.3109/10428194.2011.591013 . [DOI] [PubMed] [Google Scholar]
- 29.Nichols GL, Raines MA, Vera JC, Lacomis L, Tempst P, Golde DW. Identification of CRKL as the constitutively phosphorylated 39-kD tyrosine phosphoprotein in chronic myelogenous leukemia cells. Blood. 1994;84(9):2912–8. . [PubMed] [Google Scholar]
- 30.White D, Saunders V, Lyons AB, Branford S, Grigg A, To LB, et al. In vitro sensitivity to imatinib-induced inhibition of ABL kinase activity is predictive of molecular response in patients with de novo CML. Blood. 2005;106(7):2520–6. Epub 2005/06/16. 2005-03-1103 doi: 10.1182/blood-2005-03-1103 . [DOI] [PubMed] [Google Scholar]
- 31.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162(1):156–9. . [DOI] [PubMed] [Google Scholar]
- 32.Chaudhary PM, Roninson IB. Induction of multidrug resistance in human cells by transient exposure to different chemotherapeutic drugs. J Natl Cancer Inst. 1993;85(8):632–9. Epub 1993/04/21. . [DOI] [PubMed] [Google Scholar]
- 33.Herzog CE, Tsokos M, Bates SE, Fojo AT. Increased mdr-1/P-glycoprotein expression after treatment of human colon carcinoma cells with P-glycoprotein antagonists. J Biol Chem. 1993;268(4):2946–52. Epub 1993/02/05. . [PubMed] [Google Scholar]
- 34.Thomas J, Wang L, Clark RE, Pirmohamed M. Active transport of imatinib into and out of cells: implications for drug resistance. Blood. 2004;104(12):3739–45. Epub 2004/08/19. doi: 10.1182/blood-2003-12-4276 . [DOI] [PubMed] [Google Scholar]
- 35.Zhou SF, Wang LL, Di YM, Xue CC, Duan W, Li CG, et al. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr Med Chem. 2008;15(20):1981–2039. Epub 2008/08/12. . [DOI] [PubMed] [Google Scholar]
- 36.Szakacs Z, Beni S, Varga Z, Orfi L, Keri G, Noszal B. Acid-base profiling of imatinib (gleevec) and its fragments. J Med Chem. 2005;48(1):249–55. Epub 2005/01/07. doi: 10.1021/jm049546c . [DOI] [PubMed] [Google Scholar]
- 37.Khurana V, Minocha M, Pal D, Mitra AK. Role of OATP-1B1 and/or OATP-1B3 in hepatic disposition of tyrosine kinase inhibitors. Drug metabolism and drug interactions. 2014;29(3):179–90. doi: 10.1515/dmdi-2013-0062 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kosztyu P, Dolezel P, Mlejnek P. Can P-glycoprotein mediate resistance to nilotinib in human leukaemia cells? Pharmacological research: the official journal of the Italian Pharmacological Society. 2013;67(1):79–83. doi: 10.1016/j.phrs.2012.10.012 . [DOI] [PubMed] [Google Scholar]
- 39.Mahon FX, Hayette S, Lagarde V, Belloc F, Turcq B, Nicolini F, et al. Evidence that resistance to nilotinib may be due to BCR-ABL, Pgp, or Src kinase overexpression. Cancer Res. 2008;68(23):9809–16. Epub 2008/12/03. 68/23/9809 doi: 10.1158/0008-5472.CAN-08-1008 . [DOI] [PubMed] [Google Scholar]
- 40.Jiang X, Zhao Y, Smith C, Gasparetto M, Turhan A, Eaves A, et al. Chronic myeloid leukemia stem cells possess multiple unique features of resistance to BCR-ABL targeted therapies. Leukemia. 2007;21(5):926–35. Epub 2007/03/03. 2404609 doi: 10.1038/sj.leu.2404609 . [DOI] [PubMed] [Google Scholar]
- 41.Loscher W, Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2(1):86–98. Epub 2005/02/18. doi: 10.1602/neurorx.2.1.86 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burger H, van Tol H, Brok M, Wiemer EA, de Bruijn EA, Guetens G, et al. Chronic imatinib mesylate exposure leads to reduced intracellular drug accumulation by induction of the ABCG2 (BCRP) and ABCB1 (MDR1) drug transport pumps. Cancer Biol Ther. 2005;4(7):747–52. Epub 2005/06/23. . [DOI] [PubMed] [Google Scholar]
- 43.Abolhoda A, Wilson AE, Ross H, Danenberg PV, Burt M, Scotto KW. Rapid activation of MDR1 gene expression in human metastatic sarcoma after in vivo exposure to doxorubicin. Clin Cancer Res. 1999;5(11):3352–6. Epub 1999/12/10. . [PubMed] [Google Scholar]
- 44.Riva MA, Tascedda F, Lovati E, Racagni G. Regulation of NMDA receptor subunit messenger RNA levels in the rat brain following acute and chronic exposure to antipsychotic drugs. Brain Res Mol Brain Res. 1997;50(1–2):136–42. Epub 1997/12/24. . [DOI] [PubMed] [Google Scholar]
- 45.Yin OQ, Giles FJ, Baccarani M, le Coutre P, Chiparus O, Gallagher N, et al. Concurrent use of proton pump inhibitors or H2 blockers did not adversely affect nilotinib efficacy in patients with chronic myeloid leukemia. Cancer Chemother Pharmacol. 2012;70(2):345–50. Epub 2012/05/25. doi: 10.1007/s00280-012-1881-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slater LM, Sweet P, Stupecky M, Gupta S. Cyclosporin A reverses vincristine and daunorubicin resistance in acute lymphatic leukemia in vitro. J Clin Invest. 1986;77(4):1405–8. Epub 1986/04/01. doi: 10.1172/JCI112450 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981;41(5):1967–72. Epub 1981/05/01. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
p-CRKL determined IC50NIL was determined via incubating patient MNCs with the indicated concentrations of nilotinib in the absence and presence of a) three ABCB1 inhibitors: PSC-833, verapamil and pantoprazole and b) three ABCC6 inhibitors: pantoprazole, indomethacin, probenecid. CRKL western blotting was performed to determine the concentration of nilotinib required for 50% Bcr-Abl kinase inhibition (IC50NIL). The western blots depict one patient and are representative of typical results. NIL = nilotinib; Ver = verapamil; PP = pantoprazole; Indo = Indomethacin; Pro = probenecid.
(TIF)
p-CRKL determined IC50NIL was determined via incubating a) K562 b) K562-Dox c) K562-ABCG2 and d) KU812 cells with the indicated concentrations of nilotinib in the absence and presence of three ABCC6 inhibitors (200 μM pantoprazole, 100 μM indomethacin, 1 mM probenecid). CRKL western blotting was performed to determine the concentration of TKI required for 50% Bcr-Abl kinase inhibition (IC50NIL). The western blots are representative of typical results. The arrows depict approximate nilotinib concentration required for achievement of equal levels of CRKL and p-CRKL proteins. NIL = nilotinib. PP = pantoprazole; Indo = Indomethacin; Pro = probenecid.
(TIF)
p-CRKL determined IC50DAS was determined via incubating a) K562 b) K562-Dox c) K562-ABCG2 and d) KU812 cells with the indicated concentrations of dasatinib in the absence and presence of three ABCC6 inhibitors (200 μM pantoprazole, 100 μM indomethacin, 1 mM probenecid). CRKL western blotting was performed to determine the concentration of TKI required for 50% Bcr-Abl kinase inhibition (IC50DAS). The western blots are representative of typical results. The arrows depict approximate dasatinib concentration required for achievement of equal levels of CRKL and p-CRKL proteins. DAS = dasatinib; PP = pantoprazole; Indo = Indomethacin; Pro = probenecid.
(TIF)
p-CRKL determined IC50IM was determined via incubating a) K562 b) K562-Dox c) K562-ABCG2 and d) KU812 cells with the indicated concentrations of imatinib in the absence and presence of three ABCC6 inhibitors (200 μM pantoprazole, 100 μM indomethacin, 1 mM probenecid). CRKL western blotting was performed to determine the concentration of TKI required for 50% Bcr-Abl kinase inhibition (IC50IM). The western blot analyses are representative of typical results. The arrows depict approximate imatinib concentration required for achievement of equal levels of CRKL and p-CRKL proteins. IM = imatinib; PP = pantoprazole; Indo = Indomethacin; Pro = probenecid.
(TIF)
p-CRKL determined IC50IM and IC50DAS were determined via incubating patient MNCs with the indicated concentrations of a) dasatinib and b) imatinib in the absence and presence of three ABCC6 inhibitors: pantoprazole, indomethacin, probenecid. CRKL western blotting was performed to determine the concentration of imatinib or dasatinib required for 50% Bcr-Abl kinase inhibition (IC50). The western blots depict one patient and are representative of typical results. DAS = dasatinib; IM = imatinib; PP = pantoprazole; Indo = Indomethacin; Pro = probenecid.
(TIF)
Expression levels of ABCC6 and ABCB1 mRNA were assessed in a) K562 b-c) K562-Dox cells and c) KU812 gradually made resistant to a-b) dasatinib or c-d) imatinib by exposure to increasing concentrations of TKI over time. ABCC6 and ABCB1 levels were normalized to the housekeeping gene GUSB and fold change in resistance intermediates calculated relative to control cells (control cell fold change was set at 1). The mRNA expression represents a single experiment performed in triplicate. DAS = dasatinib; IM = imatinib; RES = resistant.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.