Abstract
While directed migration may have evolved to escape nutrient depletion, it has been adopted for an extensive range of physiological events during development and in the adult. The subversion of these movements results in disease. Though the mechanisms of propulsion and sensing are extremely diverse, most cells move by extending actin-filled protrusions called macropinosomes, pseudopodia, or lamellipodia or by extension of blebs. In addition to motility, directed migration involves polarity and directional sensing. The hundreds of gene products involved in these processes are organized into networks of parallel and interconnected pathways. Many of these components are activated or inhibited coordinately with stimulation and on each spontaneously extended protrusion. Moreover, these networks display hallmarks of excitability, including “all-or-nothing” responsiveness and wave propagation. Cellular protrusions result from signal transduction waves which propagate outwardly from an origin and drive cytoskeletal activity. The range of the propagating waves and hence the size of the protrusions can be altered by lowering or raising the threshold for network activation, with larger and wider protrusions favoring gliding or oscillatory behavior over amoeboid migration. Here, we evaluate the variety of models of excitable networks controlling directed migration and outline critical tests. We also discuss the utility of this emerging view in producing cell migration and in integrating the various extrinsic cues that direct migration.
Keywords: Chemotaxis, electrotaxis, shear stress, biochemical oscillations, inflammation, metastasis
Diversity and Importance of Directed Cell Migration
Nearly all cells in living organisms move spontaneously and with direction from extrinsic cues, although the mechanisms of propulsion and sensing are extremely diverse. It is likely that cell migration initially evolved to escape nutrient depletion although it has been adopted for an enormous variety of fascinating processes. Bacteria and many free-living eukaryotic cells such as protozoa move with flagella, cilia, or related internal or external appendages. Other free living cells, such as amoebae, and most metazoan cells move with morphological extensions such as pseudopodia, lamellipodia, or blebs although many have cilia and some, such as sperm, rely on flagella for propulsion (Figure 1). A multiplicity of extrinsic cues direct migration, including light (Armitage and Hellingwerf, 2003), chemicals (Tessier-Lavigne, 1994) (Bagorda and Parent, 2008), mechanical forces (Lo et al., 2000; Harland et al., 2011), electric fields (Zhao et al., 2006; Gao et al., 2011; Cortese et al., 2014), and temperature (Whitaker and Poff, 1980; Ramot et al., 2008). Some of these cues are illustrated in Supplemental Figure 1. Cells are capable of integrating these cues which come from the environment as well as other cells (see supplemental Table 1) (Rørth, 2011) (Haeger et al., 2015).
Figure 1.
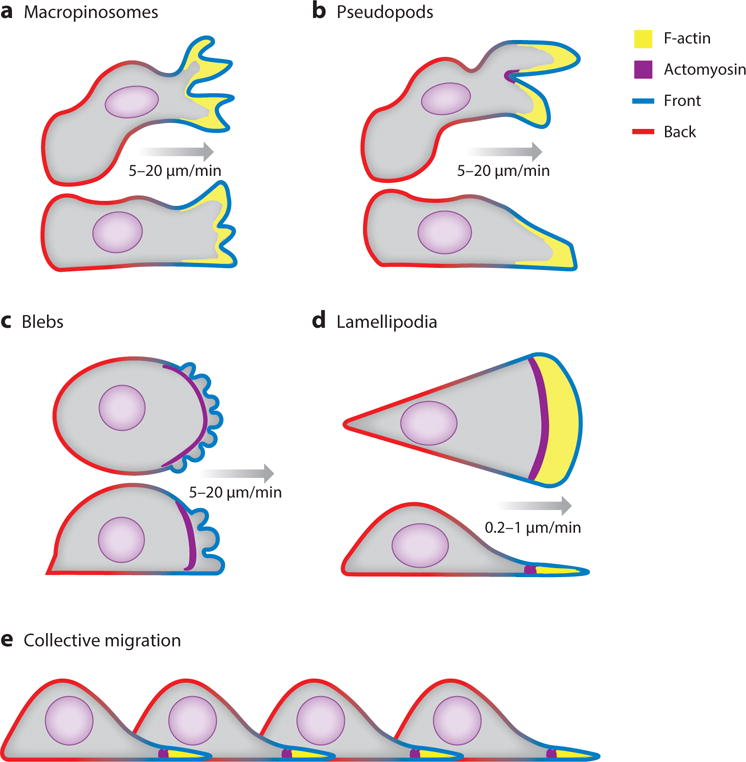
The diverse array of migratory cell projections that project the cell forward are diagrammed in coronal and sagittal slice. Green membrane represents the “front” and red represents the “back” In these cartoons, only the polymerizing F-actin and contractile actomyosin at the leading edge of cells is highlighted. A) Macropinosomes are wide cup-like shaped structures at the top and sides of the cell. B) Pseudopods are narrower protrusions usually found closer to the substrate. C) Blebs are a result of the plasma membrane detaching from the actomyosin cortex due to contractile pressure. D) Lamellipodia are sheet like structures containing distinct actin and actomyosin zones. E) Collective migration of cells connected and partially driven by cryptic lamellipodia.
Cell migration is critical for an extensive range of physiological events. During metazoan development, the concerted movements of cell sheets bring about gastrulation (Yang et al., 2002; Leptin, 2005; Keller, 2005) and neurulation (Theveneau and Mayor, 2012) and groups of cells migrate coordinately during the formation of organs and glands (Montell, 2008). Single neural crest cells travel to distal sites, participating in a wide array or different tissues and germ cells traverse through the embryo to find the gonad (Blaser et al., 2006) (Richardson and Lehmann, 2010). Neural precursors and glial cells (Klämbt, 2009)migrate directionally while neuronal growth cones, tethered to growing axons, seek specific targets (Wen and Zheng, 2006)In the adult, immune cells traverse the vessels and lymphatics and seek invading substances (Weninger et al., 2014; Nourshargh and Alon, 2014) fibroblasts and keratinocytes close wounds (Shaw and Martin, 2009) regenerative stem cells mobilize from niches (Baumann, 2014; Wright et al., 2001), and neurons remodel connections.
When these orchestrated movements occur improperly or are subverted, it results in disease. For example, defects in leukocyte migration cause sarcoidosis, infections, Wiscott-Aldrich Syndrome, and other leukopenias (Lakshman and Finn, 2001; Moulding et al., 2013). Excessive inflammatory responses, in which cellular accumulations fail to resolve, underlie the many disorders including atherosclerosis, asthma, arthritis, periodontal disease, as well as much of injury-associated pain (Lakshman and Finn, 2001). Top on the list of disease processes involving cell migration is cancer metastasis in which cells move away from primary tumors or enter the circulation and later extravasate to colonize new sites (Condeelis et al., 2005; Reymond et al., 2013).
There are salient similarities and differences in the migration behaviors of different cells (Figure 1; supplemental Table 1). Most eukaryotic cells move by extending actin-filled protrusions at the cell front coupled with acto-myosin based contraction, usually at the rear of the cell or the base of the projections. Variations on this general scheme can give rise to a diversity of migration modes. Amoeboid cells, such as leukocytes and some metastatic cancer cells, rhythmically extend and retract defined actin-filled pseudopodia producing intermittent advances in the cell front. Under some conditions, amoeboid motion can be characterized by the extension of cytoplasmic extensions called blebs in which the actin polymerization is weakened relative to the acto- myosin contraction (Yoshida and Soldati, 2006; Blaser et al., 2006). Primordial germ cells (Richardson and Lehmann, 2010; Blaser et al., 2006) and some metastatic cancer cells exclusively deploy blebs (Wolf et al., 2003; Fackler and Grosse, 2008). Keratinocytes or keratocytes move with a rocking, gliding motion, led by a wide, flat actin-filled protrusion which often covers nearly three-quarters of the cell perimeter (Keren and Theriot, 2008; Barnhart et al., 2010). Amoeboid and keratocyte-like motility is usually rapid with cells able to move at 15 to 30 μm/min (Anderson and Cross, 2000). Fibroblastic or mesenchymal migration involves gliding lamellipodia and is typically much slower with cells advancing at 0.2 to 1 μm/min (Hou et al., 2012). These disparate speeds are reflected in the transient attachments made by amoeboid and keratocyte-like cells versus the developed focal adhesions that fibroblastic cells make to the extracellular matrix (Satulovsky et al., 2008; Parsons et al., 2010; Mogilner and Keren, 2009). Ostensibly similar motions are also observed in collected groups and sheets of epithelial cells that form structures during development and regeneration. These movements are highly coordinated, likely through mechanical forces exerted directly through cell-cell attachments or adhesions to extracellular matrix (Gupton and Waterman-Storer, 2006)This review will focus primarily on the signal transduction events involved in amoeboid migration; however, many of the molecular events and concepts described below can be extended to account for other cell behaviors, including those seen during collective migration and movement through three-dimensional environments.
With this vast assortment of migratory modes and mechanisms and the large number of independent investigators studying them, there is a need to define some of the major terms. Directed migration can be described conceptually as involving motility, polarity, and directional sensing (Figure 2). Motility refers to the ability of a cell to extend protrusions, coordinated with appropriate contractions and attachments, and thereby translocate. Polarity indicates the relatively stable axis with a definite front and rear displayed by many cells which provides persistence of movement and is distinct from the momentary asymmetry displayed by a motile cell extending a protrusion. Directional sensing denotes the capacity of a cell to sense a spatially heterogeneous cue and respond biochemically independently of motility or polarity. In directed cell migration, these processes occur concurrently and coordinately. Additional terms that are useful and prevalent in describing directed migration are included in a glossary (supplemental Table 2).
Figure 2.
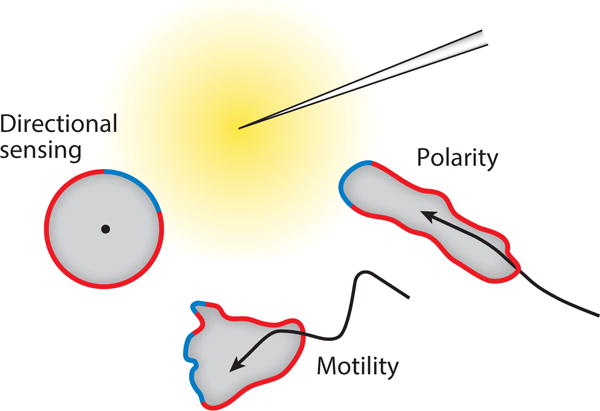
The three distinct processes that coordinate to bring about directed migration towards the chemoattractant gradient (yellow). The region of the cortex facing the needle forms the “front” (green) while the quiescent “back” is demarcated by red.
Complexity of Signal Transduction Events Involved in Directed Cell Migration
The list of signal transduction events associated with directed cell migration grows continuously. Much of this information is derived from genetic, biochemical, and biosensor analysis of Dictyostelium cells responding to chemoattractant (supplemental Table 3). Many external signals feed into a network of pathways (Figure 3). cARs and FARs (cAMP and folic acid GPCRs) and associated G-proteins are essential for migration toward the respective chemical gradients and trigger many signal transduction events, which as far as has been tested are also locally activated under guidance of electric fields (Zhao et al., 2006; Zhao et al., 2002; Miao et al., 2017; Meng et al., 2011; Allen et al., 2013) or shear force (Artemenko et al., 2016; Décave et al., 2003). Importantly, network events are activated and cells move even in the apparent absence of external cues (Sasaki et al., 2007; Bosgraaf and Van Haastert, 2009; Arai et al., 2010).
Figure 3.
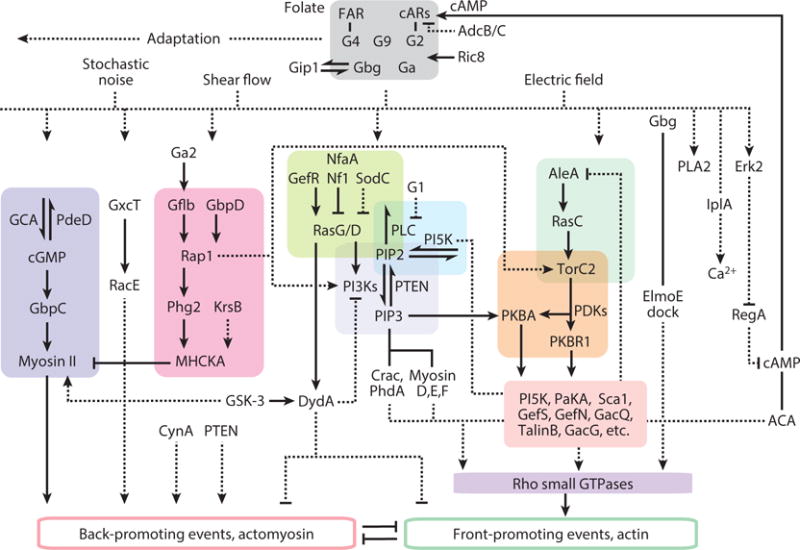
Network of signal transduction pathways involved in directed cell migration in Dictyostelium. Architecture is based on biochemical and genetic analysis and reflects an extensive series of experiments by independent investigators. In most cases connections represent interpretations of phenotypes or stimulus-induced biochemical and biosensor behavior, contrasted to wild type, in cells carrying single or multiple gene deletions. Half arrows are substrate-product relationships; solid connectors represent direct interactions between components, dashed connectors are inferred or indirect links. The references supporting these interactions are included in Supplemental Table 3.
The network has a number of notable features. First, it contains multiple parallel or compensating pathways as evidenced by the fact that individual disruptions at many nodes are relatively inconsequential whereas activating perturbations at the same ones produce striking phenotypes. For example, cells can move in the absence of PI3K signaling (Weiger and Parent, 2012; Takeda et al., 2007; Hoeller and Kay, 2007; Chen et al., 2003) but ectopic production of PIP3 is sufficient to initiate protrusions (Weiner et al., 2002; Kakumoto and Nakata, 2013; Inoue and Meyer, 2008) and excess accumulation of PIP3 leads to multiple persistent extensions (Sarraj et al., 2009; Iijima and Devreotes, 2002). Inhibition of three or four parallel pathways is required for a significant loss of protrusion formation (Supplemental Video 1) (Veltman et al., 2008; Chen et al., 2007; Artemenko et al., 2016). Second, increments in chemoattractant trigger transient changes at many points in the network, with characteristic time courses (Figure 3; Supplemental Video 2 (Caterina and Devreotes, 1991). For example, after a delay of a few seconds, PIP3 rises to a peak at 15–20 seconds, rapidly declines, and increases again in a series of secondary peaks during the next few minutes (Huang et al., 2013; Chen et al., 2003). While the initial peak of F-actin occurs before that of PIP3, it is also followed by secondary peaks (Chen et al., 2003). The initial transient burst of F-actin is global causing cells to freeze and then contract whereas the secondary peaks are localized producing spreading protrusions (Postma et al., 2003; Condeelis et al., 1990; Futrelle et al., 1982; Xiong et al., 2010). Other responses, such as PKB activation, are delayed with respect to PIP3 but most display the characteristic initial and secondary peaks (Kamimura et al., 2008). Whereas most of these events start at a basal level and increase, others begin at an elevated level and decrease (Figure 4). For example, PTEN transiently leaves the membrane and then returns (Iijima and Devreotes, 2002; Funamoto et al., 2002). Remarkably, the same signal transduction events with the similar time courses are triggered by a brief application of shear force (Artemenko et al., 2016). Third, most of these actions take place at the membrane or cortex. In migrating and chemotaxing cells, events such as Ras and PI3K activity occur at the tips of protrusions and are loosely referred to as “front” whereas others such as PTEN dissociate from the pseudopods and are designated as “back” (Figure 4; Supplemental Video 3). A list of “front” and “back” events is shown in supplemental Table 4.
Figure 4.
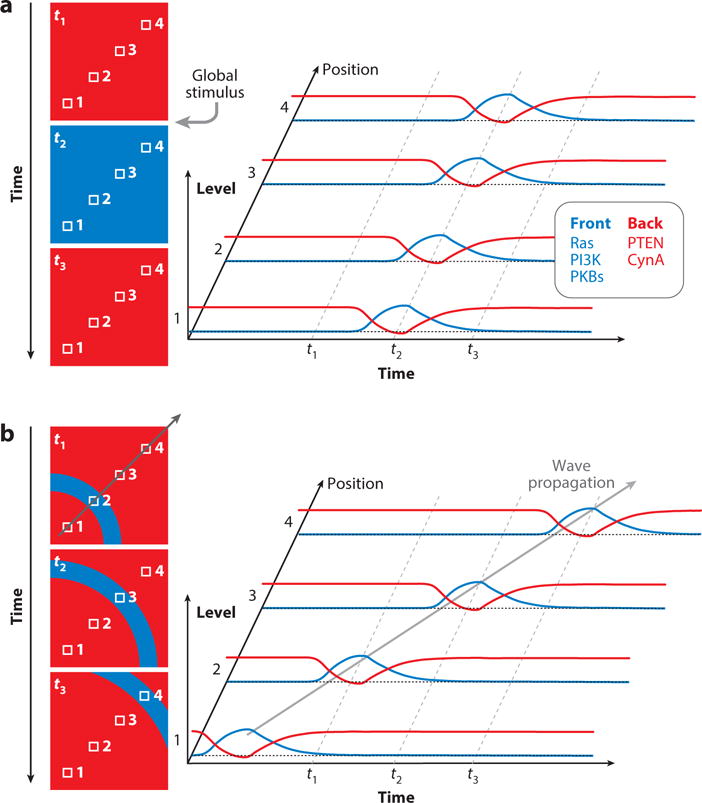
Correlation between spatial and temporal activities of front (green) and back (red) proteins. A. The colored boxes on the left represent sequential time instants. The small numbered white squares in each box denote spatial references. The plots on the right show how the global stimulus manifests as a time course at each spatial position and how the front and back proteins or biosensors (representative activities shown in the adjoining box) associate and dissociate, respectively. B. Same format as A, but showing the position of a propagating wave at three sequential times. This causes the temporal protein activities at each position to occur sequentially, in contrast to the synchronized events triggered by the global stimulus (A).
Reporting on migration associated “signaling” events in mammalian cells is somewhat limited in depth, although there are extensive observations. Many GPCRs, TRKs, and other receptors influence migration. A large set of chemokines and chemokine receptors are functionally parallel to cARs and FAR in Dictyostelium (Jin et al., 2008). Elegant studies in macrophages have shown that local activation of G-proteins is sufficient for directed migration (Supplemental Video 4) (O’Neill et al., 2016) which is consistent with earlier indirect studies in Dictyostelium and neutrophils (Wu et al., 1995; Neptune et al., 1999). Furthermore, studies of the role of Ras and PIP3 activation in mammalian cell migration are largely consistent with the Dictyostelium model (Artemenko et al., 2014). Research has focused significantly on the role of small GTPases cdc42, Rac, and Rho as activators of scAR/WAVE proteins and formins and, consequently, as key regulators of cellular protrusions. Unfortunately, direct parallels are complicated by the presence of 15 Rho family proteins in Dictyostelium which do not readily fall into the three classes based on sequence (Lim et al., 2002). Recent evidence suggests that Dictyostelium Rac 1A/C and Rac E, may function similarly to mammalian Rac1 and RhoA, respectively (Wang et al., 2013; Filić et al., 2012). With more parallel observations, such as the involvement of Ras and Rap family proteins (Khanna et al., 2016), TorC2, and PKBs upstream of the Rho family G-proteins, understanding of the networks in the different systems appears to be converging (Filić et al., 2012). However, there are persistent differences, for instance with respect to the regulation of myosin where cGMP plays a major role in Dictyostelium while ROCK and MLC kinases are key in many mammalian cells (Vicente-Manzanares et al., 2009; Bosgraaf and van Haastert, 2006).
The Signal Transduction “Excitable” Network or STEN
The triggering of these events in the signal transduction network displays properties of biochemical pexcitability, including “all-or-nothing” responses to suprathreshold stimuli and a refractory period found in Dictyostelium. First, with saturating concentrations of chemoattractant the initial peak of Ras or PI3K activity is the same whether the stimulus duration is 2 or 60 seconds (Huang et al., 2013). Second, while the response to increasing doses is graded, micrometer-sized patches behave as “all-or-nothing” elements (Nishikawa et al., 2014). Different sensitivities among patches as well as individual cells feed into the dose-response behavior. More sensitive methods have detected smaller, perhaps subthreshold, patches Ras activity that do not expand (van Haastert et al., 2017). Third, refractory periods for chemoattractant and shear force-elicited PIP3 production were demonstrated by applying short, paired stimuli and monitoring the response to the second (Nishikawa et al., 2014; Huang et al., 2013; Artemenko et al., 2016). All-or-nothing responses and refractory period are also displayed by immobilized neutrophils although the kinetics are slower (Wang et al., 2014). The term signal transduction excitable network or STEN was coined to encompass this behavior (Huang et al., 2013).
Excitability is also apparent in propagating waves of signal transduction and cytoskeletal components. “Actin waves” were first reported by Vicker in Dictyostelium (Vicker, 2002) and extensively characterized by Gerisch and colleagues (Bretschneider et al., 2004; Bretschneider et al., 2009; Gerisch et al., 2009; Gerisch, 2010; Gerisch et al., 2011; Schroth-Diez et al., 2009). Waves of the biosensors for Ras (Xiong et al., 2010) (van Haastert et al., 2017) and PI3K activation were also observed in cells during migration. Studies of the recruitment of Scar subunits, F-actin binding proteins, and biosensors for Ras, PI3K, and Rac activation and coordinated dissociation of PTEN and myosin from the cell cortex suggest that the entire signal transduction and cytoskeletal networks show this behavior (Supplemental Video 5)). There is a striking, but perhaps expected, correspondence between the temporal responses to global stimuli and the spatial distribution of the same components and activities in propagating waves (Figure 4). That is, front components and activities are associated with the leading edge of the propagating wave, while back events, present on the rest of the cortex, are transiently absent from the active zone.
Similar propagating waves or evidence for excitability of signal transduction and cytoskeletal events have been observed in neutrophils (Weiner et al., 2007), mast cells (Wu et al., 2013), fibroblast (Ryan et al., 2012; Case and Waterman, 2011) and other cells (Winans et al., 2016). In some cases, the similar phase relationships among various components has been documented. In mast cells, for example, F-actin zones correspond to troughs in PIP2 waves (Xiong et al., 2016). It remains to be determined whether these dynamic events are displayed by a few cell types under specific conditions or whether they are an unrecognized general feature of migrating cells and perhaps play further roles in cell physiology.
Many investigators have appreciated that the propagating waves reflect the properties of an excitable medium, where the activity in a restricted region is relayed forward, and a trailing refractory zone assures unidirectionality and annihilation of crossing waves. As a means of formalizing these concepts, Miao, et al., (Miao et al., 2017) proposed that local regions of the cell cortex transition between inactive, active, and refractory states designated as B, F, and R, respectively (Figure 5). The B- and F-states are mutually inhibitory, creating a positive feedback loop. The F- and R-states are related through a delayed negative feedback loop. In resting cells, most of the cortex is in the B state. Once initiated, waves propagate outwardly because diffusion of F-state components trigger activation in adjoining B but not R regions. This model also captures many of the observed features of the signal transduction networks including “all-or-nothing” and refractory behavior.
Figure 5.
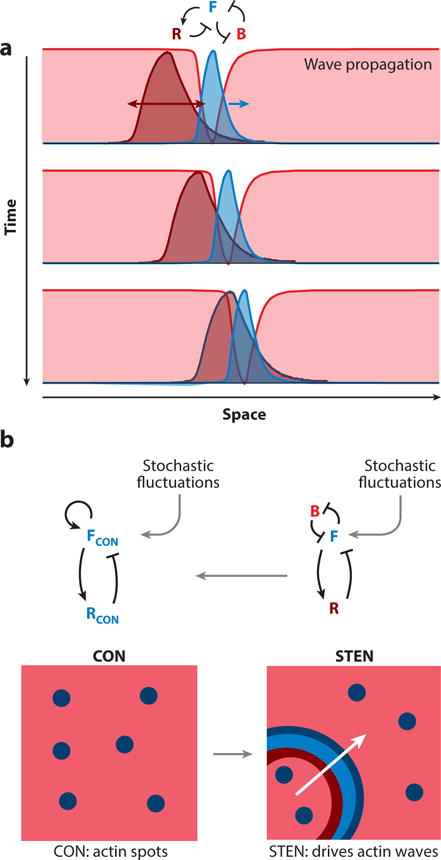
A. A cartoon of how an excitable wave propagates on the membrane. The reciprocal “front” (F) and “back” (B) zones (green and red, respectively; representative activities as in Figure 4), propagate and the “refractory” (R) zone (deep red) trails, ensuring unidirectional front propagation. B. Coupling between cytoskeletal (CON) and signaling (STEN) activities generate propagating waves of cytoskeletal activity. In the absence of STEN activity, there are flashes of actin polymerization - shown by the blue spots in the box on the left (a random 2D area on the cortex). Once STEN activity is triggered, it causes the CON spots to expand in space – driven by the excitable wave front (green) – creating actin waves.
The spontaneous signal transduction and cytoskeletal activities are independent but can couple (Figure 5). On one hand, a prescient study demonstrated that caffeine induced reciprocal waves of PIP3 and PTEN to propagate around the perimeter of immobilized Dictyostelium cells (Arai et al., 2010). Several other studies have shown that Ras, PIP3 and PTEN activity waves occur spontaneously on the basal surfaces of immobilized cells, albeit with diminished frequency (Taniguchi et al., 2013). One study shows that spontaneous, dynamic PI3K activation occurs in neutrophils which have been immobilized by a cocktail of cytoskeletal inhibitors (Wang et al., 2014). The theme of independent coupled networks was advanced by studies demonstrating a spatio-temporal association of larger waves of cytoskeletal activities with STEN reporters (Weiger et al., 2010). Furthermore, in cells migrating over perforations, F-actin is polymerized at points of high curvature but only in regions of accumulation of PIP3 (Jasnin et al., 2016). Similar PIP3-associated “actin waves” propagate along the cortex of cells in contact with ridges (Driscoll et al., 2014). On the other hand, when multiple signal transduction pathways were inhibited, the cytoskeletal reporters reverted to rapid flashes or oscillations and Huang, et al. proposed the designation cytoskeletal oscillatory network or CON (Huang et al., 2013). The flashes and oscillations do not expand as waves arguing against molecular models of propagation which rely solely on interactions among cytoskeletal components. Taken together, these studies suggest that the cytoskeletal waves are essentially a “readout” of the propagating signal transduction waves, which the spontaneous cytoskeletal events help initiate. However, one study showed that under certain conditions the oscillations could be synchronized and cells could be steered, apparently independently of STEN (Yang et al., 2016; Hoeller et al., 2016).
There are many unanswered questions surrounding the STEN wave theory of cell migration. First, the highly parallel nature of the network noted earlier, where activation at single nodes lowers threshold but no single perturbation eliminates the signal transduction waves or blocks migration, has hampered efforts to identify the core feedback loops underlying excitability and wave propagation. In the analogous case of the action potential the unifying concept of membrane potential provides a strong framework for comprehending the roles of the many participating ion channels. The realization of a similar principle would greatly facilitate the understanding of the many contributing components and responses in the STEN. Second, the molecular mechanism of coupling of the STEN waves to the cytoskeletal activity is not known although Rho family GTPases are leading candidates. In Dictyostelium, the Rac binding domain from mammalian Pak1 localizes to the leading edge of migrating cells and travels coordinately with the STEN waves that drive large protrusions, but does not associate with the unaccompanied rapid flashes and oscillations of cytoskeletal activity (Huang et al., 2013). In neutrophils, production of PIP3, without activation of the chemoattractant receptor, leads to activation of Rac (Weiner et al., 2002). Given the extensive observations of the roles of cdc42 and Rac in promoting and regulating cytoskeletal events, these small GTPases should represent a natural point of coupling between STEN and cytoskeletal activities. Third, the topological connection of laterally expanding waves on the cell cortex to the various types of protrusions that cells display (see Figure 1) is a current active area of research.
Propagating STEN Waves and the Protrusions that Underlie Motility
The coupling of the STEN waves to cytoskeletal activity appears to explain the topology of certain protrusions involved in migration of amoeboid cells, and given their flexibility, similar excitable systems may shape the great variety of protrusions made by cells (Figure 6). Careful examination of the waves propagating on the basal surfaces of cells shows that Scar subunits and actin binding proteins outline the wide advancing waves of Ras and PI3K activity in the F-state region (Weiner et al., 2007; Schroth-Diez et al., 2009; Gerisch, 2011; Huang et al., 2013). The expanding cup-like protrusions seen in lattice light sheet microscopy (Chen et al., 2014) of randomly migrating vegetative Dictyostelium cells could be explained by propagating STEN waves driving cytoskeletal activity. Consistently, actin binding proteins rim the cup-like structure which is filled with a broader zone of Ras and PI3K activity (Veltman et al., 2016; Pollitt et al., 2006). These structures, referred to as macropinosomes, are abundant in axenic cells which have been selected for fluid uptake (Maniak, 2001). The mutation that confers axenic growth capacity is a homologue of the human Ras Gap NF1, consistent with the elevated Ras activity in these cup-like structures (Bloomfield et al., 2015). However, when near the substrate, many “macropinosomes” clearly function in moving the cells, questioning the functional distinction between these structures and pseudopods. Further evidence that these structures underlie motility is the fact that changing their properties dramatically altered migration: Perturbations at key nodes of STEN lowered the threshold for network activation, increased wave speed and range, expanded the cup-like structures, and switched the mode of migration from amoeboid to keratocyte-like and oscillatory (Supplemental Videos 6 and 7) (Miao et al., 2017).
Figure 6.
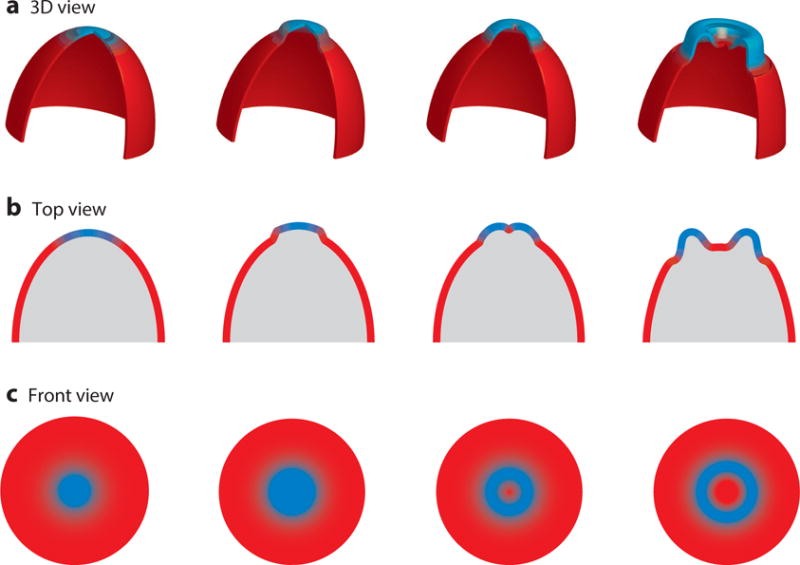
Cartoon showing the coupling between wave propagation and topology of cellular protrusions. (Top) Three-dimensional representation of the formation of cup-like structures on the cortex as a wave propagates (green and red as “front” (F) and “back” (B) activity, respectively). (Middle and Bottom) A top and front view of these structures shows how these protrusions are born out of wave propagation as the front activity spreading outward creates a hole in 2D, which translates to a cup-like protrusion in 3D.
The “pseudopodia” of migrating, differentiated Dictyostelium cells and other amoeboid cells likely have a similar basis in a spontaneous local activation of STEN and wave propagation, although there are salient differences in the structures formed. Pseudopodia are narrower and protrude further than macropinosomes. Thus, the propagating STEN wave expands differently to accommodate these different structures. These morphologically distinct protrusions can exist simultaneously in different locations on the same cell. Furthermore, under certain conditions, chemoattractants can elicit pseudopodia without abolishing the waves underlying macropinosomes (Ecke and Gerisch, 2017). We know from theoretical considerations that small changes in the set point of the excitable network can lead to structure with quite different dimensions and durations (Shi and Iglesias, 2013). The fact that small perturbations in the network can rapidly switch cup-like structures into gliding lamellipodia-like protrusions is consistent with the idea that the various types of protrusions are on a continuum arising from the same basic mechanism (Miao et al., 2017).
We speculate that further modification of the basic STEN wave scheme may account for the blebs mediating the migration of germ cells, some cancer cells, and constrained amoebae and therefore provide a clue as to the mechanism of guidance of blebbing cells. The idea is as follows: As the STEN wave expands, the response at the origin subsides and the region becomes refractory, recruiting back proteins such as myosin, causing contraction. If actin polymerization is curtailed during this process and protrusion at the rim is selectively impaired, the local contraction might cause separation of the cortex and membrane producing the bleb. Since the signaling events can be guided, a mechanism for steering blebbing cells would be provided. Consistent observations include the appearance of myosin at the base of blebs in germ cells (Blaser et al., 2006) and the fact that in Dictyostelium blebs are prevalent following the chemoattractant-elicited actin burst when myosin is highest (Langridge and Kay, 2006).
THE BIASED EXCITABLE NETWORK (BEN) CONCEPT: BIASING OF STEN ACTIVITY BY EXTERNAL CUES AND DIRECTED MIGRATION
The concept of cell migration described above, captured computationally with independent coupled excitable modules for STEN and CON, would be sufficient to allow the unstimulated cells to generate protrusions leading to random motility but directional movement requires that the protrusions be more likely in the direction of the external gradient (Figure 7). Extrinsic cues, such as chemoattractants or mechanical signals, could accomplish this by biasing the threshold for activation. For example, if the threshold level drops as receptor occupancy increases then the part of the cell facing the chemoattractant source will show a greater rate of pseudopod formation, and likely larger protrusions, than the rear. Indeed, such guidance mechanisms, broadly referred to as biased excitable network (BEN) schemes are plausible and likely operate in many systems.
Figure 7.
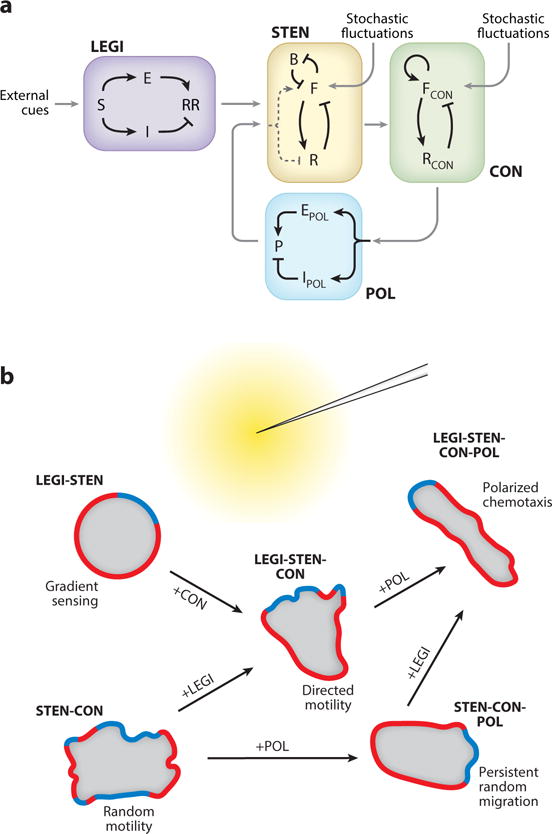
A representation of the various modules involved in directed cell migration. A. A schematic showing the coupling between the different proposed modules. B. How these modules coordinate to orchestrate different kinds of cell motility in the presence of a chemoattractant gradient (yellow), with green and red depicting the front (F) and back (B) activities, respectively.
In Dictyostelium cells and human neutrophils exposed to chemotactic gradients fewer protrusions are seen at the rear of a responding cell. Similarly, in immobilized cells, STEN activity is suppressed on the downside (Tang et al., 2014). This suggests that a shallow difference in receptor occupancy across the cell lowers the threshold at one end and raises it at the other. As this cannot be achieved using only local information, a means of sharing information globally is needed and various models incorporating global inhibitors have been proposed (Meinhardt, 1999; Levine et al., 2006; Levchenko and Iglesias, 2002). When linked to an excitable network these models are referred to as biased excitable network-global inhibitor (BENGI) and local excitation-global inhibition biased excitable network (LEGI-BEN) schemes (Tang et al., 2014). (Figure 8). These closely related classes of models differ in how the inhibitory signal is generated. In BENGI schemes, it arises as the result of a feedback loop from the activity of the excitable system (Meinhardt, 1999). This feedback may be the result of signaling components or cytoskeletal activity, as in the pseudopod-centered model (Insall, 2010). In LEGI-BEN schemes, the inhibitory signal is the result of a feedforward loop from receptor occupancy itself which generates a response regulator. Mathematical models show that both mechanisms can bias spatially the activity of STEN, cytoskeletal activity, and protrusions (Meinhardt, 1999) (Neilson et al., 2011; Hecht et al., 2011) resulting in directed motility. Experiments on Dictyostelium mutants lacking G-protein subunits and wild-type cells exposed to multiple temporal stimuli favor a LEGI circuit biasing of the STEN (Figure 8) (Tang et al., 2014). Conveniently, the global inhibitor in the feed-forward LEGI module can also account for the ability of chemotactic cells to ignore the ambient level of stimulus and respond only to the steepness of the gradient.
Figure 8.
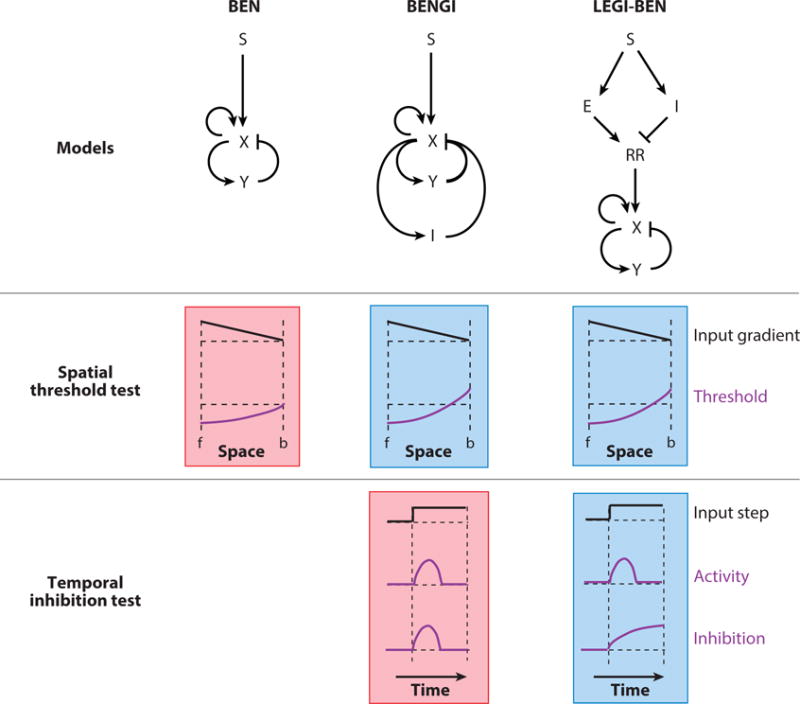
A cartoon of how LEGI-BEN emerged as a realistic model of gradient sensing. Three such proposed models (structures shown on top: S, external input; X and Y, activator and inhibitor of the excitable system; I, global inhibition; E; local excitation; RR, response regulator) are put through two specific tests. The outputs shown schematically represent the responses observed. The green and red shading represent passed and failed tests respectively. For the spatial threshold test, when an input gradient is applied (f: front, b: back of cell), all three models display a lowered threshold at the front. In the BENGI and LEGI-BEN models, which have global inhibitors, the threshold is raised at the back. This would result in better directed movement. To distinguish between these two, the cells were put through a temporal inhibition test, where the global inhibitor level is quantified for a homogenous input of chemoattractant. For the BENGI model, as the inhibition follows activator level, it dies down with time. However, in the LEGI-BEN scheme, the inhibitor is derived from the input itself causing the level of inhibition to rise with time as the step is sustained, matching real cell observations.
These models link directional sensing to motility but they do not account for the stable polarization displayed by differentiated Dictyostelium cells, human neutrophils, and many other cell types even in the absence of a gradient. Polarity can be accomplished by competing local excitatory and global inhibitory signals formed as the result of positive and negative feedback loops (POL in Figure 7). The positive feedback loop essentially lowers the threshold at locations where a firing has taken place, enabling unstimulated cells to move persistently (Shi and Iglesias, 2013; Cooper et al., 2012) (Figure 7). This or a similar mechanism may account for the low number of de novo pseudopods seen in migrating amoeboid cells. In Dictyostelium and neutrophils this intrinsic polarity mechanism is used for both memory and to sharpen the response of cells external cues (Wang et al., 2014; Janetopoulos et al., 2004). Most of the polarity is actin-dependent but an actin-independent component has been observed (Skoge et al., 2014)
From a Cacophony of Inhibitors Comes Orchestrated Movement
The inhibitors of the BENGI and LEGI-BEN models are distinct from the negative feedback loops which cause shut-off and refractoriness in the excitable networks. Depending on how they are deployed, the global inhibitors can explain the capacity of cells to cease responding to a uniform stimulus while remaining responsive to subsequent increases, referred to as adaptation (Tang et al., 2014). In Dictyostelium cells, the refractory periods of the STEN are shorter, than the time courses of adaptation and must involve faster inhibitors. This hierarchy of inhibitors with different kinetics are needed to account for the complex kinetics of many of the biochemical responses in STEN and CON such as cGMP production, Ras and PI3K activation, and actin polymerization: Uniform stimuli trigger an initial peak response which shuts-off within 45 seconds and is followed by a series secondary events that subside after a few minutes. Additional responses can be triggered by incrementing the chemoattractant or by removing it, allowing a period of recovery, and then reapplying the same level. The concatenation of these two kinetic behaviors was apparent in the earliest observations of oscillations of light scattering displayed by shaken Dictyostelium cells (Gerisch and Hess, 1974) but has led to considerable confusion in terminology, which some of the terms the glossary in supplemental Table 2 attempt to sort out.
The proposed schemes and descriptions of directed migration place numerous constraints on the molecular events that mediate inhibitory activities. There are global inhibitors, delayed feedback inhibitors, and polarity inhibitors. Supplemental Table 5 lists the proposed inhibitors. Despite the compelling phenomenology, molecular events have been only tentatively assigned to a few inhibitors. First, evidence shows that PKBs play an important role in negative feedback regulation of Ras activity since cells lacking PKBA and PKBR1 display persistently high RasC activity in pull-down assays (Charest et al., 2010) and in immobilized cells lacking the PKBs, RDB patches are more frequent but rapidly quenched by recruitment of PKBA (Miao et al., 2017). Second, in neutrophils, membrane tension has been shown to be an important negative regulator (Diz-Muñoz et al., 2016; Diz-Muñoz et al., 2013). Typically, 10–15 μm, cells induced to lengthen to 50 μm still display distinctive front and rear characteristics (Houk et al., 2012). Upon severing, thereby releasing membrane tension, the rear portion instantly forms a new Scar-decorated front. Similarly, highly polarized Dictyostelium cells lacking KrsB sometimes break during migration whereupon the rear portion instantly forms a new front (Artemenko et al., 2012). Moreover, cells lacking dynacortin, which contributes to cortical viscoelasticity, do not become as polarized as wild-type cells (Kabacoff et al., 2007). Third, in Dictyostelium, disruption of ostensible negative regulators such as SodC (Veeranki et al., 2008) the Ras Gaps NF1, Nfa (Zhang et al., 2008), and PTEN (Iijima and Devreotes, 2002) appears to lengthen the duration of chemoattractant-triggered responses and promotes protrusive behavior. Other genes appear to play a similar role, including KrsB, a set of novel RAM genes, and myosin (Lampert et al., in press). Of course, it is difficult to distinguish negative regulators from feed-forward and negative feedback inhibitors and further studies are needed to define the precise roles that each of these genes plays.
Emergence of STEN as a Controller of Migration
The concept of STEN as a regulator of cell migration has advantages for gradient sensing. First, if the external cue differentially regulates the threshold across the cell, as proposed in the BEN mechanisms incorporating global inhibitors, slight shifts in the threshold can lead to enhanced, or completely suppressed, activity at the front or the rear, respectively. While direct measurement of local thresholds on a cell exposed to a gradient is technically challenging, a difference in sensitivity at the ends of a stably polarized cell has been repeatedly demonstrated (Wang et al., 2014; Skoge et al., 2014; Jin et al., 2000) (Swanson and Taylor, 1982). Second, the excitable character of the STEN produces stochastic projections characteristic of amoeboid motion, which is effective in sensing extrinsic cues. The repeated extension of pseudopodia from a defined region at the anterior of the polarized cell coupled to contractility at the rear provide rapid motility. However, the cell’s track is readily correctable by favoring pseudopodia on one side of the front over the other during exposure to a directional cue. Less is known about the role of STEN in leukocytes, although signal transduction events similar to those observed in Dictyostelium, occur at the leading edge of migrating neutrophils. The complex ruffling at the leading edge of leukocytes, compared with the rhythmic pseudopodia of Dictyostelium, suggests that the STEN in these cells would be tuned slightly differently. At first glance, the amoeboid behavior of Dictyostelium and leukocytes seems removed from the slow gliding behavior of fibroblastic, mesenchymal cells yet some of the same signal transduction events do occur at the leading edges (Weiger et al., 2010; Kaur et al., 2006; Haugh et al., 1999). Furthermore, the fact that Dictyostelium cells can be abruptly and reversibly shifted from amoeboid to gliding migration, with protrusions similar to classic lamellipodia, suggests that the migration profiles displayed by diverse cells are slight variations of a basic mechanism (Miao et al., 2017). The behavior of mesenchymal cells may differ primarily in the much stronger adhesive properties of these cells. Interestingly, while the fast moving keratocyte-like Dictyostelium cells ignore guidance cues, fibroblastic cells can be guided, perhaps because the slow movement allows long term integration of the gradient (Miao et al., 2017).
An optimal level of protrusive activity is required for efficient motility in a given environment (Figure 9). This can be understood in light of the STEN concept. On one hand, if the set point of the network precludes stochastic noise from crossing threshold, there will be few large protrusions and cells will barely move. This is observed when multiple signal transduction pathways are inhibited in Dictyostelium, leaving only brief, uncoordinated cytoskeletal events (Veltman et al., 2008; Chen et al., 2007) (Artemenko et al., 2016). Some cells move more in uniform chemoattractant, a process referred to as chemokinesis (Petrie et al., 2009) (Vicker, 1994; Ferguson et al., 2007). According to most of the BEN schemes, chemokinesis is expected if uniform stimuli lower thresholds globally. On the other hand, the overall level of excitability can become too high, causing simultaneous projections in multiple directions, effectively blocking migration. Dictyostelium cells lacking PTEN display numerous spikey protrusions in all quadrants, as an example of this phenomenon. To a lesser extent, similar phenotypes are displayed by vegetative Dictyostelium cells, 18 regulator of adhesion and motility (RAM) mutants, and neutrophils lacking PTEN or SHIP1 (Sasaki et al., 2007) (Lampert et al., in press), (Sarraj et al., 2009; Liu et al., 1999). Where it has been tested, damping down signal transduction events or partially decoupling them from cytoskeletal activity improves migration. Thus, increased STEN activity leads to greater motility but at too high a level it becomes counterproductive. Going forward, it will be informative to ask why migration is impaired or enhanced in various contexts by focusing on the number, localization, and characteristics of protrusions.
Figure 9.
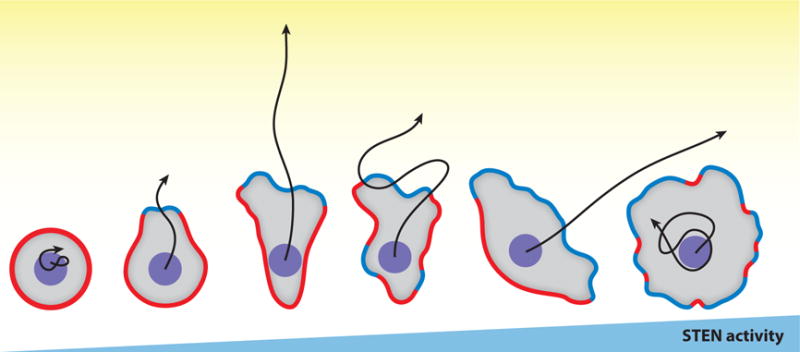
An optimal level of STEN (signal transduction excitable network) activities is required for effective directed cell migration. Cells with increasing STEN activities are illustrated from left to right, with “green” representing front events and “red” back events. Black arrows represent centroid tracks of each cell moving towards a chemoattractant gradient (yellow) in the same amount of time.
STEN is potentially the site of integration of extrinsic cues controlling migration. There is an increasing appreciation of the ability of cells to integrate environmental signals and move accordingly but the mechanisms are poorly understood (Haeger et al., 2015) (Theveneau et al., 2010). It seems evident that multiple chemoattractants can be integrated by activating the same set of signal transduction events, for instance by activating a common G-protein βγ-subunit but it is less obvious how mechanical, electrical, and optical signals are brought in. That cells migrating under shear force and in electric fields activate the same signal transduction events as chemoattractants suggests they might be integrated at this level. The fact that in Dictyostelium a brief exposure to shear force is activates STEN precisely as do chemoattractants further supports this idea. If multiple extrinsic cues controlling migration all feed into a common STEN, altering its set point, it would serve as an integrator of these signals.
There may be a profound evolutionary basis for the concurrence between the pathways involved in cell migration and cell growth. It is striking that Ras GTPases and PI3K pathways play a central role in both processes and there are parallels in the effects of mutations. For example, oncogenic mutations in Ras GTPases and PI3Ks, which promote growth in mammalian cells, lead to more active Dictyostelium cells with additional protrusions (Miao et al., 2017). Loss of tumor suppressors NF1 and PTEN and have a similar equivalence with their mammalian counterparts (Bloomfield et al., 2015) (Sarraj et al., 2009) (Iijima and Devreotes, 2002). These parallels extend further, for example to Dictyostelium Rap1 and KrsB, which are homologues of the mammalian oncogene Rap1 and, tumor suppressor Hippo/Mst1-2 respectively (Artemenko et al., 2012). Additional examples are found throughout the pathways. The effects on migration stand out in Dictyostelium, since these perturbations have minimal effects on growth rates, whereas research in mammalian cells has focused primarily on growth. We propose a possible explanation for this apparent connection between the regulation of growth and motility: Growth pathways may have evolved first in non-motile cells and as expanding colonies depleted nutrients, cells able to repurpose that same pathways for migration were able to seek further nutrients and were selected. As explained earlier the fact that macropinosomes, which engulf nutrients, mediate motility when they contact the substrate.
Conclusions
Directed cell migration, although critical in health and disease, is a fundamental physiological process displayed by nearly all eukaryotic cells. Research by numerous investigators is beginning to describe the mechanisms of migration and to understand how the hundreds of involved proteins act coordinately. Broadly, all cells displaying directed migration must link together directional sensing, motility, and polarity. We suggest that motility results from spontaneous activity within a STEN that directs cytoskeletal events. Inputs to STEN from directional sensing and polarity modules increase persistence and bring about directed migration.The coupled network or modular view of cell migration emerging from recent studies has many advantages for experimental and computation analyses. Subtle variations in the molecular composition, dynamic properties, and linkage of these modules may be sufficient to explain the vast array of migration modes and sensing abilities displayed by diverse cells throughout phylogeny.
Supplementary Material
Supplemental Figure 1.
A representation of three common types of mechanical and spatially located migratory cues. Green represents regions of stimulation and red represents unstimulated cell periphery. A) Specific amoeboid type cells travel of a gradient of shear flow cues demonstrated by the black arrows. B) Epithelial cells travel down the gradient of shear forces on the cell. C) Haptotaxis is the distribution of cues on extracellular substrate which direct cell migration. D) Durotaxis is migration along a substrate’s stiffness gradient demonstrated by increased shading in the substrate.
Footnotes
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
References
- Allen GM, Mogilner A, Theriot JA. Electrophoresis of cellular membrane components creates the directional cue guiding keratocyte galvanotaxis. Curr Biol. 2013;23:560–568. doi: 10.1016/j.cub.2013.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KI, Cross R. Contact dynamics during keratocyte motility. Curr Biol. 2000;10:253–260. doi: 10.1016/s0960-9822(00)00357-2. [DOI] [PubMed] [Google Scholar]
- Arai Y, Shibata T, Matsuoka S, Sato MJ, Yanagida T, Ueda M. Self-organization of the phosphatidylinositol lipids signaling system for random cell migration. Proc Natl Acad Sci U S A. 2010;107:12399–12404. doi: 10.1073/pnas.0908278107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage JP, Hellingwerf KJ. Light-induced behavioral responses (phototaxis’) in prokaryotes. Photosynth Res. 2003;76:145–155. doi: 10.1023/A:1024974111818. [DOI] [PubMed] [Google Scholar]
- Artemenko Y, Axiotakis L, Borleis J, Iglesias PA, Devreotes PN. Chemical and mechanical stimuli act on common signal transduction and cytoskeletal networks. Proc Natl Acad Sci U S A. 2016;113:E7500–E7509. doi: 10.1073/pnas.1608767113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artemenko Y, Batsios P, Borleis J, Gagnon Z, Lee J, Rohlfs M, et al. Tumor suppressor Hippo/MST1 kinase mediates chemotaxis by regulating spreading and adhesion. Proc Natl Acad Sci U S A. 2012;109:13632–13637. doi: 10.1073/pnas.1211304109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artemenko Y, Lampert TJ, Devreotes PN. Moving towards a paradigm: common mechanisms of chemotactic signaling in Dictyostelium and mammalian leukocytes. Cell Mol Life Sci. 2014;71:3711–3747. doi: 10.1007/s00018-014-1638-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagorda A, Parent CA. Eukaryotic chemotaxis at a glance. J Cell Sci. 2008;121:2621–2624. doi: 10.1242/jcs.018077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhart EL, Allen GM, Jülicher F, Theriot JA. Bipedal locomotion in crawling cells. Biophys J. 2010;98:933–942. doi: 10.1016/j.bpj.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann K. Stem cells: moving out of the niche. Nat Rev Mol Cell Biol. 2014;15:79. doi: 10.1038/nrm3747. [DOI] [PubMed] [Google Scholar]
- Blaser H, Reichman-Fried M, Castanon I, Dumstrei K, Marlow FL, Kawakami K, et al. Migration of zebrafish primordial germ cells: a role for myosin contraction and cytoplasmic flow. Dev Cell. 2006;11:613–627. doi: 10.1016/j.devcel.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Bloomfield G, Traynor D, Sander SP, Veltman DM, Pachebat JA, Kay RR. Neurofibromin controls macropinocytosis and phagocytosis in Dictyostelium. Elife. 2015;4 doi: 10.7554/eLife.04940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosgraaf L, van Haastert PJ. The regulation of myosin II in Dictyostelium. Eur J Cell Biol. 2006;85:969–979. doi: 10.1016/j.ejcb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Bosgraaf L, Van Haastert PJ. The ordered extension of pseudopodia by amoeboid cells in the absence of external cues. PLoS One. 2009;4:e5253. doi: 10.1371/journal.pone.0005253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case LB, Waterman CM. Adhesive F-actin waves: a novel integrin-mediated adhesion complex coupled to ventral actin polymerization. PLoS One. 2011;6:e26631. doi: 10.1371/journal.pone.0026631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Devreotes PN. Molecular insights into eukaryotic chemotaxis. FASEB J. 1991;5:3078–3085. [PubMed] [Google Scholar]
- Charest PG, Shen Z, Lakoduk A, Sasaki AT, Briggs SP, Firtel RA. A Ras signaling complex controls the RasC-TORC2 pathway and directed cell migration. Dev Cell. 2010;18:737–749. doi: 10.1016/j.devcel.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BC, Legant WR, Wang K, Shao L, Milkie DE, Davidson MW, et al. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science. 2014;346:1257998. doi: 10.1126/science.1257998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Iijima M, Tang M, Landree MA, Huang YE, Xiong Y, et al. PLA2 and PI3K/PTEN pathways act in parallel to mediate chemotaxis. Dev Cell. 2007;12:603–614. doi: 10.1016/j.devcel.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Janetopoulos C, Huang YE, Iijima M, Borleis J, Devreotes PN. Two phases of actin polymerization display different dependencies on PI(3,4,5)P3 accumulation and have unique roles during chemotaxis. Mol Biol Cell. 2003;14:5028–5037. doi: 10.1091/mbc.E03-05-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J, Bresnick A, Demma M, Dharmawardhane S, Eddy R, Hall AL, et al. Mechanisms of amoeboid chemotaxis: an evaluation of the cortical expansion model. Dev Genet. 1990;11:333–340. doi: 10.1002/dvg.1020110504. [DOI] [PubMed] [Google Scholar]
- Condeelis J, Singer RH, Segall JE. The great escape: when cancer cells hijack the genes for chemotaxis and motility. Annu Rev Cell Dev Biol. 2005;21:695–718. doi: 10.1146/annurev.cellbio.21.122303.120306. [DOI] [PubMed] [Google Scholar]
- Cooper RM, Wingreen NS, Cox EC. An excitable cortex and memory model successfully predicts new pseudopod dynamics. PLoS One. 2012;7:e33528. doi: 10.1371/journal.pone.0033528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese B, Palamà IE, D’Amone S, Gigli G. Influence of electrotaxis on cell behaviour. Integr Biol (Camb) 2014;6:817–830. doi: 10.1039/c4ib00142g. [DOI] [PubMed] [Google Scholar]
- Décave E, Rieu D, Dalous J, Fache S, Brechet Y, Fourcade B, et al. Shear flow-induced motility of Dictyostelium discoideum cells on solid substrate. J Cell Sci. 2003;116:4331–4343. doi: 10.1242/jcs.00726. [DOI] [PubMed] [Google Scholar]
- Diz-Muñoz A, Fletcher DA, Weiner OD. Use the force: membrane tension as an organizer of cell shape and motility. Trends Cell Biol. 2013;23:47–53. doi: 10.1016/j.tcb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz-Muñoz A, Thurley K, Chintamen S, Altschuler SJ, Wu LF, Fletcher DA, Weiner OD. Membrane Tension Acts Through PLD2 and mTORC2 to Limit Actin Network Assembly During Neutrophil Migration. PLoS Biol. 2016;14:e1002474. doi: 10.1371/journal.pbio.1002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll MK, Sun X, Guven C, Fourkas JT, Losert W. Cellular contact guidance through dynamic sensing of nanotopography. ACS Nano. 2014;8:3546–3555. doi: 10.1021/nn406637c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecke M, Gerisch G. Co-existence of Ras activation in a chemotactic signal transduction pathway and in an autonomous wave - forming system. Small GTPases. 2017:1–9. doi: 10.1080/21541248.2016.1268666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. J Cell Biol. 2008;181:879–884. doi: 10.1083/jcb.200802081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson GJ, Milne L, Kulkarni S, Sasaki T, Walker S, Andrews S, et al. PI(3)Kgamma has an important context-dependent role in neutrophil chemokinesis. Nat Cell Biol. 2007;9:86–91. doi: 10.1038/ncb1517. [DOI] [PubMed] [Google Scholar]
- Filić V, Marinović M, Faix J, Weber I. A dual role for Rac1 GTPases in the regulation of cell motility. J Cell Sci. 2012;125:387–398. doi: 10.1242/jcs.089680. [DOI] [PubMed] [Google Scholar]
- Funamoto S, Meili R, Lee S, Parry L, Firtel RA. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Futrelle RP, Traut J, McKee WG. Cell behavior in Dictyostelium discoideum: preaggregation response to localized cyclic AMP pulses. J Cell Biol. 1982;92:807–821. doi: 10.1083/jcb.92.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao RC, Zhang XD, Sun YH, Kamimura Y, Mogilner A, Devreotes PN, Zhao M. Different roles of membrane potentials in electrotaxis and chemotaxis of dictyostelium cells. Eukaryot Cell. 2011;10:1251–1256. doi: 10.1128/EC.05066-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch G. Actin switches in phagocytosis. Commun Integr Biol. 2011;4:344–345. doi: 10.4161/cib.4.3.15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch G, Hess B. Cyclic-AMP-controlled oscillations in suspended Dictyostelium cells: their relation to morphogenetic cell interactions. Proc Natl Acad Sci U S A. 1974;71:2118–2122. doi: 10.1073/pnas.71.5.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125:1361–1374. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Haeger A, Wolf K, Zegers MM, Friedl P. Collective cell migration: guidance principles and hierarchies. Trends Cell Biol. 2015;25:556–566. doi: 10.1016/j.tcb.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Harland B, Walcott S, Sun SX. Adhesion dynamics and durotaxis in migrating cells. Phys Biol. 2011;8:015011. doi: 10.1088/1478-3975/8/1/015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugh JM, Huang AC, Wiley HS, Wells A, Lauffenburger DA. Internalized epidermal growth factor receptors participate in the activation of p21(ras) in fibroblasts. J Biol Chem. 1999;274:34350–34360. doi: 10.1074/jbc.274.48.34350. [DOI] [PubMed] [Google Scholar]
- Hecht I, Skoge ML, Charest PG, Ben-Jacob E, Firtel RA, Loomis WF, et al. Activated membrane patches guide chemotactic cell motility. PLoS Comput Biol. 2011;7:e1002044. doi: 10.1371/journal.pcbi.1002044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeller O, Kay RR. Chemotaxis in the absence of PIP3 gradients. Curr Biol. 2007;17:813–817. doi: 10.1016/j.cub.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Hoeller O, Toettcher JE, Cai H, Sun Y, Huang CH, Freyre M, et al. Gβ Regulates Coupling between Actin Oscillators for Cell Polarity and Directional Migration. PLoS Biol. 2016;14:e1002381. doi: 10.1371/journal.pbio.1002381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Hedberg S, Schneider IC. Differences in adhesion and protrusion properties correlate with differences in migration speed under EGF stimulation. BMC biophysics. 2012 doi: 10.1186/2046-1682-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk AR, Jilkine A, Mejean CO, Boltyanskiy R, Dufresne ER, Angenent SB, et al. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell. 2012;148:175–188. doi: 10.1016/j.cell.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CH, Tang M, Shi C, Iglesias PA, Devreotes PN. An excitable signal integrator couples to an idling cytoskeletal oscillator to drive cell migration. Nat Cell Biol. 2013;15:1307–1316. doi: 10.1038/ncb2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 2002;109:599–610. doi: 10.1016/s0092-8674(02)00745-6. [DOI] [PubMed] [Google Scholar]
- Inoue T, Meyer T. Synthetic activation of endogenous PI3K and Rac identifies an AND-gate switch for cell polarization and migration. PLoS One. 2008;3:e3068. doi: 10.1371/journal.pone.0003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insall RH. Understanding eukaryotic chemotaxis: a pseudopod-centred view. Nat Rev Mol Cell Biol. 2010;11:453–458. doi: 10.1038/nrm2905. [DOI] [PubMed] [Google Scholar]
- Janetopoulos C, Ma L, Devreotes PN, Iglesias PA. Chemoattractant-induced phosphatidylinositol 3,4,5-trisphosphate accumulation is spatially amplified and adapts, independent of the actin cytoskeleton. Proc Natl Acad Sci U S A. 2004;101:8951–8956. doi: 10.1073/pnas.0402152101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnin M, Ecke M, Baumeister W, Gerisch G. Actin Organization in Cells Responding to a Perforated Surface, Revealed by Live Imaging and Cryo-Electron Tomography. Structure. 2016;24:1031–1043. doi: 10.1016/j.str.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Jin T, Xu X, Hereld D. Chemotaxis, chemokine receptors and human disease. Cytokine. 2008;44:1–8. doi: 10.1016/j.cyto.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Zhang N, Long Y, Parent CA, Devreotes PN. Localization of the G protein betagamma complex in living cells during chemotaxis. Science. 2000;287:1034–1036. doi: 10.1126/science.287.5455.1034. [DOI] [PubMed] [Google Scholar]
- Kabacoff C, Xiong Y, Musib R, Reichl EM, Kim J, Iglesias PA, Robinson DN. Dynacortin facilitates polarization of chemotaxing cells. BMC Biol. 2007;5:53. doi: 10.1186/1741-7007-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakumoto T, Nakata T. Optogenetic control of PIP3: PIP3 is sufficient to induce the actin-based active part of growth cones and is regulated via endocytosis. PLoS One. 2013;8:e70861. doi: 10.1371/journal.pone.0070861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura Y, Xiong Y, Iglesias PA, Hoeller O, Bolourani P, Devreotes PN. PIP3-independent activation of TorC2 and PKB at the cell’s leading edge mediates chemotaxis. Curr Biol. 2008;18:1034–1043. doi: 10.1016/j.cub.2008.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Park CS, Lewis JM, Haugh JM. Quantitative model of Ras-phosphoinositide 3-kinase signalling cross-talk based on co-operative molecular assembly. Biochem J. 2006;393:235–243. doi: 10.1042/BJ20051022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Cell migration during gastrulation. Curr Opin Cell Biol. 2005;17:533–541. doi: 10.1016/j.ceb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Keren K, Theriot JA. Biophysical aspects of actin-based cell motility in fish epithelial keratocytes. Cell motility 2008 [Google Scholar]
- Khanna A, Lotfi P, Chavan AJ, Montaño NM, Bolourani P, Weeks G, et al. The small GTPases Ras and Rap1 bind to and control TORC2 activity. Sci Rep. 2016;6:25823. doi: 10.1038/srep25823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klämbt C. Modes and regulation of glial migration in vertebrates and invertebrates. Nat Rev Neurosci. 2009;10:769–779. doi: 10.1038/nrn2720. [DOI] [PubMed] [Google Scholar]
- Lakshman R, Finn A. Neutrophil disorders and their management. J Clin Pathol. 2001;54:7–19. doi: 10.1136/jcp.54.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge PD, Kay RR. Blebbing of Dictyostelium cells in response to chemoattractant. Exp Cell Res. 2006;312:2009–2017. doi: 10.1016/j.yexcr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Leptin M. Gastrulation movements: the logic and the nuts and bolts. Dev Cell. 2005;8:305–320. doi: 10.1016/j.devcel.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Levchenko A, Iglesias PA. Models of eukaryotic gradient sensing: application to chemotaxis of amoebae and neutrophils. Biophys J. 2002;82:50–63. doi: 10.1016/S0006-3495(02)75373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine H, Kessler DA, Rappel WJ. Directional sensing in eukaryotic chemotaxis: a balanced inactivation model. Proc Natl Acad Sci U S A. 2006;103:9761–9766. doi: 10.1073/pnas.0601302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CJ, Spiegelman GB, Weeks G. Cytoskeletal regulation by Dictyostelium Ras subfamily proteins. J Muscle Res Cell Motil. 2002;23:729–736. doi: 10.1023/a:1024471527153. [DOI] [PubMed] [Google Scholar]
- Liu Q, Sasaki T, Kozieradzki I, Wakeham A, Itie A, Dumont DJ, Penninger JM. SHIP is a negative regulator of growth factor receptor-mediated PKB/Akt activation and myeloid cell survival. Genes Dev. 1999;13:786–791. doi: 10.1101/gad.13.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniak M. Fluid-phase uptake and transit in axenic Dictyostelium cells. Biochim Biophys Acta. 2001;1525:197–204. doi: 10.1016/s0304-4165(01)00105-2. [DOI] [PubMed] [Google Scholar]
- Meinhardt H. Orientation of chemotactic cells and growth cones: models and mechanisms. J Cell Sci. 1999;112:2867–2874. doi: 10.1242/jcs.112.17.2867. [DOI] [PubMed] [Google Scholar]
- Meng X, Arocena M, Penninger J, Gage FH, Zhao M, Song B. PI3K mediated electrotaxis of embryonic and adult neural progenitor cells in the presence of growth factors. Exp Neurol. 2011;227:210–217. doi: 10.1016/j.expneurol.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Bhattacharya S, Edwards M, Cai H, Inoue T, Iglesias PA, Devreotes PN. Altering the threshold of an excitable signal transduction network changes cell migratory modes. Nat Cell Biol. 2017 doi: 10.1038/ncb3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogilner A, Keren K. The shape of motile cells. Curr Biol. 2009;19:R762–71. doi: 10.1016/j.cub.2009.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell DJ. Morphogenetic cell movements: diversity from modular mechanical properties. Science. 2008;322:1502–1505. doi: 10.1126/science.1164073. [DOI] [PubMed] [Google Scholar]
- Moulding DA, Record J, Malinova D, Thrasher AJ. Actin cytoskeletal defects in immunodeficiency. Immunol Rev. 2013;256:282–299. doi: 10.1111/imr.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson MP, Veltman DM, van Haastert PJ, Webb SD, Mackenzie JA, Insall RH. Chemotaxis: a feedback-based computational model robustly predicts multiple aspects of real cell behaviour. PLoS Biol. 2011;9:e1000618. doi: 10.1371/journal.pbio.1000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune ER, Iiri T, Bourne HR. Galphai is not required for chemotaxis mediated by Gi-coupled receptors. J Biol Chem. 1999;274:2824–2828. doi: 10.1074/jbc.274.5.2824. [DOI] [PubMed] [Google Scholar]
- Nishikawa M, Hörning M, Ueda M, Shibata T. Excitable signal transduction induces both spontaneous and directional cell asymmetries in the phosphatidylinositol lipid signaling system for eukaryotic chemotaxis. Biophys J. 2014;106:723–734. doi: 10.1016/j.bpj.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41:694–707. doi: 10.1016/j.immuni.2014.10.008. [DOI] [PubMed] [Google Scholar]
- O’Neill PR, Kalyanaraman V, Gautam N. Subcellular optogenetic activation of Cdc42 controls local and distal signaling to drive immune cell migration. Mol Biol Cell. 2016;27:1442–1450. doi: 10.1091/mbc.E15-12-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010;11:633–643. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10:538–549. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollitt AY, Blagg SL, Ibarra N, Insall RH. Cell motility and SCAR localisation in axenically growing Dictyostelium cells. Eur J Cell Biol. 2006;85:1091–1098. doi: 10.1016/j.ejcb.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Postma M, Roelofs J, Goedhart J, Gadella TW, Visser AJ, Van Haastert PJ. Uniform cAMP stimulation of Dictyostelium cells induces localized patches of signal transduction and pseudopodia. Mol Biol Cell. 2003;14:5019–5027. doi: 10.1091/mbc.E03-08-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramot D, MacInnis BL, Lee HC, Goodman MB. Thermotaxis is a robust mechanism for thermoregulation in Caenorhabditis elegans nematodes. J Neurosci. 2008;28:12546–12557. doi: 10.1523/JNEUROSCI.2857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond N, d’Água BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer. 2013;13:858–870. doi: 10.1038/nrc3628. [DOI] [PubMed] [Google Scholar]
- Richardson BE, Lehmann R. Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat Rev Mol Cell Biol. 2010;11:37–49. doi: 10.1038/nrm2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rørth P. Whence directionality: guidance mechanisms in solitary and collective cell migration. Dev Cell. 2011;20:9–18. doi: 10.1016/j.devcel.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Ryan GL, Watanabe N, Vavylonis D. A review of models of fluctuating protrusion and retraction patterns at the leading edge of motile cells. Cytoskeleton (Hoboken) 2012;69:195–206. doi: 10.1002/cm.21017. [DOI] [PubMed] [Google Scholar]
- Sarraj B, Massberg S, Li Y, Kasorn A, Subramanian K, Loison F, et al. Myeloid-specific deletion of tumor suppressor PTEN augments neutrophil transendothelial migration during inflammation. J Immunol. 2009;182:7190–7200. doi: 10.4049/jimmunol.0802562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki AT, Janetopoulos C, Lee S, Charest PG, Takeda K, Sundheimer LW, et al. G protein-independent Ras/PI3K/F-actin circuit regulates basic cell motility. J Cell Biol. 2007;178:185–191. doi: 10.1083/jcb.200611138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satulovsky J, Lui R, Wang YL. Exploring the control circuit of cell migration by mathematical modeling. Biophys J. 2008;94:3671–3683. doi: 10.1529/biophysj.107.117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroth-Diez B, Gerwig S, Ecke M, Hegerl R, Diez S, Gerisch G. Propagating waves separate two states of actin organization in living cells. HFSP J. 2009;3:412–427. doi: 10.2976/1.3239407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw TJ, Martin P. Wound repair at a glance. Journal of cell science. 2009 doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Iglesias PA. Excitable behavior in amoeboid chemotaxis. Wiley Interdiscip Rev Syst Biol Med. 2013;5:631–642. doi: 10.1002/wsbm.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoge M, Yue H, Erickstad M, Bae A, Levine H, Groisman A, et al. Cellular memory in eukaryotic chemotaxis. Proc Natl Acad Sci U S A. 2014;111:14448–14453. doi: 10.1073/pnas.1412197111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JA, Taylor DL. Local and spatially coordinated movements in Dictyostelium discoideum amoebae during chemotaxis. Cell. 1982;28:225–232. doi: 10.1016/0092-8674(82)90340-3. [DOI] [PubMed] [Google Scholar]
- Takeda K, Sasaki AT, Ha H, Seung HA, Firtel RA. Role of phosphatidylinositol 3-kinases in chemotaxis in Dictyostelium. J Biol Chem. 2007;282:11874–11884. doi: 10.1074/jbc.M610984200. [DOI] [PubMed] [Google Scholar]
- Tang M, Wang M, Shi C, Iglesias PA, Devreotes PN, Huang CH. Evolutionarily conserved coupling of adaptive and excitable networks mediates eukaryotic chemotaxis. Nat Commun. 2014;5:5175. doi: 10.1038/ncomms6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi D, Ishihara S, Oonuki T, Honda-Kitahara M, Kaneko K, Sawai S. Phase geometries of two-dimensional excitable waves govern self-organized morphodynamics of amoeboid cells. Proc Natl Acad Sci U S A. 2013;110:5016–5021. doi: 10.1073/pnas.1218025110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Lavigne M. Axon guidance by diffusible repellants and attractants. Curr Opin Genet Dev. 1994;4:596–601. doi: 10.1016/0959-437x(94)90078-h. [DOI] [PubMed] [Google Scholar]
- Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, Mayor R. Collective chemotaxis requires contact-dependent cell polarity. Dev Cell. 2010;19:39–53. doi: 10.1016/j.devcel.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theveneau E, Mayor R. Neural crest delamination and migration: from epithelium-to-mesenchyme transition to collective cell migration. Dev Biol. 2012;366:34–54. doi: 10.1016/j.ydbio.2011.12.041. [DOI] [PubMed] [Google Scholar]
- van Haastert PJ, Keizer-Gunnink I, Kortholt A. Coupled Excitable Ras and F-actin activation mediate spontaneous pseudopod formation and directed cell movement. Mol Biol Cell. 2017 doi: 10.1091/mbc.E16-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranki S, Kim B, Kim L. The GPI-anchored superoxide dismutase SodC is essential for regulating basal Ras activity and for chemotaxis of Dictyostelium discoideum. J Cell Sci. 2008;121:3099–3108. doi: 10.1242/jcs.030056. [DOI] [PubMed] [Google Scholar]
- Veltman DM, Keizer-Gunnik I, Van Haastert PJ. Four key signaling pathways mediating chemotaxis in Dictyostelium discoideum. J Cell Biol. 2008;180:747–753. doi: 10.1083/jcb.200709180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltman DM, Williams TD, Bloomfield G, Chen BC, Betzig E, Insall RH, Kay RR. A plasma membrane template for macropinocytic cups. Elife. 2016;5 doi: 10.7554/eLife.20085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicker MG. The regulation of chemotaxis and chemokinesis in Dictyostelium amoebae by temporal signals and spatial gradients of cyclic AMP. J Cell Sci. 1994;107:659–667. doi: 10.1242/jcs.107.2.659. [DOI] [PubMed] [Google Scholar]
- Wang MJ, Artemenko Y, Cai WJ, Iglesias PA, Devreotes PN. The directional response of chemotactic cells depends on a balance between cytoskeletal architecture and the external gradient. Cell Rep. 2014;9:1110–1121. doi: 10.1016/j.celrep.2014.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ku CJ, Zhang ER, Artyukhin AB, Weiner OD, Wu LF, Altschuler SJ. Identifying network motifs that buffer front-to-back signaling in polarized neutrophils. Cell Rep. 2013;3:1607–1616. doi: 10.1016/j.celrep.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiger MC, Ahmed S, Welf ES, Haugh JM. Directional persistence of cell migration coincides with stability of asymmetric intracellular signaling. Biophys J. 2010;98:67–75. doi: 10.1016/j.bpj.2009.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiger MC, Parent CA. Phosphoinositides in chemotaxis. Subcell Biochem. 2012;59:217–254. doi: 10.1007/978-94-007-3015-1_7. [DOI] [PubMed] [Google Scholar]
- Weiner OD, Marganski WA, Wu LF, Altschuler SJ, Kirschner MW. An actin-based wave generator organizes cell motility. PLoS Biol. 2007;5:e221. doi: 10.1371/journal.pbio.0050221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner OD, Neilsen PO, Prestwich GD, Kirschner MW, Cantley LC, Bourne HR. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat Cell Biol. 2002;4:509–513. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Zheng JQ. Directional guidance of nerve growth cones. Curr Opin Neurobiol. 2006;16:52–58. doi: 10.1016/j.conb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Weninger W, Biro M, Jain R. Leukocyte migration in the interstitial space of non-lymphoid organs. Nat Rev Immunol. 2014;14:232–246. doi: 10.1038/nri3641. [DOI] [PubMed] [Google Scholar]
- Whitaker BD, Poff KL. Thermal adaptation of thermosensing and negative thermotaxis in Dictyostelium. Exp Cell Res. 1980;128:87–93. doi: 10.1016/0014-4827(80)90390-0. [DOI] [PubMed] [Google Scholar]
- Winans AM, Collins SR, Meyer T. Waves of actin and microtubule polymerization drive microtubule-based transport and neurite growth before single axon formation. Elife. 2016;5:e12387. doi: 10.7554/eLife.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, et al. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- Wu L, Valkema R, Van Haastert PJ, Devreotes PN. The G protein beta subunit is essential for multiple responses to chemoattractants in Dictyostelium. J Cell Biol. 1995;129:1667–1675. doi: 10.1083/jcb.129.6.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Wu X, De Camilli P. Calcium oscillations-coupled conversion of actin travelling waves to standing oscillations. Proc Natl Acad Sci U S A. 2013;110:1339–1344. doi: 10.1073/pnas.1221538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong D, Xiao S, Guo S, Lin Q, Nakatsu F, Wu M. Frequency and amplitude control of cortical oscillations by phosphoinositide waves. Nat Chem Biol. 2016;12:159–166. doi: 10.1038/nchembio.2000. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Huang CH, Iglesias PA, Devreotes PN. Cells navigate with a local-excitation, global-inhibition-biased excitable network. Proc Natl Acad Sci U S A. 2010;107:17079–17086. doi: 10.1073/pnas.1011271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HW, Collins SR, Meyer T. Locally excitable Cdc42 signals steer cells during chemotaxis. Nat Cell Biol. 2016;18:191–201. doi: 10.1038/ncb3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Dormann D, Münsterberg AE, Weijer CJ. Cell movement patterns during gastrulation in the chick are controlled by positive and negative chemotaxis mediated by FGF4 and FGF8. Dev Cell. 2002;3:425–437. doi: 10.1016/s1534-5807(02)00256-3. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Soldati T. Dissection of amoeboid movement into two mechanically distinct modes. J Cell Sci. 2006;119:3833–3844. doi: 10.1242/jcs.03152. [DOI] [PubMed] [Google Scholar]
- Zhang S, Charest PG, Firtel RA. Spatiotemporal regulation of Ras activity provides directional sensing. Curr Biol. 2008;18:1587–1593. doi: 10.1016/j.cub.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Jin T, McCaig CD, Forrester JV, Devreotes PN. Genetic analysis of the role of G protein-coupled receptor signaling in electrotaxis. J Cell Biol. 2002;157:921–927. doi: 10.1083/jcb.200112070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1.
A representation of three common types of mechanical and spatially located migratory cues. Green represents regions of stimulation and red represents unstimulated cell periphery. A) Specific amoeboid type cells travel of a gradient of shear flow cues demonstrated by the black arrows. B) Epithelial cells travel down the gradient of shear forces on the cell. C) Haptotaxis is the distribution of cues on extracellular substrate which direct cell migration. D) Durotaxis is migration along a substrate’s stiffness gradient demonstrated by increased shading in the substrate.


