Abstract
The objective of this study was to define the behavioral, electrophysiological, and morphological characteristics of spontaneous autoimmune peripheral polyneuropathy (SAPP) in female B7-2 deficient non-obese diabetic (NOD) mice. A cohort of 77 female B7-2 deficient and 31 wild-type control NOD mice were studied from 18 to 40 weeks of age. At pre-defined time points, the dorsal caudal tail and sciatic motor nerve conduction studies (MNCS) were performed. Sciatic nerves were harvested for morphological evaluation. SAPP mice showed slowly progressive severe weakness in hind and forelimbs without significant recovery after 30 weeks of age. MNCS showed progressive reduction in mean compound motor action potential amplitudes and conduction velocities, and increase in mean total waveform duration from 24 to 27 weeks of age, peaking between 32 and 35 weeks of age. Toluidine blue-stained, semi-thin plastic-embedded sections demonstrated focal demyelination associated with mononuclear cell infiltration early in the disease course, with progressively diffuse demyelination and axonal loss associated with more intense mononuclear infiltration at peak severity. Immunohistochemistry confirmed macrophage-predominant inflammation. This study verifies SAPP as a progressive, unremitting chronic inflammatory demyelinating polyneuropathy with axonal loss.
Keywords: chronic neuritis, histopathology, inflammatory neuropathy, mouse models, nerve electrophysiology
Introduction
Spontaneous autoimmune peripheral polyneuropathy (SAPP) is a recently developed reproducible mouse model of chronic peripheral nerve inflammation observed in female non-obese diabetic (NOD) mice deficient in co-stimulatory molecule, B7-2 (B7-2−/−; also known as CD86). Earliest onset of SAPP in B7-2−/− NOD mice was 20 weeks of age, with 100% of females and 30% of males affected by 32 weeks of age (Salomon et al., 2001). The immunopathogenesis of SAPP was determined in female mice, and verified using severe combined immunodeficiency NOD mice and a myelin protein zero-specific T-cell receptor transgenic mouse strain, in which fulminant peripheral neuritis was observed when these mice were bred to a recombination activating gene background. There is evidence that SAPP is an interferon-γ, CD4+ T-cell-mediated disorder, with autoreactive T-cells and autoantibodies directed against myelin protein zero. This disorder is also associated with a reduction in antigen-specific CD4+ Foxp3+ regulatory T-cells (Salomon et al., 2001; Louvet et al., 2009). Female B7-2−/− NOD mice are protected from diabetes, unlike female wild-type (B7-2+/+) mice that develop severe diabetes (>70%) due to pancreatic insulin-secreting cell inflammation starting at 15 weeks of age. However, none of the diabetic mice developed peripheral neuropathy or myopathy based on electrophysiological and histopathological evaluation (Salomon et al., 2001).
The initial characterization of the SAPP model demonstrated some electrophysiological and histopathological evidence for demyelination in severe cases. There was some evidence for partial conduction block and axonal damage in severe SAPP based on peak-to-peak amplitude measurements and ongoing denervation in foot intrinsic muscles. These findings were associated with significant infiltration of dendritic cells and CD4+ and CD8+ T-lymphocytes into sciatic nerves (without central nervous system involvement) compared with unaffected controls (Salomon et al., 2001). We had previously demonstrated some degree of conduction block in 50% of dorsal caudal tail (DCT) nerves studied by motor nerve electrophysiology at maximal severity (Xia et al., 2010b).
It is unclear if SAPP is an “all-or-none” chronic neuritis that spontaneously develops with maximal involvement from the onset in some mice, progresses in a stepwise fashion and persists without resolution, or is a slowly progressive or relapsing–remitting demyelinating neuritis in a large cohort of susceptible NOD mice. Detailed information on the electrophysiological characterization of demyelination and axonal loss in SAPP during its disease course is lacking. It is also unclear whether macrophages, the predominant leukocyte found in immune-mediated polyneuropathies and implicated in demyelination and axonal degeneration in human peripheral nerves are present in affected SAPP nerves (Rizzuto et al., 1998; Bouchard et al., 1999; Kiefer et al., 2001; Kieseier et al., 2002; Köller et al., 2005; Meyer zu Hörste et al., 2007). The extent of axonal injury that may occur in SAPP during its disease course is also not known.
Deducing the natural history of SAPP using behavioral assessments of muscle weakness (i.e., neuromuscular severity scores [NMSS]), detailed motor nerve electrophysiology, and nerve histopathology is necessary, as it validates SAPP as a model to evaluate pathogenic mechanisms and signaling pathways responsible for demyelination and axonal injury in chronic polyneuritis. Furthermore, validated behavioral, electrophysiological, and histopathological assessments provide objective, quantitative, or semi-quantitative measures of peripheral nerve function that can be used in pathogenic and pre-clinical treatment studies in SAPP mice. We sought to address these knowledge gaps by comparing these variables in a cohort of female B7-2−/− and B7-2+/+ NOD mice.
Materials and Methods
Animals
B7-2−/− (NOD.129S4-Cd86tm1Shr/JbsJ) and B7-2+/+ (NOD/ShiLtJ) NOD mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and bred homozygous × homozygous in the specific pathogen-free Transgenic Mouse Facility (a barrier level 3 facility) at Baylor College of Medicine. Genotyping and strain susceptibility to diabetes were confirmed by the supplier. Mice were kept in micro-isolator cages with chow and water provided ad libitum, maintaining a 12 h light–dark cycle. All animal procedures were reviewed and approved by the Baylor College of Medicine Institutional Animal Care and Use Committee in compliance with the National Research Council Guide for the Care and Use of Laboratory Animals.
Behavioral assessments
NMSS and weights were obtained twice a week in a cohort of 77 female B7-2−/− and 31 wild-type B7-2+/+ controls from 18 to 40 weeks of age. Severity of weakness was determined using a published 6-point scale (0 = normal strength, 1 = mild tail weakness, 2 = mild/moderate fore or hind limb weakness, 3 = severe fore or hind limb weakness, 4 = mild/moderate fore and hind limb weakness, 5 = severe fore and hind limb weakness) (Xia et al., 2010a; 2010c). The neuromuscular severity score was recorded and motor nerve electrophysiology performed on mice with external signs of distress prior to euthanasia. Intermediate scores were given for asymmetrical hind or forelimb weakness.
Nerve electrophysiology
DCT and sciatic motor nerve conduction studies (MNCS) were performed bilaterally on each mouse at pre-defined time intervals during the study using a portable Keypoint® v5.11 electrodiagnostic system (Alpine Biomed Corporation, Fountain Valley, CA, USA) with waveforms displayed on a Tecra S3 LCD monitor (Toshiba America, Irvine, CA, USA), as previously described (Xia et al., 2010a; 2010b; 2010c). Distal and proximal compound motor action potential (CMAP) amplitudes (measured from baseline to negative peak [in mV]), conduction velocity (automated calculation based on distance between distal and proximal stimulating points [in mm] divided by the difference between distal and proximal onset latencies [in ms]), and total waveform durations (measured from initial negative waveform deflection until return to baseline [in ms]) were measured.
Sciatic nerve histopathology
Following nerve electrophysiology, anesthetized mice were euthanized by cervical disarticulation. The sciatic nerves were immediately dissected from the region of the sciatic notch to the posterior popliteal fossa proximal to or including its trifurcation on both sides and placed on filter paper cards to maintain longitudinal alignment. Nerve specimens were placed in either 3% glutaraldehyde in 0.1 M phosphate buffer for plastic embedding or placed in Tissue-Tek® optimum cutting temperature compound (Sakura Finetek USA Inc., Torrance, CA, USA) and immediately stored at −80°C for cryostat sectioning. Glutaraldehyde-fixed nerve sections from pre-defined time points were post-fixed in 1% osmium tetroxide and prepared for and embedded in epoxy resin for semi-thin (1 μm) Tolui-dine blue-stained sections as previously described (Xia et al., 2010a). These were evaluated for morphological evidence of leukocyte infiltration, demyelination, and axonal degeneration/loss during the course of SAPP, and compared with unaffected age-matched controls.
Indirect immunohistochemistry (to characterize the inflammatory infiltrates and further demonstrate demyelination and axonal loss) was performed on 10 μm acetone-fixed, sciatic nerve axial cryostat sections of SAPP-affected and control mice, using previously described protocols (Xia et al., 2010a). The following primary antibodies were used sequentially on separate slides: polyclonal rabbit anti-mouse neurofilament heavy polypeptide (NF-H, an axonal marker) IgG, polyclonal rabbit anti-mouse S100β (Schwann cell marker) IgG [both from Santa Cruz Biotechnology, Santa Cruz, CA, USA], monoclonal rat anti-mouse F4/80 (mouse macrophage marker) IgG2b (AbD Serotec, Oxford, UK), monoclonal rat-anti-mouse CD3 (T-cell marker) IgG2b, and rat anti-mouse CD19 (B-cell marker) IgG2a (all from SouthernBiotech, Birmingham, AL, USA). The following secondary antibodies were used (all from SouthernBiotech): polyclonal goat anti-rabbit IgG-Texas Red (for NF-H), polyclonal goat anti-rabbit IgG-Fluorescein isothiocyanate (for S100β), polyclonal goat anti-rat IgG-Texas Red (for F4/80), and polyclonal goat anti-rat IgG-Fluorescein isothiocyanate (for CD3 and CD19).
Statistical analysis
Wilcoxon-Kruskall’s Rank Sum Test was used to determine statistically significant differences between nonparametric neuromuscular severity scores while Student’s t-test (or analysis of variance for multiple comparisons) was used for electrophysiological parameters that were normally distributed based on tests of skewness and kurtosis. Means are displayed ± standard errors. Statistical significance is defined as p-value <0.05.
Results
Neuromuscular severity scores
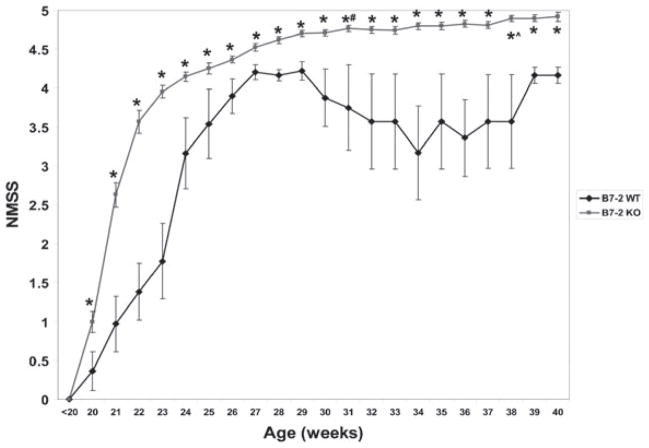
Survival in B7-2−/− NOD mice over 40 weeks was 92%, compared with 50% in NOD B7-2+/+ wild-type mice. B7-2−/− mice were compared with B7-2+/+ wild-type control mice to evaluate whether the immune-mediated polyneuropathy was specific to the genetic alterations that occur following B7-2 deletion or was a natural consequence of aging in the NOD strain. As previously described, the earliest onset of SAPP occurred at 20 weeks of age. SAPP mice typically presented with bilateral mild hind limb weakness that progressed to severe fore and hind limb weakness. NMSS gradually worsened with age, reaching a statistically significant plateau by 31 weeks (mean NMSS 4.77 vs. 4.53 at 27 weeks of age, p = 0.0004). There was a small, but significant worsening in mean NMSS from 38 weeks of age onwards (mean NMSS 4.90, p = 0.013 compared to 31 weeks) in this cohort of female B7-2−/− NOD mice. B7-2+/+ female NOD mice became less active and developed generalized mild muscle weakness (associated with significant weight loss, polyuria and polydipsia [data not shown]) later in the disease course that was behaviorally difficult to distinguish from SAPP-affected mice with mild tetraparesis. This weakness was an “all-or-none” response that became more prevalent in surviving wild-type female mice with age. NMSS were statistically significantly worse in female B7-2−/− NOD mice from 20 weeks of age compared to age-matched female B7-2+/+ NOD mice at all time points (Fig. 1).
Figure 1.
Natural history of neuromuscular weakness in SAPP. This figure shows the mean neuromuscular severity scores (NMSS) from a cohort of 77 B7-2 knockout (B7-2 KO) and 31 B7-2 wild-type (B7-2 WT) female NOD mice. There is progressive weakness in B7-2 KO mice (red lines), with the most rapid progression seen from 20 to 24 weeks of age. There is gradual progression in weakness reaching a plateau by 31 weeks, with slight worsening seen after 38 weeks of age in this cohort. Based on NMSS, SAPP can be divided into a disease onset phase (20–24 weeks), early progressive phase (25–30 weeks), plateau (31–37 weeks), and late progressive phase (>38 weeks). Weakness is also observed in age-controlled B7-2 WT female mice affected with diabetes (blue lines) in an “all-or-none” fashion (resulting in wider error bars) that reached a plateau by 27 weeks of age. Few B7-2 WT mice survived after 30 weeks of age, resulting in wider variations in mean NMSS between 29 and 39 weeks of age. B7-2 KO mice are weaker than WT controls from ages 20–40 weeks. * indicates p < 0.05 comparing age-matched B7-2 KO and B7-2 WT mice, # indicates p < 0.05 between 27 weeks (early progressive) and 31 weeks (plateau) in SAPP, and ^ indicates p < 0.05 between 31 weeks (plateau) and late progressive phase (38 weeks).
Motor nerve electrophysiology
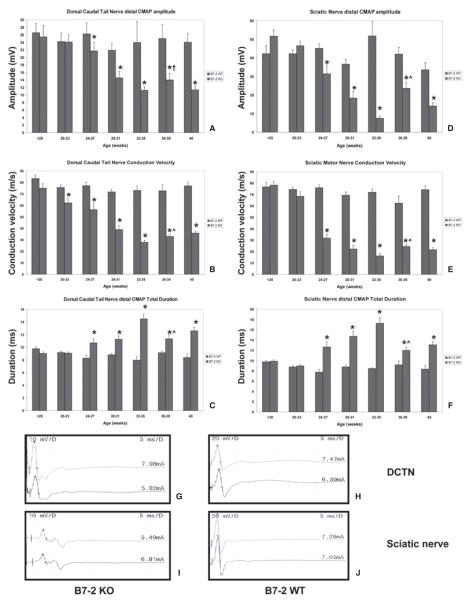
CMAP amplitudes provide a measure of total motor axonal integrity; conduction velocity is a measure of saltatory conduction in the fastest conducting motor axons (mainly a measure of the degree of myelination in these axons), while total waveform duration indicates the degree of synchrony in motor axonal conduction (a measure of conduction variability in myelinated axons). In the DCT and sciatic MNCS, there were statistically significant progressive reductions in mean CMAP amplitudes and conduction velocities, with increases in mean total waveform duration from 24 to 27 weeks of age, reaching a nadir or peak between 32 and 35 weeks of age in SAPP mice compared with controls (Fig. 2). B7-2+/+ female NOD mice did not develop a polyneuropathy based on these parameters, implying that the weakness observed was most likely related to generalized malaise secondary to severe diabetes with muscle wasting, confirming previous observations (Salomon et al., 2001). At peak severity, the mean DCT distal CMAP amplitude in SAPP-affected mice (compared to wild-type controls) was 11.2 ± 1.0 mV (vs. 24.0 ± 5.7 mV; p = 0.001), conduction velocity 27.8 ± 1.9 m/s (vs. 73.1 ± 3.9 m/s; p < 0.0001) and total waveform duration of 14.5 ± 0.7 ms (vs. 8.0 ± 0.5 ms; p < 0.0001) as shown in Figs. 2A–C. The mean sciatic motor NCS parameters at peak severity were as follows: mean distal CMAP amplitude 7.5 ± 1.0 mV (vs. 51.8 ± 7.7 mV; p < 0.0001), conduction velocity 16.2 ± 2.0 m/s (vs. 72.1 ± 2.9 m/s; p < 0.0001), and distal waveform duration 17.7 ± 1.1 ms (vs. 8.5 ± 0.2 ms; p < 0.0001), as shown in Figs. 2D–F.
Figure 2.
Motor nerve electrophysiology and representative waveforms in SAPP. The mean dorsal caudal tail (A–C) and sciatic (D–F) motor nerve electrophysiological studies of a cohort of B7-2 WT and B7-2 KO mice, grouped by age is shown. A reduction in mean dorsal caudal tail motor conduction velocity starts at 20–23 weeks of age in B7-2 KO mice compared to B7-2 WT controls (B), implying that demyelination of large myelinated axons is the earliest feature of SAPP. Reduction in mean CMAP amplitude and increase in total CMAP duration become apparent by 24–27 weeks of age (A, C), implying associated axonal injury and further demyelination of small- and medium-sized axons, respectively. Significant changes in these parameters in the sciatic nerves occur between 24 and 27 weeks (D–F), implying diffuse demyelination with axonal injury/loss. There are no statistically significant changes in these electrophysiological parameters in B7-2 WT mice. The progressive reductions in mean CMAP amplitude and conduction velocity reach a nadir, and the increase in total CMAP duration reaches a peak between 32 and 35 weeks age. There are small but significant increases in conduction velocity and reduction in total CMAP duration between 36 and 39 weeks of age, suggesting axonal remyelination in both nerves. There is no significant change in mean dorsal caudal tail nerve CMAP amplitudes between 36 and 39 weeks of age; however, a statistically significant increase in sciatic nerve CMAP amplitudes is observed, suggesting some degree of axonal regeneration or resolution in distal conduction block. * indicates p < 0.05 relative to age-matched B7-2 WT and <20-week-old B7-2 KO mice, † indicates p > 0.05 between 32–35 week and 36–39 week data, and ^ indicates p < 0.05 between 32–35 week and 36–39 week data. Representative distal (upper) and proximal (lower) CMAP waveforms from the dorsal caudal tail nerve (DCTN) and sciatic nerve of a female B7-2 KO mouse with SAPP at peak severity (G and I, respectively) are compared with an age-matched unaffected B7-2 WT mouse (H and J). There are reductions in CMAP amplitudes and increases in total waveform durations in SAPP compared to controls. The reduction in CMAP amplitudes is generally uniformly diffuse in the sciatic nerves (I), while approximately 25%–50% reduction in proximal amplitudes compared to distal responses is commonly observed in the DCTN (G), suggesting a component of conduction block between distal and proximal stimulation sites. Numbers to the upper left depict sensitivity in mV per division (mV/D), numbers to the upper right depict sweep speed in ms per division (ms/D) while numbers above each waveform to the right represent supramaximal stimulus current (in mA).
In the DCT and sciatic nerves, there were statistically significant increases in mean MNCS conduction velocity and reductions in total distal waveform duration at 36–39 weeks of age compared to peak severity at 32–35 weeks of age in SAPP mice (at 36–39 weeks: DCT conduction velocity 33.0 ± 1.8 m/s, p = 0.05; waveform duration 11.3 ± 0.7 ms, p = 0.003; sciatic nerve conduction velocity 24.5 ± 2.1 m/s, p = 0.01; waveform duration 12.0 ± 0.6 ms, p = 0.0003). There was a statistically significant increase in mean sciatic nerve CMAP amplitudes between 36 and 39 weeks of age (21.7 ± 3.1 mV, p < 0.0001) without improvement in the DCTN CMAP amplitudes (14.0 ± 1.7 mV, p = 0.17). These data imply that some remyelination or resolution of conduction block or both may take place in SAPP nerves between 36 and 39 weeks of age; however, there was persistent evidence of demyelination and axonal compromise at 40 weeks of age, as seen in Fig. 2. Representative electrophysiological waveforms comparing SAPP at peak severity with unaffected age-matched controls are shown in Figs. 2G–K. These data suggest that SAPP is a slowly progressive chronic demyelinating polyneuropathy with associated axonal compromise that persists with little electrophysiological resolution for up to 40 weeks of age in female B7/2−/− NOD mice.
Peripheral nerve histopathology
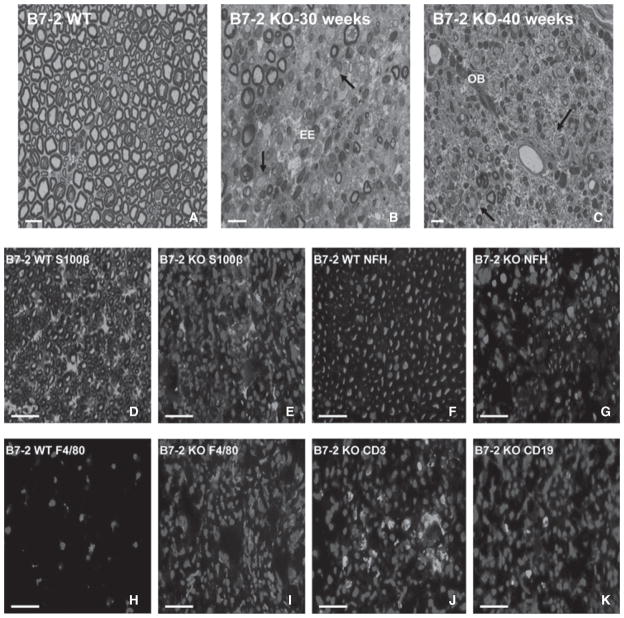
Toluidine blue-stained semi-thin plastic-embedded sections demonstrated focal demyelination associated with mononuclear cell infiltration early in the disease course, with progressive, more diffuse demyelination and axonal loss associated with intense mononuclear infiltration and endoneurial edema. Wild-type B7-2+/+ control mice at all ages and B7-2−/− mice at 18 weeks of age showed normal myelin and axonal architecture, with rare endoneurial leukocytes (Fig. 3A). This supports the normal motor nerve electrophysiological studies in control mice. During early disease states, there were areas with minimal leukocyte infiltration or demyelination, areas with foci of mononuclear cells associated with thinly myelinated or demyelinated axons without pathological evidence for Wallerian degeneration and areas with inflammatory demyelination with active Wallerian degeneration and intraendoneurial edema. Increased mononuclear leukocyte infiltration associated with worsening demyelination, Wallerian degeneration and reduction in axonal density, and endoneurial edema were observed at peak severity (Fig. 3B). During the later stages of disease, onion-bulb formation (due to repetitive demyelination-remyelination), persistent mononuclear cell infiltration with demyelinated axons and reduced axonal density were present, as shown in Fig. 3C. These features are compatible with a chronic demyelinating neuritis, and consistent with the motor nerve electrophysiological data.
Figure 3.
Pathological characterization of sciatic nerves in SAPP. Toluidine blue-stained photomicrographs of representative 1 μm semi-thin axial sections of plastic-embedded sciatic nerves at different ages are shown (A–C). There is normal axonal density with large, medium, and small myelinated axons in B7-2 WT mice at all ages (A), with rare endoneurial leukocytes seen. Increased leukocyte infiltration (red arrows) with uniformly persistent demyelination (black arrows) and endoneurial edema (EE) are seen in the sciatic nerves at peak severity, as observed at 30 weeks of age in a severely affected B7-2 KO mouse (B). During the late stages of SAPP (C), there is onion-bulb formation (OB), persistence of thinly myelinated and demyelinated axons (black arrows) and foci of infiltrated leukocytes (red arrow), as commonly observed in CIDP nerve biopsies. Digitally merged photomicrographs of representative indirect immunohistochemical 10 μm axial cryostat sections of sciatic nerves stained to detect S100β (Schwann cell/myelin marker: D and E), neurofilament heavy chain (NF-H; axon marker: F and G), F4/80 (macrophage marker: H and I), CD3 (T-cell receptor: J) and CD19 (B-cell marker; K) in B7-2 WT and KO NOD mice at 40 weeks of age are shown. The normal honeycomb appearance of myelinated axons (green immunoreactivity) with scattered nuclei (DAPI stain: blue immunoreactivity) seen in B7-2 WT mice (D) is disrupted, with increased leukocyte infiltration (increased blue immunoreactivity) seen in a SAPP-affected B7-2 KO mouse (E). Normal axonal density (red immunoreactivity) in the same B7-2 WT mouse is seen (F), in contrast to significant axonal loss associated with leukocyte infiltration in the SAPP-affected mouse (G). Rare macrophages (red immunoreactivity) are seen in an unaffected B7-2 WT mouse (H) in contrast to intense macrophage infiltration seen in SAPP-affected B7-2 KO mice (I). Foci of T-cells and scattered B-cells (green immunoreactivity) are also observed in affected mice (J and K, respectively) while these cells are rarely detected in B7-2 WT mouse sciatic nerves. Macrophages are the predominant leukocyte subpopulation seen in SAPP. These histopathological features are diffuse and consistently observed in SAPP-affected B7-2 KO mice >30 weeks of age. Magnification bars = 10 μm in A–C and 20 μm in D–K.
Peripheral nerve immunohistochemistry
Indirect immunohistochemistry demonstrated significant loss of S-100β and NF-H immunoreactivity in SAPP-affected B7-2−/− mice compared to unaffected B7-2+/+ controls (Figs. 3D–G). These observations further support Schwann cell injury/demyelination and axonal loss, respectively, as suggested by motor nerve electrophysiology and demonstrated on semi-thin plastic-embedded sections. There was significant infiltration of mononuclear leukocytes (F4/80+ macrophages followed by CD3+ T-cells and CD19+ B-cells) into the sciatic nerves of SAPP-affected mice at peak severity that persisted for up to 40 weeks of age (Figs. 3H–K), as described in immune-mediated polyneuropathies such as chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). These data show that the SAPP is a mouse model with essential electrophysiological and histopathological features of a severe immune-mediated demyelinating polyneuritis.
Discussion
This study establishes the natural history of SAPP in a large cohort of female B7-2−/− NOD mice over a defined period (age 18–40 weeks), providing detailed behavioral, electrophysiological, and histopathological data. Our results indicate that SAPP is a chronic progressive, inflammatory, predominantly demyelinating polyneuropathy with secondary axonal degeneration and cumulative axonal loss over time. The inflammatory and neuropathic features are not present in age- and gender-matched wild-type controls, emphasizing an important role of B7-2 in the dysregulation of cellular immunity, as well as tissue-specific inflammation and injury of peripheral nerves in NOD mice. Notably, the disease penetrance is 100% in females, and the severity of peripheral nerve injury is quite uniform in this model. These features, as well as its spontaneous onset and disease chronicity, make SAPP a suitable model for pathogenic and preclinical studies focusing on chronic inflammatory demyelinating neuropathies. This contrasts with active or passive transfer experimental autoimmune neuritis, which typically has a monophasic course and inherent variability in disease incidence and severity that occurs with induction protocols using extrinsic agents (Meyer zu Hörste et al., 2007).
This study confirms initial observations that SAPP is a predominantly demyelinating neuropathy (Salomon et al., 2001; Xia et al., 2010b). Our electrophysiological studies show that the demyelinating process is diffuse and progressive (based on a gradual reduction in mean conduction velocities and increase in mean total waveform durations in two motor nerves bilaterally) and is most marked when mice are most severely affected clinically. Furthermore, motor electrophysiological studies demonstrated progressive reductions in mean CMAP amplitudes that reached a nadir when mice were most severely affected. These electrophysiological data suggest that axonal injury/loss or distal conduction block may also occur in SAPP.
We show that the pathological changes in peripheral nerves evolve over time in this model. Early on, multi-focal demyelination associated with mononuclear leukocyte infiltration that predominantly involved large myelinated axons was seen in SAPP-affected nerves. This progressed to diffuse demyelination with more intense inflammation, Wallerian degeneration, and subsequent axonal loss. Although distal conduction block may occur in SAPP, these morphological observations imply that axonal degeneration with subsequent loss occurs in this model, as described in CIDP patients (Bouchard et al., 1999). Macrophage-predominant infiltration was demonstrated by immunohistochemistry, supporting the observation that SAPP is a chronic inflammatory neuropathy with primary targeted autoimmune attack against myelin protein zero (Louvet et al., 2009), the most prevalent protein found in mammalian myelin (Garbay et al., 2000). Why deficiency in a general co-stimulatory molecule (B7-2) required for T-cell activation following interactions with antigen presenting cells in NOD mice results in a restricted inflammatory polyneuropathy associated with autoimmunity against myelin protein zero remains a fascinating question. NOD mice are quite susceptible to autoimmune disorders, with a restricted chronic demyelinating peripheral neuritis also described in intercellular adhesion molecule-1-deficient mice (Meyer zu Hörste et al., 2011), peripheral neuritis, sialadenitis and gastritis in programmed cell death-1-deficient mice with the anti-diabetogenic major histocompatibility complex haplo-type, H-2(b) (Yoshida et al., 2008; Jiang et al., 2009), and myositis, sensory ganglionitis, and central nervous system inflammation in mice deficient in inducible costimulator (ICOS) and ICOS-ligand (Prevot et al., 2010). In all of these models, homozygous knockout mice were protected from developing diabetes, as observed in B7-2−/− NOD mice.
The observation that at the early stages of disease, hematogenous leukocyte infiltration associates with demyelination without axonal degeneration/loss raises the possibility that demyelinated axons are more susceptible to injury in the chronic inflammatory milieu within the endoneurium in SAPP. If effective strategies that can modulate chronic inflammation and/or demyelination were to become available, then this preclinical model provides an opportunity to test whether inhibition of peripheral nerve leukocyte infiltration and/or demyelination could prevent subsequent axonal loss. This issue has potential relevance to many disorders including CIDP, where disability correlates to the extent of axonal compromise in humans (Bouchard et al., 1999; Nagamatsu et al., 1999). Determining the molecular mediators and signaling pathways responsible for early and persistent inflammation and demyelination in this model could have translational implications for chronic demyelinating polyneuropathies in humans. In summary, SAPP is a progressive chronic demyelinating polyneuritis with associated axonal loss restricted to the peripheral nervous system. This is a reliable and reproducible pre-clinical model that may prove useful to the study of disease effector mechanisms in chronic demyelinating polyneuritis.
Acknowledgments
This study is supported in part by the GBS/CIDP Foundation International Research Grant and Baylor College of Medicine New Investigator start-up award to E. E. U. and NIH Grants NS42888 and NS54962 to K. A. S.
Footnotes
Presented in part as an abstract at the Peripheral Nerve Society Satellite meeting in Sydney, Australia in July 2010.
References
- Bouchard C, Lacroix C, Planté V, Adams D, Chedru F, Guglielmi J, Said G. Clinicopathologic findings and prognosis of chronic inflammatory demyelinating polyneuropathy. Neurology. 1999;52:498–503. doi: 10.1212/wnl.52.3.498. [DOI] [PubMed] [Google Scholar]
- Garbay B, Heape A, Sargueil F, Cassagne C. Myelin synthesis in the peripheral nervous system. Prog Neurobiol. 2000;61:267–304. doi: 10.1016/s0301-0082(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Jiang F, Yoshida T, Nakaki F, Terawaki S, Chikuma S, Kato Y, Okazaki IM, Honjo T, Okazaki T. Identification of QTLs that modify peripheral neuropathy in NOD.H2b-Pdcd1−/−mice. Int Immunol. 2009;21:499–509. doi: 10.1093/intimm/dxp020. [DOI] [PubMed] [Google Scholar]
- Kiefer R, Kieseier BC, Stoll G, Hartung HP. The role of macrophages in immune-mediated damage to the peripheral nervous system. Prog Neurobiol. 2001;64:109–127. doi: 10.1016/s0301-0082(00)00060-5. [DOI] [PubMed] [Google Scholar]
- Kieseier B, Dalakas M, Hartung H. Immune mechanisms in chronic inflammatory demyelinating neuropathy. Neurology. 2002;59:S7–S12. doi: 10.1212/wnl.59.12_suppl_6.s7. [DOI] [PubMed] [Google Scholar]
- Köller H, Kieseier BC, Jander S, Hartung HP. Chronic inflammatory demyelinating polyneuropathy. N Engl J Med. 2005;352:1343–1356. doi: 10.1056/NEJMra041347. [DOI] [PubMed] [Google Scholar]
- Louvet C, Kabre B, Davini D, Martinier N, Su M, DeVoss J, Rosenthal W, Anderson M, Bour-Jordan H, Bluestone J. A novel myelin P0-specific T cell receptor transgenic mouse develops a fulminant autoimmune peripheral neuropathy. J Exp Med. 2009;206:507–514. doi: 10.1084/jem.20082113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer zu Hörste G, Hartung H, Kieseier B. From bench to bedside – experimental rationale for immune-specific therapies in the inflamed peripheral nerve. Nat Clin Pract Neurol. 2007;3:198–211. doi: 10.1038/ncpneuro0452. [DOI] [PubMed] [Google Scholar]
- Meyer zu Hörste G, Mausberg AK, El-Haddad H, Martin S, Hartung H-P, Kieseier BC. Spontaneous demyelinating autoimmune neuropathy in ICAM-1 deficient NOD mice. J Peripher Nerv Syst. 2011;16:S87. [Google Scholar]
- Nagamatsu M, Terao S, Misu K, Li M, Hattori N, Ichimura M, Sakai M, Yamamoto H, Watanabe H, Riku S, Ikeda E, Hata J, Oda M, Satake M, Nakamura N, Matsuya S, Hashizume Y, Sobue G. Axonal and perikaryal involvement in chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry. 1999;66:727–733. doi: 10.1136/jnnp.66.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevot N, Briet C, Lassmann H, Tardivel I, Roy E, Morin J, Mak TW, Tafuri A, Boitard C. Abrogation of ICOS/ICOS ligand costimulation in NOD mice results in autoimmune deviation toward the neuromuscular system. Eur J Immunol. 2010;40:2267–2276. doi: 10.1002/eji.201040416. [DOI] [PubMed] [Google Scholar]
- Rizzuto N, Morbin M, Cavallaro T, Ferrari S, Fallahi M, Galiazzo Rizzuto S. Focal lesions area feature of chronic inflammatory demyelinating polyneuropathy (CIDP) Acta Neuropathol. 1998;96:603–609. doi: 10.1007/s004010050941. [DOI] [PubMed] [Google Scholar]
- Salomon B, Rhee L, Bour-Jordan H, Hsin H, Montag A, Soliven B, Arcella J, Girvin A, Padilla J, Miller S, Bluestone J. Development of spontaneous autoimmune peripheral polyneuropathy in B7-2-deficient NOD mice. J Exp Med. 2001;194:677–684. doi: 10.1084/jem.194.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia R, Yosef N, Ubogu E. Clinical, electrophysiological and pathologic correlations in a severe murine experimental autoimmune neuritis model of Guillain-Barré syndrome. J Neuroimmunol. 2010a;219:54–63. doi: 10.1016/j.jneuroim.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Xia R, Yosef N, Ubogu E. Dorsal caudal tail and sciatic motor nerve conduction studies in adult mice: technical aspects and normative data. Muscle Nerve. 2010b;41:850–856. doi: 10.1002/mus.21588. [DOI] [PubMed] [Google Scholar]
- Xia RH, Yosef N, Ubogu EE. Selective expression and cellular localization of pro-inflammatory chemokine ligand/receptor pairs in the sciatic nerves of a severe murine experimental autoimmune neuritis model of Guillain-Barré syndrome. Neuropathol Appl Neurobiol. 2010c;36:388–398. doi: 10.1111/j.1365-2990.2010.01092.x. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Jiang F, Honjo T, Okazaki T. PD-1 deficiency reveals various tissue-specific autoimmunity by H-2b and dose-dependent requirement of H-2g7 for diabetes in NOD mice. Proc Natl Acad Sci USA. 2008;105:3533–3538. doi: 10.1073/pnas.0710951105. [DOI] [PMC free article] [PubMed] [Google Scholar]